Abstract
Although perception begins when a stimulus is transduced by a sensory neuron, numerous perceptual mechanisms can modify sensory information as it is processed by an animal's nervous system. One such mechanism is categorical perception, in which (1) continuously varying stimuli are labelled as belonging to a discrete number of categories and (2) there is enhanced discrimination between stimuli from different categories as compared with equally different stimuli from within the same category. We have shown previously that female zebra finches (Taeniopygia guttata) categorically perceive colours along an orange–red continuum that aligns with the carotenoid-based coloration of male beaks, a trait that serves as an assessment signal in female mate choice. Here, we demonstrate that categorical perception occurs along a blue–green continuum as well, suggesting that categorical colour perception may be a general feature of zebra finch vision. Although we identified two categories in both the blue–green and the orange–red ranges, we also found that individuals could better differentiate colours from within the same category in the blue–green as compared with the orange–red range, indicative of less clear categorization in the blue–green range. We discuss reasons why categorical perception may vary across the visible spectrum, including the possibility that such differences are linked to the behavioural or ecological function of different colour ranges.
Keywords: colour perception, discrimination, vision, zebra finch, sexual selection, signalling
1. Introduction
Colour plays a critical role in animal behaviour, from communication to foraging to camouflage [1]. Colour stimuli vary continuously and, in studies of colour perception, it is often implicitly assumed that a viewer's perception is also continuous; that is, that any given change across a colour spectrum will result in a similar change in the viewer's perception of that colour. This assumption is often untested, however, and increasing evidence suggests that the perception of many kinds of stimuli, including colour, can be categorical [2–8]. Categorical perception refers to a perceptual mechanism by which a sensory system sorts continuously varying stimuli into distinct groups [9]. The two hallmarks of categorical perception are that (1) continuously varying stimuli appear to be labelled as belonging to a discrete number of perceptual categories and (2) stimuli from different sides of the category boundary are discriminated more readily than stimuli that differ by a similar predicted perceptual distance, but that fall within the same category [10,11]. Whether the perception of variation in a given colour stimulus is continuous or categorical can have important implications for our understanding of the function and evolution of coloration in animals.
Categorical perception was first described in the context of the discrimination of phonemes in human speech, where it helps compensate for variation in the articulation of speech sounds in different word contexts and across different individuals [11,12]. Categorical perception has since been shown to play a role in the perception of natural acoustic signals across a variety of animals, including frogs, crickets and birds [2–4]. Humans also perceive continuous colour variation as separate colour categories [5,6], and work with macaques has demonstrated categorical colour perception in non-human primates as well [7]. We recently described categorical colour perception in a songbird, the zebra finch (Taeniopygia guttata), the first demonstration of this phenomenon outside of primates [8]. Specifically, we showed that females categorically perceive an orange to red colour range that aligns with the carotenoid-based coloration of male beaks [8], a signal of male quality used by females to assess potential mates [13]. Songbirds represent an important study system for understanding colour perception given that they are highly visual animals that exhibit numerous adaptations for colour vision [14,15] and famously use a wide range of colours in displays across many behavioural contexts [16].
An unanswered question in the study of categorical perception is whether it occurs only in contexts where discrimination between stimulus variants has an especially important behavioural or ecological function, for example, in discriminating between different speech phonemes [11] or birdsong note types [4], predators versus conspecifics [3] or potential mates [2,8]. Alternatively, categorical perception could be a general feature of perceptual processing. Here we ask whether categorical colour perception in zebra finch females is confined to colour ranges having a signalling function or is instead a more general feature of their colour perception. We examined whether female zebra finches exhibit categorical perception in a colour range from blue to green, a part of the visual spectrum having no known signal function in this species and that is unlikely to play a role in other important behavioural contexts such as foraging or territorial defence.
2. Material and methods
(a). Subjects
Throughout, we follow methods described in [8], except we trained and tested birds with colours ranging from blue to green, rather than with colours that ranged from orange to red. Also, the blue–green colours were of approximately equal brightness (as would be detected by zebra finch photoreceptors [17]), whereas the orange–red colours used in [8] varied in brightness as well as hue in order to match natural variation in zebra finch beak coloration. We used 17 birds in this study, all of which were sexually mature female zebra finches (age range: 15–62 months at the start of experimental testing) from a colony maintained by Dr Richard Mooney (Duke University, Durham, NC; IACUC A258-14-10). All of the birds used in this study had previously been used in a study of the colour perception of an orange–red range [8] and were re-trained on blue–green stimuli at the start of the present study. Later retesting on the original orange–red range showed that successive training on additional colour ranges results in no change in the categorical perception previously observed (electronic supplementary material, figure S1). Housing and diet were as described in [8] (Duke University, Durham, NC; IACUC A004-17-01).
(b). Selecting colour stimuli
Colour stimuli were made using Munsell colour paper (Pantone LLC, Carlstadt NJ, USA). We calculated relative zebra finch photon catch (Q), a measure of photoreceptor stimulation, for 152 shades of blue, green and blue–green Munsell colours, over wavelengths (λ) from 300 to 700 nm, using the following formula:
where Sr is the sensitivity of receptor type r, Rc is the reflectance of colour c and I is the irradiance of the illuminant. We used normalized sensitivity data from the zebra finch [17,18] to calculate photon catches for the SW, MW, LW and double cones. We also calculated relative photon catches for the ultraviolet (UV) cone and confirmed that our stimuli viewed under experimental lighting conditions did not result in substantial photon catch by the UV cone (UV photon catch represented less than or equal to 2.6% of total photon catch). Because of the minor contribution of the UV cone to total photon catch, we did not further consider the contribution of UV light to birds' perception of our stimuli.
