Abstract
Winning or losing contests can impact subsequent competitive behaviour and the duration of these effects can be prolonged. While it is clear effects depend on social and developmental environments, the extent to which they are heritable, and hence evolvable, is less clear and remains untested. Furthermore, theory predicts that winner and loser effects should evolve independently of actual fighting ability, but again tests of this prediction are limited. Here we used artificial selection on replicated beetle populations to show that the duration of loser effects can evolve, with a realized heritability of about 17%. We also find that naive fighting ability does not co-evolve with reductions in the duration of the loser effect. We discuss the implications of these findings and how they corroborate theoretical predictions.
Keywords: winner effect, loser effect, fighting, contest, realized heritability
1. Introduction
In many animals aggressive contests occur for limited resources like territories, food and mates [1]. Additionally, individuals frequently engage in repeated contests, and previous fighting experience often influences current contest outcomes [2]. Thus prior winning often increases the probability of winning subsequent contests, and prior losing decreases the probability, phenomena known as winner and loser effects respectively. While the precise underlying causes of winner/loser effects are often unclear, they are assumed to have some genetic underpinnings (reviewed in [3]). However, despite the widespread occurrence of winner/loser effects [2,3], this claim is rarely tested—in fact we could find no examples where explicit tests of a genetic basis to winner/loser effects had been undertaken. Additionally, although we expect genetic variation to underpin behavioural phenotypes [4], this might not always be the case [5].
One explanation for winner and loser effects is that prior experience shapes future contests by providing contestants information about their relative resource-holding potential (RHP) or fighting ability [6], and two non-mutually exclusive hypotheses for the effects have been proposed [3]. Individuals either gain information on their own RHP (a self-assessment mechanism) or winning and losing produces status-related cues that affect the assessment of subsequent opponents (a social-cue mechanism). With a social-cue mechanism, individuals are predicted to detect previous winning or losing by their opponents from visible or chemical ‘cues’ emitted by them, and should adjust self-behaviours based on an opponent's previous experience [3]. Social-cues include signs of exhaustion or injuries [7], and odours [8]. Both hypotheses require there to be variation in fighting ability in the population so that there is value in working out who to fight and who not to fight [9]. A typical example of self-assessment is learning through prior fighting [10,11]. Here, individuals adjust their behaviour based on their previous experience (e.g. [12–14]), and there is abundant evidence that individuals vary in their behavioural adjustments, including in contest duration [2] and the type of adjustments employed [14–16]. Additionally, variation in behavioural adjustment may be underpinned by differences in perception [10] and learning ability [11], and this variation can be related to behavioural syndromes or personality [17]. Finally, although the evolution of winner and loser effects can be inferred from such among-individual differences [3,11], direct evidence for genetic variation and responses to selection of winner/loser effects appears to be lacking. This may be because these effects arise from experience, effectively the environment. But of course the environment is responsible for all manner of gene expression variation that generates physiological changes in an individual, and any genetic variation in gene expression (e.g. [18]) will mean genetic variation for winner/loser effects. Thus genetics will also be important [2].
Here we only focused on loser effects and their duration. This is primarily because theory suggests loser effects can evolve without corresponding winner effects, while the reverse is not true [3,19]. This loser only evolution should occur when the costs of fighting (C: the rate of increase in costs of over-estimating RHP in terms of heightened risk of getting into and losing escalated fights) are moderate and the fitness benefits of dominance (V: relative fitness of dominant individuals) are substantial (e.g. V > C > 0), a pattern reported for several taxa (e.g. [20–22]). Furthermore, although experience effects are generally short-lived, as noted above, variable durations are found within and across taxa (reviewed in [3]). For example, effects can persist from 10 min to 10 days (e.g. [23,24]), and although there is limited evidence for intra-specific variation in loser effects [2], variation has been found in the cricket Gryllus bimaculatus [23,25–27]. Variation in the duration of effects is thought to be influenced by the frequency of social interactions and population density [22,28], as well as the costs and benefits of fighting [2], which all implies that these effects can evolve. Interestingly, effects may be owing to perception only. That is, absolute fighting ability need not reflect the duration of loser effects and vice versa. So loser effects could potentially evolve without affecting fighting ability, although this remains to be demonstrated experimentally.
Broad-horned flour beetles (Gnatocerus cornutus) are increasingly well studied, especially with respect to their fighting behaviour and its consequences (e.g. [14,29–36]). Males freely engage in combat for access to females [14] and experience a loser effect when they are beaten in these fights. The loser effect lasts for about 4 days, during which time fewer than 25% of losers will engage in combat (75% of losers will not fight), and there is no apparent decay of the effect during that 4 day period [14]. Rather than fighting, losing males tend to disperse to new territories (which may or may not contain other males) and increase their investment in sperm production [14,35]. It should be emphasized that there is no modulation of male behaviour due to winning (i.e. winners are not different from naive males), which is consistent with theoretical predictions that loser effects can evolve alone [3,19]. Here we investigated whether the duration of the loser effect could evolve through artificial selection in experimental populations of G. cornutus. Any response to selection would then facilitate estimating the heritability of the response duration and enable testing for correlated evolution of male fighting behaviour. Furthermore, demonstrating such evolutionary responsiveness would establish the broad-horned flour beetle system as a model for explicit testing of theoretical predictions about the conditions under which pure loser effects are expected to evolve [19].