Reflectance spectra from each colour sample were measured using an integrating sphere with a built-in tungsten-halogen light source (ISP-REF; Ocean Optics Inc., Dunedin, FL). All measurements were taken with reference to a Spectralon 99% white reflectance standard (Labsphere, Congleton, UK). As our measure of ambient light, we used a standard tungsten bulb illuminance spectrum (CIE Illuminant A, colour temperature 2856 K; spectrum in electronic supplementary material), which is similar to the spectrum of ambient light provided by the halogen bulbs in our experiment (colour temperature 2900 K, model number H&PC-61361, Philips Lighting, Somerset, NJ; raw spectra in electronic supplementary material). Lastly, we calculated the relative quantum catch of the zebra finch double cone [17] for each Munsell colour, since it is thought that in birds the double cones encode brightness information [19,20] (reviewed in [21]).
We also measured the ambient light of the room in which we performed our behavioural trials (see spectrum in electronic supplementary material) using a spectrometer (USB 2000; Ocean Optics Inc.; average 3000 scans per spectrum, 1000 scans per second, spectral resolution = 0.1 nm), calibrated using a tungsten light source with a colour temperature of 3100 K. Calculations of quantum catch and chromatic distance (see below) were unchanged if we made calculations using measures of the halogen ambient light present in our experimental room instead of Illuminant A (electronic supplementary material, table S1). We have reported the distances calculated using Illuminant A so as to maximize the replicability of our calculations.
We then used the receptor noise-limited (RNL) model [22] to calculate the chromatic distance (ΔS), a measure of predicted discriminability, between colours. ΔS is sometimes equated with just-noticeable differences (JNDs); however, here we refer only to ΔS throughout, as behaviourally measured JNDs are often greater than ΔS = 1 (e.g. honeybees [23]). We then plotted chromatic distances between colours in a perceptually uniform, two-dimensional chromaticity space for tri-chromats (appropriate here because we did not include UV quantum catches) in which Euclidean distance corresponds to the model-derived ΔS [23]. From this visualization, we selected seven colours that (1) covered the entire Munsell range from the most blue (colour 1) to the most green (colour 7); (2) were of approximately equal brightness as measured by zebra finch double cone [17] quantum catch; (3) were separated by approximately equal ΔS (figure 1); and (4) covered approximately the same total range in ΔS (total = 19.2) as the previously used orange–red colours (total = 19.1; see [8] for details).
Figure 1.
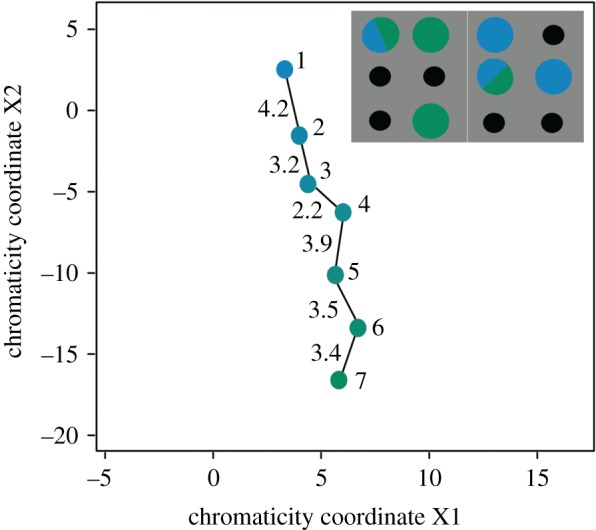
Chromaticity coordinates for the Munsell colours used to create stimuli and example set-up. Dots are coloured to represent corresponding Munsell colours (the number that we assign to each colour is shown to the right of each dot; exact colours in the figure may vary due to computer screens or printing process). Numbers to the left of each line segment show the chromatic distance between colours. The inset is a schematic of the foraging grid set-up for an example trial. Munsell colour names for the colours used are 1 = 10B 5/10, 2 = 5B 5/8, 3 = 7.5BG 5/8, 4 = 5BG 5/8, 5 = 10G 5/8, 6 = 5G 5/8, 7 = 2.5G 5/8. (Online version in colour.)
(c). Experimental protocol
We tested perception using a protocol in which birds remove discs covering wells to access a food reward. Discs were made of two semicircles of Munsell colour paper, glued together to form a circle and then covered with a clear epoxy cover. Disc halves were either different (bicolour) or the same (solid) in colour. During trials, barriers were placed between cages such that birds could not see one another perform the task, and halogen lights and a vellum paper diffuser above each cage provided consistent, even lighting. We then presented each bird with a grid of 12 wells, of which six were covered, two by bicolour discs and four by solid discs (two of each colour in the bicolour discs).
Using discs comprising colours 1 and 7 (1|7) and using a food reward to bait only bicolour discs, we trained the birds to search for food under discs they perceived as comprising two different colours (see [8,24] for details on stages used to train birds on the disc-flipping task). Birds passed a trial if they flipped both bicolour discs before flipping any solid discs within 2 min. We chose this pass criterion because birds would be expected to flip both bicolour discs first by chance in only 1/15 of trials (our results were robust to changes in this pass criterion, electronic supplementary material, table S2). Birds that passed six out of seven consecutive training trials began experimental trials, in which the number of each type of disc was the same, but the colours making up each set of discs varied (see below). In each trial, we randomized the location of each disc using the ‘sample’ function in R [25]. We also performed trials using discs that were the same colour on each half (1|1 and 7|7) to control for the possibility that birds detected seeds by olfaction. In both cases, the birds performed no better than chance at these colour combinations, indicating that they did not use olfactory cues to determine which discs to flip.
(d). Labelling tests
Labelling (or ‘categorization’) trials used bicolour discs that included colour 1 in combination with all other colours (i.e. 1|2, 1|3, 1|4, etc.) and colour 7 in combination with all other colours (i.e. 7|6, 7|5, 7|4, etc.) to test for discontinuities in how females perceive colours in this range. Birds were scored as either passing or not, depending on whether they flipped the two bicolour discs before flipping any other discs within 2 min of the start of the trial. Each bird was given 10 trials with each combination of colours and we calculated the proportion of trials that a bird passed for each combination (hereafter ‘pass frequency’). We removed from our dataset those trials in which a bird either made no selection or selected only one disc within 2 min.
We examined pass frequency data for each combination of colours and identified steps along our colour continuum (hereafter ‘colour steps’) where large changes in pass frequency occurred. The locations of these large changes yield hypotheses for where category boundaries might exist. For example, if birds passed 1|3 trials much more frequently than they passed 1|2 trials and also passed 2|7 trials much more frequently than they passed 3|7 trials, those results would suggest a category boundary between colours 2 and 3.
(e). Discrimination tests
After identifying putative boundaries using labelling tests, we performed discrimination tests, which assay whether discrimination of two colours is enhanced when those colours fall on opposite sides of the putative category boundary as compared to equally spaced colours that both fall on the same side of the putative boundary. In each discrimination trial, birds were asked to discriminate between colours that were either one colour apart (e.g. 2|3, 3|4, 4|5, etc.), two colours apart (e.g. 2|4, 3|5, 4|6, etc.), three colours apart (e.g. 1|4, 2|5, 3|6, 4|7) or four colours apart (e.g. 1|5, 2|6, 3|7). As with the labelling trials, each bird was given 10 discrimination trials with each colour combination.
(f). Statistical analyses
We analysed discrimination tests by comparing the average pass frequency of birds when comparisons either did or did not cross the putative boundary. For each bird, we first calculated the average pass frequency for all discrimination trials of a given perceptual distance that crossed the boundary and then compared this rate with that bird's average pass frequency for trials of the same distance that did not cross the boundary. We used paired two-sided t-tests to test for differences in pass frequency in those comparisons that either did or did not cross the putative boundary. We used the lme4 package [26] in R [25] to build a mixed-effects model examining the effect of each colour step on birds' pass frequency, described below. All data met the assumptions of parametric tests.
3. Results
(a). Labelling tests
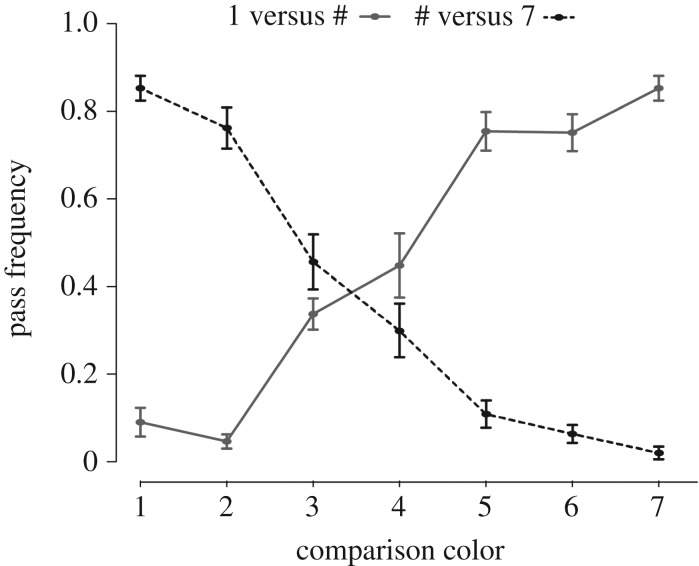
We found that pass frequency generally increased with increasing chromatic distance (electronic supplementary material, figure S6 and table S4) between colours 1 or 7 and the comparison colour. However, we observed large changes in pass frequency at two points along our colour continuum, suggestive of potential perceptual boundaries between colours 2 and 3 and between colours 4 and 5 (figure 2). Pass frequency for 1|3 was 29 percentage points higher than pass frequency for 1|2, and pass frequency for 7|2 was 30 percentage points higher than for 7|3. Similarly, pass frequency for 1|5 was 31 percentage points higher than for 1|4, and for 7|4 was 19 percentage points higher than for 7|5 (figure 2). The mean (and median) increase in pass frequency between all other colour steps on the blue–green continuum was only 6 percentage points (standard deviation ± 7 percentage points; see electronic supplementary material, figure S3). Overall, our labelling results indicated that the 2–3 and 4–5 colour steps had greater effects on pass frequency than any other colour steps, leading to the hypothesis that category boundaries existed at one or both of these locations. We tested this hypothesis using discrimination data and a linear mixed-effects model.