2. Material and methods
The G. cornutus beetle culture originated from adults collected in Miyazaki City (31°54′ N, 131°25′ E), Japan, and has been maintained in the laboratory of the National Food Research Institute, Japan, for approximately 50 years on whole meal enriched with yeast. The stock contains 1500–2000 beetles per generation. This beetle is a stored product pest, and thus, the laboratory conditions very closely mimic what have become natural conditions over the last 4500 years [37]. All rearing and subsequent experimentation was conducted in a chamber maintained at 25°C, 60% relative humidity and with a photoperiod cycle of 14 : 10 h light : dark.
To obtain virgin adults for experiments, one final instar larva was placed in each well of a 24-well tissue culture plate with 1 g of food (Cellstar; Greiner Bio-One, Frickenhausen, Germany) [14,32]. Individuals were placed in the wells immediately after eclosion, and did not interact with conspecifics until the start of the experiments. Thus, we ensured that animals were virgin and had no previous fighting experience. Adults 15–20 days old (after final eclosion) were used for the experiments (for a more detailed description of the stock culture see [14,32]). The body size (prothorax width: [14,32,38]) of each experimental individual was measured (±0.01 mm), using a dissecting microscopic monitoring system (VM-60; Olympus, Tokyo, Japan) (see [32] for landmarks).
(a). Identifying losers
Following established protocols [14], adult males with no fighting experience were collected from the stock culture (collected as final instar larvae and housed alone until adulthood). To control for the effect of body size on fighting success, males were paired so that the difference in body size between contestants was less than 0.01 mm, thus competitors differed in size by less than 2% [14]. Pairs were placed on filter-paper (17 mm diameter) in a plastic container (17 mm diameter, 20 mm high) and allowed to interact (and fight) for 1 h—previous work has shown that male fights occur in almost all trials when staged in this manner [14]. Males that pushed opponents and chased them were denoted the winner [14]. Losers (L-males) were those that retreated from the winner. For a more detailed description of the methods, see [14]. Subsequently, each L-male was placed in one well of a 24-well tissue culture plate with food (1 g), as described above, until testing for the selection and control populations. These focal males were marked with white or pink spots [Mitsubishi Paint-Marker] on their elytra; in half of the trials, focal L-males were white, and in half of the trials, focal L-males were pink.
(b). Selection protocol
As shown previously [14], loser effects last about 4 days, with no apparent decay in the proportion of males affected during that period (and again note there is no modulation of behaviour due to winning fights). Here we selected for a reduced duration of the loser effect after losing fights. We first collected males from the stock culture to manipulate the loser effect, as described above, to establish three selection and three control populations (initially with ca 75 males population−1). To investigate whether the loser effect influenced the outcome of a subsequent fight, each loser male (males that lost initial fights) was matched with an opponent male collected from the stock culture (tester male), at 4 days after first fight losses. The tester males had no fighting experience in these or other experiments. Contestants were matched for body size (as above) and outcomes were assessed as above. We then selected the 12 losers that won these second fights (i.e. males that had not modulated their behaviour until day 4 owing to their previous losing experience) as sires of the reduced loser-effect-duration populations (RLE populations). To propagate control populations (C population) 12 random (with respect to their fighting behaviour in these second bouts) (previous) losers were selected to act as sire. That is, control males had also lost initial fights, but we did not take their subsequent win/lose status into account when choosing them as sires. The 12 males population−1 were randomly divided into four groups (three males in each), and each group was placed in a plastic cup (7 cm diameter, 2.5 cm height) with 20 g of medium and three females collected from the stock culture. Groups were maintained this way for two months with males able to mate with females and females were allowed to lay eggs in each group, until final instar larvae were obtained [38]. Final instar larvae were collected (as above) to obtain the adults for subsequent generations. When the adults reached 10–15 days old, 144 males population−1 were randomly collected and 72 male pairs population−1 were matched within each population and tested again as above. We then took losers from these fights and selected the 12 losers that won second fights against tester males (4 days later) to propagate RLE populations, and randomly selected 12 previous losers regardless of their winner or loser status in second fights to propagate controls (C populations). Females were randomly chosen as dams from within each experimental population. This regime continued for 10 generations. We randomly collected 50 males from each experimental population at generation 5 and 10 and examined whether the duration of the loser effect had decayed at day 4; we examined whether males were attacked first by or lost fights to tester males, again noting that usually almost all losers will not fight so not initiating attacks is a measure of loser effect duration [14]. Winning or losing a fight provides an estimate of fighting ability.
To compare population rates of attacking first and losing fights, we applied a generalized linear model (GLM) with a binomial distribution, a logit-link function, and overdispersion test. Replicate (population) was nested within selection regime (RLE = reduced loser effect duration and C = control (no artificial selection on loser effect duration)). Losing (losing = 1, winning = 0) and attacks (attacked = 1, attacking = 0) were the response variables. All model assumptions were met. All statistical analyses were carried out using JMP 7 [39]. The realized heritabilities were calculated according to the liability model [40] as cumulative response to selection divided by cumulative selection differential (also see [41]).