Figure 2.
Labelling trial data. Females were 29% more likely to pass 1|3 than 1|2 trials and 30% more likely to pass 7|2 than 7|3 trials. They were 31% more likely to pass 1|5 than 1|4 trials and 19% more likely to pass 7|4 than 7|5 trials. Grey dots and grey solid line represent pass frequency results for 1|# trials; black dots and black dashed line represent results for #|7 trials. Vertical bars with each dot represent s.e.
(b). Discrimination tests
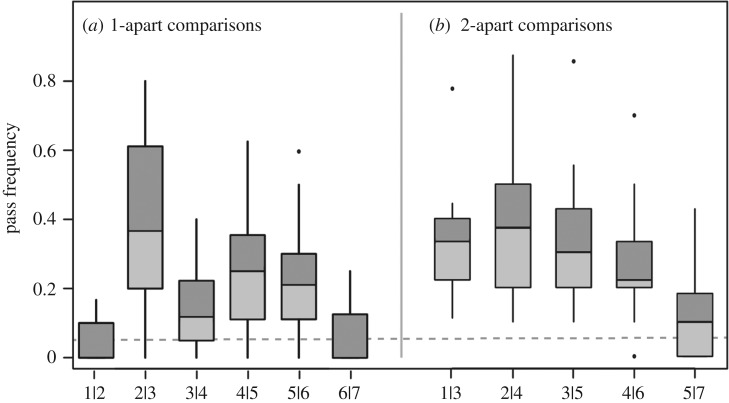
For discrimination comparisons of colour pairs that were one step apart, the pass frequency for the colour pair that crossed the 2–3 boundary was 24 percentage points higher than the mean pass frequency of all comparisons that did not (figure 3a; 95% confidence interval = 12–36 percentage points; paired two-tailed t-test: t = 4.28, p = 0.0008, Bonferroni-corrected α2 = 0.025). Likewise, for the two-apart comparisons, the pass frequency for comparisons that crossed the 2–3 boundary was on average 12 percentage points higher than comparisons that did not (figure 3b; 95% confidence interval = 6–17; paired two-tailed t-test: t = 4.49, p = 0.0004, Bonferroni-corrected α2 = 0.025), providing support for a category boundary between colours 2 and 3. Results for the three-apart comparisons were similar (electronic supplementary material, figure S4).
Figure 3.
Discrimination of stimuli that are (a) one and (b) two colour steps apart. In both panels, pass frequency was higher for comparisons that cross the 2–3 boundary (in (a), 2|3; in (b), 1|3 and 2|4) compared with comparisons that do not. However, there is not a similar increase for comparisons crossing the 4–5 boundary compared with those that did not. For each comparison, the median (horizontal line), 25 and 75th percentiles (boxes) and 1.5× interquartile range (whiskers), for pass frequency are presented. The grey horizontal line shows expected pass frequency if birds flip discs at random.
By contrast, we found no support for a category boundary between colours 4 and 5. Pass frequency for the one-apart comparison that crossed the 4–5 boundary was only six percentage points higher than the mean of comparisons that did not, a non-significant difference (figure 3a; 95% confidence interval = −2–15; paired two-tailed t-test: t = 1.56, p = 0.14, Bonferroni-corrected α2 = 0.025). Similarly, two-apart comparisons crossing the 4–5 boundary had a pass frequency only two percentage points higher than the mean of comparisons that did not, also a non-significant difference (figure 3b; 95% confidence interval = −2–6; t = 1.1, p = 0.29, Bonferroni-corrected α2 = 0.025). In sum, examining the one- and two-apart discrimination data supported the hypothesis that a category boundary exists between colours 2 and 3, but not between 4 and 5. Results for the three-apart comparisons were similar (electronic supplementary material, figure S4).
(c). Linear mixed model of combined labelling and discrimination data
Discrimination tests, which demonstrate enhanced discrimination of stimuli across a category boundary relative to stimuli that fall within a category, are considered a hallmark of demonstrating categorical perception [9,27]. However, because we tested for differences in discrimination across two putative boundaries simultaneously, we were unable to fully isolate the effects of the 2–3 or 4–5 boundary using the analysis described above. For example, the collection of two-apart discrimination trials that did not cross the putative 4–5 boundary also included tests of birds’ ability to discriminate 1|3 and 2|4, which did cross the putative 2–3 boundary. Similarly, two-apart trials that did not cross the putative 2–3 boundary include 3|5 and 4|6 trials, which did cross the putative 4|5 boundary. To address the non-independence between trials that did or did not cross the 2|3 and 4|5 boundaries, we built a linear mixed-effects model that combined data from all of our trials. This model isolated the effects of each colour step and estimated the effect of each colour step on pass frequency, independent of other colour steps.