In addition to testing for an impact of selection on loser effect at day 4, we also tested effects of losing on males at days 1–5 after they lost their initial fights (using the methods previously described, with different males used for each day—each male only fought twice). On each day after initial losses, 20 losers (per day) were observed per population (n = 600 in total), in which the experimental losers competed against tester males from the stock culture. Population rates of being attacked first or losing to by tester males were compared using GLMs with a binomial distribution, a logit-link function, and overdispersion test. Replicate was again nested within selection regime (RLE and C), and this and test time (day after initial loss 1–5) were used as the explanatory variables. Losing (losing = 1, winning = 0) and attacks (attacked = 1, attacking = 0) were the response variables. When significant interaction terms (selection regime × day) were observed, as a post hoc test, we compared population rate at each day using GLMs with a binomial distribution, a logit-link function, and overdispersion test. Replicate was nested within selection regime (RLE and C), and this was used as the explanatory variable. We note here that when we used model selection (electronic supplementary material, table S1) inferences were unchanged.
(c). Correlated responses in fighting success
At generation 10, adults were also collected to assess the fighting success of naive males as a correlated response to selection on loser effect duration. Males from each of the six experimental populations (RLE and C) were used to assess fighting success when they had experienced no previous fighting—their first fights when they were naive. We observed 30 contests per population (n = 180 in total) in which focal experimental males competed against a tester male (as described above). Trials were then continuously observed until fight outcomes could be scored. Population rates of being attacked by and losing to tester males were compared using GLM with a binomial distribution, a logit-link function, and overdispersion test. Replicate was nested within selection regime (RLE and C) and this was used as the explanatory variable. Losing (losing = 1, winning = 0) and attacks (attracted = 1, attack = 0) were the response variables.
(d). Correlated response in body size
Thirty males were also randomly chosen from each of the six experimental populations (RLE and C) at generation 10 and were used to assess body size (prothorax width). Body size was compared using GLM with a normal distribution and an identity-link function. Replicate was nested within selection regime (RLE and C), which was the primary explanatory variable.
3. Results
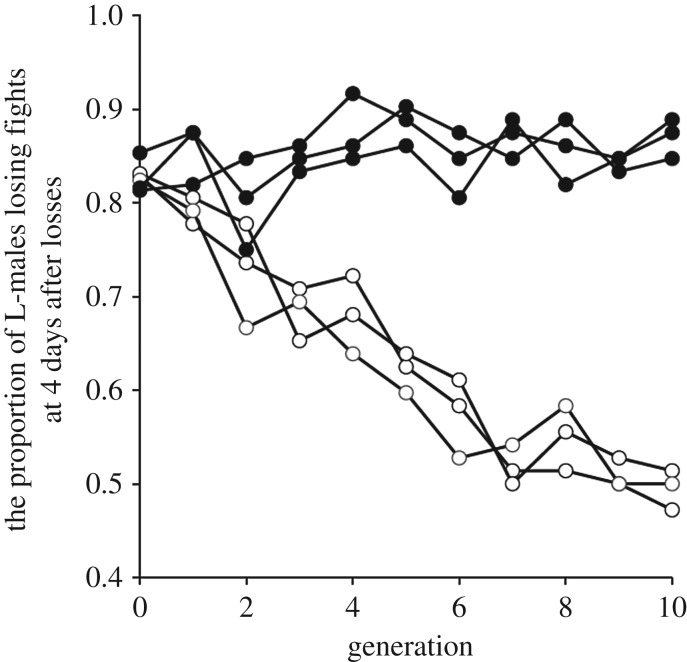
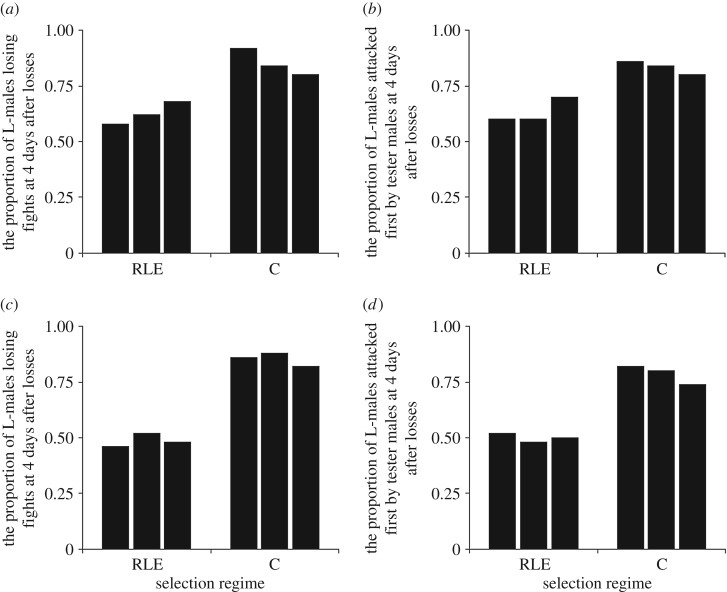
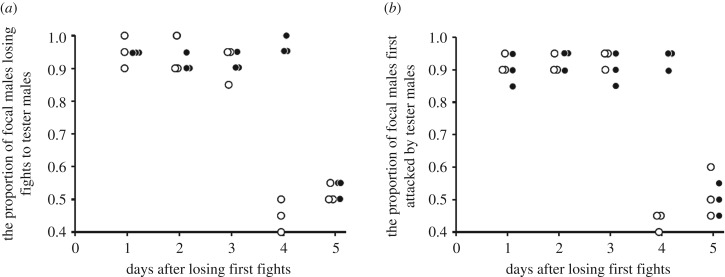
The proportion of previous losing males that lost focal fights to tester males at 4 days after initial losses showed a clear direct response to selection (figure 1), with a steady divergence between selection and control populations (generation 5: selection, χ12 = 21.51, p < 0.001; replicate (within selection), χ24 = 4.27, p = 0.31; generation 10: selection, χ12 = 47.87, p < 0.001; replicate (within selection), χ42 = 1.11, p = 0.89; figures 1 and 2a,c). After 10 generations of selection, the proportion of males losing their second fights 4 days after initial losses in the RLE populations had fallen to around 50%, whereas it was always higher than 80% in control populations. Similar results were observed in the proportion of previous losing males that were first attacked by tester males at 4 days after initial losses (generation 5: selection, χ21 = 15.52, p < 0.001; replicate (within selection), χ24 = 2.12, p = 0.71; generation 10: selection, χ21 = 27.69, p < 0.001; replicate (within selection), χ24 = 1.18, p = 0.88; figure 2b,d). However, males of the RLE populations had not become completely immune to losing fights. In the first 3 days after initial fight loss, the RLE males behaved much the same as control males losing second contests about 85% of the time (figure 3a), while the control males did not fall to the day 4 levels of selection male success (50% of fights won) until 5 days after initial losses (figure 3a). Similar results were found when we compared which males attacked first, with RLE males tending to become more aggressive only at day 4 and control males not achieving this level of aggression until day 5 after initial losses (figure 3b). This all indicates that loser effect decayed after 3 days in the RLE populations, and after 4 days in control populations. Thus, we found a significant difference in the duration of loser effects between experimental treatments.