For example, a two-apart trial with 2|4 bicolour discs includes steps 2–3 and 3–4, while a three-apart trial using 2|5 bicolour discs includes steps 2–3, 3–4, and 4–5. The difference in a bird's pass frequency in 2|5 trials as compared with 2|4 trials therefore provides information that helps the model estimate the effect of specifically crossing the 4|5 boundary. Combining all of our labelling and discrimination data into a single model gives a more reliable estimate of the contribution of each colour step to pass frequency than separate analyses of the one-apart, two-apart or three-apart data. The model included pass frequency for a given comparison as the response variable, each of the six colour steps as binary fixed effects and bird ID as a random effect (table 1).
Table 1.
Point estimates and 95% confidence intervals of the contribution of each colour step to birds’ pass frequency in separate analyses of blue–green (present study) and orange–red (data from [8]). Colour steps that have significantly greater effects than any other colour step along their respective colour range (category boundaries) are noted in italics (see main text for statistical tests).
| analysis | colour step | point estimate | 95% CI lower boundary | 95% CI upper boundary |
|---|---|---|---|---|
| blue–green | 1–2 | 0.03 | −0.01 | 0.07 |
| 2–3 | 0.29 | 0.25 | 0.33 | |
| 3–4 | 0.13 | 0.09 | 0.17 | |
| 4–5 | 0.20 | 0.15 | 0.24 | |
| 5–6 | 0.11 | 0.06 | 0.15 | |
| 6–7 | 0.00 | −0.04 | 0.05 | |
| orange–red | 1–2 | 0.05 | 0.01 | 0.09 |
| 2–3 | 0.04 | 0 | 0.08 | |
| 3–4 | 0.07 | 0.03 | 0.1 | |
| 4–5 | 0.09 | 0.05 | 0.12 | |
| 5–6 | 0.25 | 0.21 | 0.29 | |
| 6–7 | 0.12 | 0.08 | 0.16 | |
| 7–8 | 0.02 | −0.01 | 0.06 |
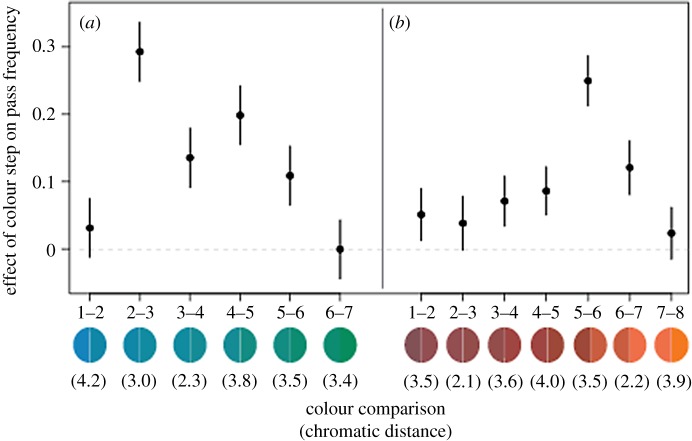
If perception over the blue–green range is continuous, we would expect an approximately equal contribution to pass frequency for each colour step. By contrast, the presence of category boundaries at 2–3 and/or 4–5 would be supported if one or both of these colour steps contribute significantly more to birds' ability to distinguish two colours than the other colour steps (1–2, 3–4, 5–6 and 6–7). We found that different colour steps yielded different contributions to pass frequency, with the 2–3 colour step having the largest effect (table 1, figure 4a), and a model that included each colour step performed much better than a model that considered only chromatic distance (electronic supplementary material, table S4, ΔAIC = 142). Importantly, the colour steps with the greatest effect on pass frequency were not those that span the greatest chromatic distance (figure 4a, electronic supplementary material, figure S5). The effect of the 2–3 step was significantly greater than each of the other steps, including the 4–5 colour step (two-tailed t-tests, t ≥ 2.9 in all cases, p ≤ 0.004 in all cases, Bonferroni-corrected α5 = 0.01). By contrast, the effect of the 4–5 step was significantly smaller than the effect of the 2–3 step and was not significantly greater than the 3–4 colour step (two-tailed t-test, t = 2.15, p = 0.03, Bonferroni-corrected α4 = 0.013), although it was significantly greater than the 1–2, 5–6 and 6–7 steps (two-tailed t-tests, t ≥ 2.9, p ≤ 0.003, Bonferroni-corrected α5 = 0.01). Taken together, these results support the presence of categorical perception of colour over the range of blue–green colours that we tested, with a perceptual boundary between colours 2 and 3. While our labelling data showed that pass frequency increased between colours 4 and 5, neither our discrimination data nor our mixed-effects models offer strong support for a perceptual boundary between these colours.
Figure 4.
Comparing results from the (a) blue–green and (b) orange–red datasets. The effects of each colour step on pass frequency, resulting from a model which simultaneously considers data from colour steps 1, 2, 3, 4, 5 and 6 steps apart. Points and bars represent estimates and 95% confidence intervals corresponding to the contribution of each colour step to birds' pass frequency. The coloured circles indicate the two Munsell colours that fall on either end of a given step. Note that the colour steps with the greatest effect on pass frequency are not those that span the greatest chromatic distance (indicated in brackets). (Online version in colour.)
(d). Comparing blue–green and orange–red categorical perception
Our model suggests that categorical perception in the blue–green colour range is less well-defined than what we observed previously in the orange–red range; that is, it appears that within-category discrimination was greater along the blue–green colour range as compared to the orange–red colour range. We tested this possibility by using data from [8] to create a linear mixed effects model for our orange–red dataset that allowed us to directly compare categorical perception in the two colour ranges (table 1 and figure 4).