Figure 1.
Responses to selection on loser-effect duration. The proportion of males with losing experience (L-males) that lost subsequent fights to a tester male at 4 days after losing initial fights (our measure of loser effect duration). White circles, are the populations where we selected for reduced duration of the loser effect (reduced loser effect duration: RLE). Black circles are the control populations (C) that were not subjected to selection on the duration of the loser effect.
Figure 2.
The proportion of males with losing experience (L-males) that lost subsequent fights to a tester male at 4 days after losing fight (our measure of loser effect duration) and L-males that were attacked first by a tester male (i.e. focal males that did not initiate attacks) at 4 days after losing fight at generations 5 (a,b) and 10 (c,d). RLE populations are those where we selected for reduced duration of the loser effect (reduced loser effect duration). The control populations (C) were not subjected to selection on the duration of the loser effect.
Figure 3.
Loser effects at each day after losing initial fights in focal experimental males—white circles, are the populations where we selected for reduced duration of the loser effect (reduced loser effect duration: RLE). Black circles are the control populations (C) that were not subjected to selection on the duration of the loser effect. (a) is the proportion of focal males that lost subsequent fights, and (b) is the proportion of focal males attacked first by tester males (i.e. focal males that did not initiate attacks). There was neither an effect of selection regime nor replication, but there was a significant interaction between selection regime and day (selection × day, χ42 = 30.26, p < 0.001).
This rapid response to the selection indicated heritable variation in the effects losing has on males. Realized heritabilities were significantly different from zero for all RLE populations (h2 (±s.e.) - RLE I, 0.188 (0.015): RLE II, 0.179 (0.021); RLE III: 0.161 (0.024); all p < 0.001), with 16–19% of the variation in the duration of the loser effect estimated to be due to additive gene action.
Selection on the duration of the loser effect did not affect male fighting success and likelihood of initiating attacks on rivals when males had no previous fighting experience. Naive males from the selection populations attacked as much and won/lost as much in their initial fights as males from control populations (initiate attacks—RLE, 0.53, 0.50, 0.57: C, 0.47, 0.43, 0.53: selection, χ21 = 0.56, p = 0.46; replicate (within selection), χ24 = 0.89, p = 0.93; fights lost—RLE, 0.43, 0.53, 0.53: C, 0.50, 0.57, 0.47: selection, χ21 = 0.02, p = 0.88, replicate (within selection), χ24 = 1.43, p = 0.84). Furthermore body size did not evolve as a correlated response to selection on loser effect duration (body size (mm ± s.e.): RLE, 1.214 (0.006), 1.222 (0.006), 1.214 (0.005): C, 1.217 (0.007), 1.207 (0.005), 1.208 (0.007): selection, χ21 = 1.42, p = 0.23, replicate (within selection), χ24 = 2.84, p = 0.58).
4. Discussion
Our major findings here were that the duration of loser effects can evolve, with narrow sense heritabilities of about 17%, and furthermore, the evolved, reduced duration of the loser effect was not simply owing to a general loss of the effect. Additionally there appeared to be no general change in fighting ability (as measured by fighting success in first fights) or body size that evolved as correlated responses to selection on loser effect duration. We discuss these findings further below.
Perhaps the most interesting finding was that in the populations that evolved shorter loser-effect durations, fighting success in contests between naive animals did not evolve—there was no difference in success rates between control and experimental populations. This suggests that actual fighting ability in these dyadic contests had not evolved in response to our selection, but clearly there was a reduction in the effects losing had on subsequent behaviours in the experimental populations. This contrasts somewhat with crickets where winning is associated with a broader range of fighting tactics [42], but the fact that beetle populations evolving reduced impacts of losing had not changed their fighting success (% naive wins) only serves to highlight the differences between fighting ability and the impacts of losing. Indeed, the fact that loser effects can evolve independently of fighting ability establishes the broad-horned flour beetle as an ideal system to test formal theoretical predictions about when loser effects are expected to evolve by themselves [19]. That is, in testing how fighting costs and dominance benefits affect the disconnect between loser and winner effects, and for example, testing whether increasing variation in fighting ability within populations selects for stronger loser effects as predicted by theory [9,19]. Future work could therefore manipulate key parameters in different populations and quantify any concomitant evolutionary change in loser effects.
Body size also did not evolve as a correlated response to selection, which given the lack of change in fighting ability is arguably not surprising. Size frequently determines RHP, and RHP should correlate with an individual's absolute probability of winning fights [6]. However, fighting ability is also associated to other factors like fighting skills and physical performance [43–45]. Indeed, recent work has shown that fighting ability can be linked to measureable functional traits such as bite force (reviewed in [43]), and the loser effect is associated with a decrease in bite force in the cricket Acheta domesticus [44]. Further studies are required to investigate precisely what determines fighting ability in G. cornutus, but our results suggest that the loser effect and fighting ability of naive (with respect to fighting) males are not closely genetically linked in this species—males from populations selected for reduced duration of the loser effect did not win more initial fights than control males (nor where they larger), so it appears functional traits linked to absolute ability did not coevolve with reduced loser effects. This finding corroborates assumptions in the theoretical literature, which posit that loser (and winner) effects reflect changes in subjective estimates of the distribution of fighting abilities in the population but not changes in individual fighting abilities per se [9].
While experience effects are often short-lived, they vary in their durations and duration can be affected by costs and benefits of fighting and social interaction frequency [2,22]. These general inferences are mirrored in a theoretical study of G. cornutus fighting behaviour, which predicted that the optimal duration of the loser effect would depend on the frequency of social interactions, the mating success derived from fighting (benefit) and the decrease in longevity resulting from fighting (cost) [28]. Again, these findings all suggest effects can evolve, as we have shown here. Interestingly, the heritability of the loser effect we report is on the low side for a behaviour [46] and this probably reflects the fact that there are many links in the causal pathway generating the effect. That is, we may have selected on memory retention or metabolic rate for example, but have not directly estimated the heritability of memory or metabolism. Additionally, a number of studies have implicated biogenic amines such as octopamine or dopamine as neurochemical mechanisms of winner/loser effects (e.g. [23,47]; reviewed in [48]). Thus by selecting on the duration of the loser effect we may well have altered the time course of octopamine effects, or those of an octopamine agonist. We did not test for these possible changes, and there are of course mechanisms other than these that could be involved in generating the evolutionary change we document, including a raft of other physiological and neurological processes [49,50] that could have been altered by the artificial selection we applied. We finally note that realized heritabilities are only approximations of base-population heritabilities [40], and that there was no evolution of effects in the control lines.
The relatively low heritability also implies that, as expected, much of the variation in the loser effect is environmental. Outcomes of direct physical fights will obviously depend on opponents and will provide reliable information enabling self-assessment of one's own fighting ability relative to others in the population. Thus the social environment and an individuals' developmental environment must influence winner/loser effects to a large degree and thus contribute much to phenotypic variation in these effects (e.g. [51,52]).
Behavioural modulations resulting from winning fights have not been recorded in G. cornutus [14] even though the loser effect has a relatively long duration. This matches a general pattern of effect decay, with loser effects generally lasting longer than winner effects [2]. For example, losing fights impacts sticklebacks for around 6 h, but the winner effect has largely disappeared after about 3 h [53]. From a proximate perspective, it has been suggested that this asymmetry is a consequence of fundamental learning processes: losers may have more control over situation outcomes (i.e. they can retreat but individuals cannot determine whether a fight will occur or not as that depends on opponent behaviour) and hence links (activity-outcome) are easier to establish and remember [19,54].
Given the methodological impacts on winner/loser effect assessment, it is important to note that individuals in our investigation were self-selecting (sensu 2) (i.e. we did not randomly allocate subjects to winner/loser treatments), were isolated for much of their lives and had very few encounters with competitors. Each of these factors can potentially affect individual experience [2]. In our beetles, individuals are normally likely to encounter multiple rivals throughout their lives, these multiple encounters will probably result in more complex effects, with each individual experience potentially contributing to cumulative effects on future contest outcomes (e.g. [55,56]).
Fighting experience effects can also impact multiple behaviours and ecological processes that we did not assess here (e.g. [57,58]). Indeed, the loser effect can impact various reproductive and dispersal strategy in G. cornutus beyond the fighting outcome itself [14,35]. Similarly, theory predicts that many factors can influence the strength of loser effects, including age and experience (e.g. [59], reviewed in [9]), and many of these are untested in flour beetles. Furthermore, the effects of male experience could impact female reproductive behaviours in this beetle. There are direct fitness costs imposed on females by aggressive, competitively superior males [60]. These males are highly aggressive towards rival males [38] but also attack females [60]. Thus female fitness-costs are probably side-effects of misdirected male aggression, as suggested for the dung fly, Sepsis cynipsea [61]. Many studies have now demonstrated that highly competitive males can be harmful to females (e.g. [62–66]) and in G. cornutus, indicate female mate-preference may be constrained, because although females prefer males that fight less, they frequently do not get to mate with them [33,60].