This model, which was identical in structure to that used for the blue–green data, confirmed the presence of the orange–red category boundary we described previously [8], with the effect of the 5–6 colour step in the orange–red dataset being significantly greater than the effect of the second highest colour step and thus each of the other five steps as well (figure 4b, two-tailed t-test, t = 4.8, p < 0.0001, Bonferroni-corrected α7 = 0.007). Both visual and quantitative comparisons of the blue–green and orange–red models suggest that there is greater within-category discrimination in the blue–green dataset as compared with the orange–red dataset (figure 4 and table 1). For example, among colour steps that do not cross the 2–3 category boundary in the blue–green model (table 1), the three steps with the greatest effect on discrimination were each estimated to have a greater effect on pass frequency (0.20, 0.13, 0.11) than the equivalent three steps in the orange–red model (0.12, 0.09, 0.07). Furthermore, in the blue–green dataset, the effect of the 2–3 colour step was 1.5 times the effect of the second largest colour step (4–5) and 3.1 times the mean effect of all five other colour steps. In the orange–red dataset, the effect of the 5–6 step was 2.1 times that of the second largest step (6–7) and 3.9 times the mean effect of all six other colour steps (electronic supplementary material, table S3). This comparison suggests that there is greater discrimination within the categories we identified in the blue–green colour range as compared with discrimination within the categories previously identified in the orange–red, and thus that categorical perception is less strongly expressed in the blue–green range as compared with the orange–red.
4. Discussion
Our results indicate that zebra finch females exhibit categorical perception of colours in the blue–green range, but with greater within-category discrimination, and thus less enhancement of cross-boundary discrimination, as compared with the orange–red colour range. By demonstrating categorical colour perception across two different portions of the visible spectrum, our results provide evidence that this phenomenon may be a general feature of colour perception in female zebra finches, as it is in humans [28]. Categorical colour perception was once thought to be a uniquely human trait, perhaps associated with the use of language to name colours (e.g. [5,29]). This view was challenged with the discovery of categorical colour perception in macaques [7] and in prelingual infants (e.g. [30,31]). Our discovery that colours across a wide range of the visible spectrum are perceived in a categorical fashion by a songbird further erodes this view and opens the question of which other animals capable of colour vision might also exhibit this perceptual mechanism.
Our finding that categorical perception in the blue–green range differs from what we observed previously for orange–red coloration parallels findings on human colour categorization. Humans are known to exhibit categorical colour perception [32–35], and although no study has directly compared categorical perception in an orange–red versus a blue–green colour range in humans, our ability to categorize and discriminate colours is less well defined in the blue–green than in the orange–red (e.g. [36]). In humans, this difference could be the consequence of physiological constraints (i.e. the wavelength discrimination function of photoreceptors [37]) or, as some have argued, due in part to the consequence of linguistic constructs. For example, some languages use only a single colour term to describe the entire blue–green region [38,39], and across diverse languages the most common semantic identity—that is, when the same word is applied to two or more colours—equates blue and green [40].
Linguistic constraints would not apply to zebra finches, of course, but several explanations could account for the differences we observed in categorical perception in the blue–green versus orange–red colour ranges. First, the orange–red colours used in our earlier study varied in brightness as well as hue in order to match the range of natural male zebra finch beak colours, which also vary in brightness [8]. By contrast, the blue–green colours used here were approximately equiluminant, as indicated by cone catch for the zebra finch double cone [17]. Brightness alone did not account for the categorical perception we observed in the orange–red range, as shown by experiments performed with hue information removed [8], but brightness differences may provide additional information that birds use when categorizing the orange–red colours associated with male beaks, yielding better-defined categories.
Another possible reason for the presence of less distinct categories in the blue–green range relates to the possibility that the evolution of visual perception in general has been influenced by the reflectance properties of natural objects (e.g. [36,41]). In terrestrial environments, light reflected from many natural objects (for example tree bark, dead leaves, flowers, fruits or melanin- and carotenoid-based coloration typical of many animals) contains more variation in the long-wavelength portion of the visible spectrum than the short-wavelength portion [42]. Thus, categorical colour perception by terrestrial organisms overall may be better defined for long-wavelength coloration (i.e. orange–red) given the reflectance spectra of the environments in which they evolved.
Finally, the differences in categorical perception along the blue–green and orange–red colour ranges may occur because carotenoid-based coloration is especially salient for female zebra finches, given its role in visual signalling [43,44]. Previous studies have shown the categorical perception of behaviourally or ecologically important stimuli, for example, in discriminating between different speech phonemes [11] or birdsong note types [4], predators versus conspecifics [3], or potential mates [2,8], but studies have not compared the perception of similar stimuli across different functional contexts. Male zebra finch beak colours range from light orange to dark red [13], and females show a mating preference for males having redder beaks [43,44], a preference thought to be driven by the fact that the expression of carotenoid-based red coloration correlates positively with variation in cell-mediated immunity in this species [13,45,46]. The sharper category boundary observed in the orange–red colour range may therefore be the result of selection on communicative function in the context of mate choice.