To conclude, we used artificial selection to cause micro-evolution of the duration of the loser effect. We also found that the reduction in response duration was not associated with a change in fighting ability (as measured by the likelihood of fighting success), which supports theoretical predictions. Further investigations of these effects and on the precise neural/physiological mechanism underpinning the outcomes of our artificial selection are warranted. Our work also suggests broad-horned flour-beetles are an excellent model to explicitly test theoretical predictions about the conditions under which pure loser effects are expected to evolve.
Supplementary Material
Acknowledgements
We thank Alastair Wilson, and Anna Duarte for discussion of aggression and loser effects and three anonymous referees for comments that greatly improved the manuscript.
Data accessibility
Data supporting this paper can be found in the Dryad Digital Repository: https://doi.org/10.5061/dryad.34b1466 [67].
Authors' contributions
K.O. collected data, carried out the statistical analyses, participated in the design of the study and drafted the manuscript; Y.O., S.R.X.D. and D.J.H. conceived of the study, coordinated the design of the study and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (Kakenhi 18K0641700 to K.O., Kakenhi 18H04815, 17H05938, 17K19381 to Y.O.).
References
- 1.Huntingford FA, Turner AK. 1987. Animal conflict. London, UK: Chapman & Hall. [Google Scholar]
- 2.Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74. ( 10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 3.Rutte C, Taborsky M, Brinkhof MW. 2006. What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21. ( 10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- 4.Hosken DJ, Hunt J, Wedell N. 2019. Genes and behaviour: beyond nature-nurture. Chichester, UK: Wiley. [Google Scholar]
- 5.Blows MW, Hoffmann AA. 2005. A reassessment of genetic limits to evolutionary change. Ecology 86, 1371–1384. ( 10.1890/04-1209) [DOI] [Google Scholar]
- 6.Parker GA. 1974. Assessment strategies and the evolution of fighting behavior. J. Theor. Biol. 47, 223–243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 7.Taylor PW, Jackson RR. 2003. Interacting effects of size and prior injury in jumping spider conflicts. Anim. Behav. 65, 787–794. ( 10.1006/anbe.2003.2104) [DOI] [Google Scholar]
- 8.Obermeier M, Schmitz B. 2003. Recognition of dominance in the big-clawed snapping shrimp (Alpheus heterochaelis Say 1818) part II: analysis of signal modality. Mar. Freshw. Behav. Physiol. 36, 17–29. ( 10.1080/1023624031000088949) [DOI] [Google Scholar]
- 9.Mesterton-Gibbons M, Dai Y, Goubault M. 2016. Modeling the evolution of winner and loser effects: a survey and prospectus. Math. Biosci. 274, 33–44. ( 10.1016/j.mbs.2016.02.002) [DOI] [PubMed] [Google Scholar]
- 10.Yurkovic A, Wang O, Basu AC, Kravitz EA. 2006. Learning and memory associated with aggression in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 103, 17 519–17 524. ( 10.1073/pnas.0608211103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 12.Whitehouse MEA. 1997. Experience influences male-male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 53, 913–923. ( 10.1006/anbe.1996.0313) [DOI] [Google Scholar]
- 13.Hsu Y, Lee IH, Lu CK. 2009. Prior contest information: mechanisms underlying winner and loser effects. Behav. Ecol. Sociobiol. 63, 1247–1257. ( 10.1007/s00265-009-0791-9) [DOI] [Google Scholar]
- 14.Okada K, Miyatake T. 2010. Effect of losing on male fights of broad-horned flour beetle, Gnatocerus cornutus. Behav. Ecol. Sociobiol. 64, 361–369. ( 10.1007/s00265-009-0852-0) [DOI] [Google Scholar]
- 15.Emlen DJ. 1997. Alternative reproductive tactics and male dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341. ( 10.1007/s002650050) [DOI] [Google Scholar]
- 16.Moczek AP, Emlen DJ. 2000. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466. ( 10.1006/anbe.1999.1342) [DOI] [PubMed] [Google Scholar]
- 17.Briffa M, Sneddon LU, Wilson AJ. 2015. Animal personality as a cause and consequences of contest behaviour. Biol. Lett. 11, 20141007 ( 10.1098/rsbl.2014.1007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DT, Hosken DJ, ffrench-Constant RH, Wedell N. 2009. Variation in sex peptide expression in D. melanogaster. Genet. Res. 91, 237–242. ( 10.1017/S0016672309000226) [DOI] [PubMed] [Google Scholar]
- 19.Mesterton-Gibbons M. 1999. On the evolution of pure winner and loser effects: a game-theoretic model. Bull. Math. Biol. 61, 1151–1186. ( 10.1006/bulm.1999.0) [DOI] [PubMed] [Google Scholar]
- 20.Francis RC. 1983. Experimental effects on agonistic behavior in the paradise fish, Macropodus opercularis. Behaviour 8, 292–313. ( 10.1163/156853983X00273) [DOI] [Google Scholar]