Further work is needed on categorical colour perception with species that do not use carotenoid coloration in signalling, or that use other colours instead, to better understand if and how selection for communication or other behavioural functions may impact the expression of this perceptual mechanism. Additional work is also needed to understand the mechanisms by which categorical perception occurs and to elucidate the conditions under which it is most likely to be observed. Our conclusions about categorical perception hinge on differences between measured behavioural responses to colour stimuli and predicted chromatic distance from the RNL model, which only considers visual inputs at the photoreceptor level. The RNL model is agnostic regarding any processing of inputs above the photoreceptor level. Both the results we present here and our previous findings in the orange–red range suggest that categorical perception is likely to be the result of processing beyond the photoreceptor, although exactly how this processing occurs remains an open question. Specifically, our results (electronic supplementary material, figure S6) suggest that, using this discrimination assay, behavioural colour discrimination fits the predictions of categorical perception when two colours differ by less than some chromatic distance threshold. Under our experimental conditions, colour pairs whose difference exceeds this threshold all appear to be readily discriminable.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Lauren Deehan and John Bollinger for assistance running trials, and Dr Thomas Cronin for comments on an earlier draft of this manuscript.
Ethics
These experiments were approved by Duke University, Durham, NC (IACUC A004-17-01).
Data accessibility
Data available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.3gp17g0 [47].
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Duke University Office of the Provost.
References
- 1.Cott H. 1940. Adaptive coloration in animals. London, UK: Methuen. [Google Scholar]
- 2.Baugh AT, Akre KL, Ryan MJ. 2008. Categorical perception of a natural, multivariate signal: mating call recognition in túngara frogs. Proc. Natl Acad. Sci. USA 105, 8985–8988. ( 10.1073/pnas.0802201105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyttenbach RA, May ML, Hoy RR. 1996. Categorical perception of sound frequency by crickets. Science 273, 1542–1544. ( 10.1126/science.273.5281.1542) [DOI] [PubMed] [Google Scholar]
- 4.Nelson DA, Marler P. 1989. Categorical perception of a natural stimulus continuum: birdsong. Science 244, 976–978. ( 10.1126/science.2727689) [DOI] [PubMed] [Google Scholar]
- 5.Roberson D, Davies I, Davidoff J. 2000. Color categories are not universal: replications and new evidence from a stone-age culture. J. Exp. Psychol. Gen. 129, 369–398. ( 10.1037/0096-3445.129.3.369) [DOI] [PubMed] [Google Scholar]
- 6.Skelton AE, Catchpole G, Abbott JT, Bosten JM, Franklin A. 2017. Biological origins of color categorization. Proc. Natl Acad. Sci. USA 114, 5545–5550. ( 10.1073/pnas.1612881114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandell JH, Gross CG, Bornstein MH. 1979. Color categories in macaques. J. Comp. Physiol. Psychol. 93, 626–635. ( 10.1037/h0077594) [DOI] [PubMed] [Google Scholar]
- 8.Caves EM, Green PA, Zipple MN, Peters S, Johnsen S, Nowicki S. 2018. Categorical perception of colour signals in a songbird. Nature 560, 365–367. ( 10.1038/s41586-018-0377-7) [DOI] [PubMed] [Google Scholar]
- 9.Harnad SR. 1987. Categorical perception: the groundwork of cognition. Cambridge, UK: University of Cambridge Press. [Google Scholar]
- 10.Studdert-Kennedy M, Liberman AM, Harris KS, Cooper FS. 1970. Motor theory of speech perception: a reply to Lane's critical review. Psychol. Rev. 77, 234–249. ( 10.1037/h0029078) [DOI] [PubMed] [Google Scholar]
- 11.Liberman AM, Harris KS, Hoffman HS, Griffith BC. 1957. The discrimination of speech sounds within and across phoneme boundaries. J. Exp. Psychol. 54, 358–368. ( 10.1037/h0044417) [DOI] [PubMed] [Google Scholar]
- 12.Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. 1967. Perception of the speech code. Psychol. Rev. 74, 431–461. ( 10.1037/h0020279) [DOI] [PubMed] [Google Scholar]
- 13.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127. ( 10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- 14.Walls G. 1942. The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: The Cranbook Press. [Google Scholar]
- 15.Hart NS. 2001. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 20, 675–703. ( 10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- 16.Hill GE, McGraw KJ (eds). 2006. Bird coloration, volume 2: function and evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Lind O. 2016. Colour vision and background adaptation in a passerine bird, the zebra finch (Taeniopygia guttata). R. Soc. open sci. 3,160383 ( 10.1098/rsos.160383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. 1997. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 37, 2183–2194. ( 10.1016/S0042-6989(97)00026-6) [DOI] [PubMed] [Google Scholar]
- 19.Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and color vision. Proc. R. Soc. B 272, 1745–1752. ( 10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osorio D, Jones C, Vorobyev M. 1999. Accurate memory for colour but not pattern contrast in chicks. Curr. Biol. 9, 199–202. ( 10.1016/S0960-9822(99)80089-X) [DOI] [PubMed] [Google Scholar]