- 21.McDonald AL, Heimstra NW, Damkot DK. 1968. Social modification of agonistic behavior in fish. Anim. Behav. 16, 437–441. ( 10.1016/0003-3472(68)90037-7) [DOI] [PubMed] [Google Scholar]
- 22.Schuett GW. 1997. Body size and agonistic experience affect dominance and mating success in male copperheads. Anim. Behav. 54, 213–224. ( 10.1006/anbe.1996.0417) [DOI] [PubMed] [Google Scholar]
- 23.Adamo SA, Hoy RR. 1995. Agonsitic behaviour in male and female field crickets, Gryllus bimaculatus, and how behavioral context influences its expression. Anim. Behav. 49, 1491–1501. ( 10.1016/0003-3472(95)90070-5) [DOI] [Google Scholar]
- 24.Dodson GN, Swaab AT. 2001. Body size, leg autonomy and prior experience as factors in fighting success in male crab spiders, Misumenoides formosipes. J. Insect Behav. 14, 841–855. ( 10.1023/A:101304560) [DOI] [Google Scholar]
- 25.Adamo SA, Linn CE, Hoy RR. 1995. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691–1700. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki M, Delago A, Nishino H, Aonuma H. 2006. Effects of previous experience on the agonistic behaviour of male crickets, Gryllus bimaculatus. Zool. Sci. 23, 863–872. ( 10.2108/zsj.23.863) [DOI] [PubMed] [Google Scholar]
- 27.Hofmann HA, Stevenson PA. 2000. Flight restores fight in crickets. Nature 403, 613 ( 10.1038/35001137) [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Okada K, Kajiwara T, Miyatake T. 2010. On the optimal duration of memory of losing a conflict—a mathematical model approach. J. Biol. Dyn. 4, 270–281. ( 10.1080/17513750903161036) [DOI] [PubMed] [Google Scholar]
- 29.Harano T, Okada K, Nakayama S, Miyatake T, Hosken DJ. 2010. Intralocus sexual conflict unresolved by sex-limited trait expression. Curr. Biol. 20, 2036–2039. ( 10.1016/j.cub.2010.10.023) [DOI] [PubMed] [Google Scholar]
- 30.House CM, Jensen K, Rapkin J, Lane S, Okada K, Hosken DJ, Hunt J. 2016. Macronutrient balance mediates the growth of sexually selected weapons but not genitalia in male broad horned beetles. Funct. Ecol. 30, 769–779. ( 10.1111/1365-2435.12567) [DOI] [Google Scholar]
- 31.Katsuki M, Harano T, Miyatake T, Okada K, Hosken DJ. 2012. Intralocus sexual conflict and offspring sex ratio. Ecol. Lett. 15, 193–197. ( 10.1111/j.1461-0248.2011.01725.x) [DOI] [PubMed] [Google Scholar]
- 32.Okada K, Miyatake T. 2010. Plasticity of size and allometry in multiple sexually selected traits in an armed beetle Gnatocerus cornutus. Evol. Ecol. 24, 1339–1351. ( 10.1007/s10682-010-9370-9) [DOI] [Google Scholar]
- 33.Okada K, Katsuki M, Sharma MD, House CM, Hosken DJ. 2014. Sexual conflict over mating in Gnatocerus cornutus: females prefer lovers not fighters. Proc. R. Soc. B 281, 20140388 ( 10.1098/rspb.2014.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada K, Archer CR, Katsuki M, Suzaki Y, Sharma MD, House CM, Hosken DJ. 2015. Polyandry and fitness in female horned flour beetles (Gnatocerus cornutus). Anim. Behav. 106, 11–16. ( 10.1016/j.anbehav.2015.05.008) [DOI] [Google Scholar]
- 35.Okada K, Yamane T, Miyatake T. 2010. Ejaculatory strategies associated with experience of losing. Biol. Lett. 6, 593–596. ( 10.1098/rsbl.2010.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamane T, Okada K, Nakayama S, Miyatake T. 2010. Dispersal and ejaculatory strategies associated with exaggeration of weapon in an armed beetle. Proc. R. Soc. B 277, 1705–1710. ( 10.1098/rspb.2009.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaddick PR, Leek FF. 1972. Further specimens of stored products insects found in ancient Egyptian tombs. J. Stored Prod. Res. 8, 83–86. ( 10.1016/0022-474X(72)90023-9) [DOI] [Google Scholar]
- 38.Okada K, Miyatake T. 2009. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 77, 1057–1065. ( 10.1016/j.anbehav.2009.01.008) [DOI] [Google Scholar]
- 39.Institute SAS. 2007. JMP release 7. Cary, NC: SAS Institute Inc. [Google Scholar]
- 40.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. New York, NY: Longman. [Google Scholar]
- 41.Unrug J, Tomkins JL, Radwan J. 2004. Alternative phenotypes and sexual selection: can dichotomous handicaps honestly signal quality? Proc. R. Soc. Lond. B 271, 1401–1406. ( 10.1098/rspb.2004.2729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hack MA. 1997. Assessment strategies in the contests of male crickets, Acheta domesticus. Anim. Behav. 53, 733–747. ( 10.1006/anbe.1996.0310) [DOI] [Google Scholar]
- 43.Lailvaux SP, Irschick DJ. 2006. A functional perspective on sexual selection: insights and future prospects. Anim. Behav. 72, 263–273. ( 10.1016/j.anbehav.2006.02.003) [DOI] [Google Scholar]