- 21.Martin GR, Osorio D. 2008. Vision in birds. In The senses: a comprehensive reference (eds Masland D, Albright RM), vol. 1, pp. 25–52. San Diego, CA: Academic Press. [Google Scholar]
- 22.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hempel De Ibarra N, Giurfa M, Vorobyev M.. 2002. Discrimination of coloured patterns by honeybees through chromatic and achromatic cues. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 503–512. ( 10.1007/s00359-002-0322-x) [DOI] [PubMed] [Google Scholar]
- 24.Anderson RC, Searcy WA, Peters S, Hughes M, DuBois AL, Nowicki S. 2017. Song learning and cognitive ability are not consistently related in a songbird. Anim. Cogn. 20, 309–320. ( 10.1007/s10071-016-1053-7) [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 26.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 27.Calder AJ, Young AW, Perrett DI, Etcoff NL, Rowland D. 1996. Categorical perception of morphed facial expressions. Vis. cogn. 3, 81–117. ( 10.1080/713756735) [DOI] [Google Scholar]
- 28.Bornstein MH. 1987. Perceptual categories in vision and audition. In Categorical perception: the groundwork of cognition (ed. Harnad SR.), pp. 287–300. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Berlin B, Kay P. 1969. Basic color terms: their university and evolution. Berkeley, CA: University of California Press. [Google Scholar]
- 30.Bornstein MH, Kessen W, Weiskopf S. 1976. Color vision and hue categorization in young human infants. J. Exp. Psychol. Hum. Percept. Perform. 2, 115–129. ( 10.1037/0096-1523.2.1.115) [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Kanazawa S, Yamaguchi MK, Kuriki I. 2016. Cortical response to categorical color perception in infants investigated by near-infrared spectroscopy. PNAS 113, 2370–2375. ( 10.1073/pnas.1512044113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin A, Drivonikou GV, Bevis L, Davies IRL, Kay P, Regier T. 2008. Categorical perception of color is lateralized to the right hemisphere in infants, but to the left hemisphere in adults. Proc. Natl Acad. Sci. USA 105, 3221–3225. ( 10.1073/pnas.0712286105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberson D, Pak H, Hanley JR. 2008. Categorical perception of colour in the left and right visual field is verbally mediated: evidence from Korean. Cognition 107, 752–762. ( 10.1016/j.cognition.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 34.Gilbert AL, Regier T, Kay P, Ivry RB. 2006. Whorf hypothesis is supported in the right visual field but not the left. Proc. Natl Acad. Sci. USA 103, 489–494. ( 10.1073/pnas.0509868103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster MA, Kay P. 2012. Color categories and color appearance. Cognition 122, 375–392. ( 10.1016/j.cognition.2011.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves D, Lotto RB. 2011. Why we see what we do redux: a wholly empirical theory of vision. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 37.Nathans J. 1999. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron 24, 299–312. ( 10.1016/S0896-6273(00)80845-4) [DOI] [PubMed] [Google Scholar]
- 38.Kay P, Regier T. 2003. Resolving the question of color naming universals. Proc. Natl Acad. Sci. USA 100, 9085–9089. ( 10.1073/pnas.1532837100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kay P, Maffi L. 1999. Color appearance and the emergence and evolution of basic color lexicons. Am. Anthropol. 101, 1–32. ( 10.1525/aa.1999.101.4.743) [DOI] [Google Scholar]
- 40.Bornstein MH. 1973. Color vision and color naming: a psychophysiological hypothesis of cultural difference. Psychol. Bull. 80, 257–285. ( 10.1037/h0034837) [DOI] [PubMed] [Google Scholar]
- 41.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 42.Osorio D, Bossomaier TRJ. 1992. Human cone-pigment spectral sensitivities and the reflectances of natural surfaces. Biol. Cybern. 67, 217–222. ( 10.1007/BF00204394) [DOI] [PubMed] [Google Scholar]
- 43.Burley N, Coopersmith CB. 1987. Bill color preferences of zebra finches. Ethology 151, 133–151. ( 10.1111/j.1439-0310.1987.tb00679.x) [DOI] [Google Scholar]
- 44.Collins SA, ten Cate C. 1996. Does beak colour affect female preference in zebra finches? Anim. Behav. 52, 105–112. ( 10.1006/anbe.1996.0156) [DOI] [Google Scholar]
- 45.George DB, Schneider BC, McGraw KJ, Ardia DR. 2017. Carotenoids buffer the acute phase response on fever, sickness behavior, and rapid bill color change in zebra finches. J. Exp. Biol. 220(Pt.16), 2957–2964. ( 10.1242/jeb.155069) [DOI] [PubMed] [Google Scholar]
- 46.Weaver RJ, Santos ESA, Tucker AM, Wilson AE, Hill GE. 2018. Carotenoid metabolism strengthens the link between feather coloration and individual quality. Nat. Commun. 9, 73 ( 10.1038/s41467-017-02649-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zipple MN, Caves EM, Green PA, Peters S, Johnsen S, Nowicki S. 2019. Data from: Categorical colour perception occurs in both signalling and non-signalling colour ranges in a songbird Dryad Digital Repository. ( 10.5061/dryad.3gp17g0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zipple MN, Caves EM, Green PA, Peters S, Johnsen S, Nowicki S. 2019. Data from: Categorical colour perception occurs in both signalling and non-signalling colour ranges in a songbird Dryad Digital Repository. ( 10.5061/dryad.3gp17g0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.3gp17g0 [47].