- 44.Condon C, Lailvaux SP. 2016. Losing reduces maximum bite performance in house cricket contests. Funct. Ecol. 30, 1660–1664. ( 10.1111/1365-2435.12654) [DOI] [Google Scholar]
- 45.Briffa M, Lane SM. 2017. The role of skill in animal contests: a neglected component of fighting ability. Proc. R. Soc. B 284, 20171596 ( 10.1098/rspb.2017.1596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mousseau TA, Roff DA. 1987. Natural selection and the evolution of fitness components. Heredity 59, 181–187. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 47.Rillich J, Stevenson PA. 2011. Winning fights induces hyperaggression via the action of the biogenic amine octopamine in crickets. PLoS ONE 6, e28891 ( 10.1371/journal.pone.0028891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bubak AN, Grace JL, Watt MJ, Renner KJ, Swallow JG. 2014. Neurochemistry as a bridge between morphology and behavior: perspectives on aggression in insects. Curr. Zool. 60, 778–790. ( 10.1093/czoolo/60.6.778) [DOI] [Google Scholar]
- 49.Kravitz EA, Huber R. 2003. Aggression in invertebrates. Curr. Opin. Neurobiol. 13, 736–743. ( 10.1016/j.conb.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 50.Stevenson PA, Hofmann HA, Schoch K, Schildberger K. 2000. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 43, 107–120. () [DOI] [PubMed] [Google Scholar]
- 51.Moore AJ, Brodie ED, Wolf JB. 1997. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362. ( 10.1111/j.1558-5646.1997.tb01458.x) [DOI] [PubMed] [Google Scholar]
- 52.Wilson AJ, Gelin U, Perron M-C, Real D. 2009. Indirect genetic effects and the evolution of aggression in a vertebrate system. Proc. R. Soc. B 276, 533–541. ( 10.1098/rspb.2008.1193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakker TCM, Feuth-De Bruijn E, Sevensster P.. 1989. Asymmetrical effect of prior winning and losing on dominance in sticklebacks (Gasterosteus aculeatus). Ethology 82, 224–229. ( 10.1111/j.1439-0310.1989.tb00502.x) [DOI] [Google Scholar]
- 54.Staddon JER. 1983. Adaptive behaviour and learning. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 55.Hsu Y, Wolf LL. 1999. The winner and loser effect: integrating multiple experiences. Anim. Behav. 57, 903–910. ( 10.1006/anbe.1998.1049) [DOI] [PubMed] [Google Scholar]
- 56.Stuart-Fox DM, Firth D, Moussalli A, Whiting MJ. 2006. Multiple signals in chameleon contests: designing and analysing animal contests as a tournament. Anim. Behav. 71, 1263–1271. ( 10.1016/j.anbehav.2005.07.028) [DOI] [Google Scholar]
- 57.Taborsky B, Oliveira RF. 2012. Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679–688. ( 10.1016/j.tree.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 58.Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU. 2007. Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc. R. Soc. B 274, 333–339. ( 10.1098/rspb.2006.3751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fawcett TW, Johnstone RA. 2010. Learning your own strength: winner and loser effects should change with age and experience. Proc. R. Soc. B 277, 1427–1434. ( 10.1098/2Frspb.2009.2088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiyose K, Katsuki M, Suzaki Y, Okada K. 2015. Competitive males but not attractive males reduce female fitness in Gnatocerus cornutus. Anim. Behav. 109, 265–272. ( 10.1016/j.anbehav.2015.09.002) [DOI] [Google Scholar]
- 61.Teuschl Y, Hosken DJ, Blanckenhorn WU. 2007. Is reduced female survival after mating a by-product of male-male competition in the dung fly Sepsis cynipsea? BMC Evol. Biol. 7, 194 ( 10.1186/1471-2148-7-194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hongo Y. 2012. Mating interaction of the Japanese horned beetle Trypoxylus dichotomus septentrionalis: does male-excluding behavior induce female resistance? Acta Ethol. 15, 195–201. ( 10.1007/s10211-012-0128-y) [DOI] [Google Scholar]
- 63.Moore AJ, Gowaty PA, Wallin WG, Moore PJ. 2001. Sexual conflict and the evolution of female mate choice and male social dominance. Proc. R. Soc. B 268, 517–523. ( 10.1098/rspb.2000.1399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore AJ, Gowaty PA, Moore PJ. 2003. Females avoid manipulative males and live longer. J. Evol. Biol. 16, 523–530. ( 10.1046/j.1420-9101.2003.00527.x) [DOI] [PubMed] [Google Scholar]
- 65.Moore AJ, Moore PJ. 1999. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. B 266, 711–716. ( 10.1098/rspb.1999.0694) [DOI] [Google Scholar]
- 66.Pitnick S, García-González F. 2002. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B 269, 1821–1828. ( 10.1098/rspb.2002.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okada K, Okada Y, Dall SRX, Hosken DJ. 2019. Data from: Loser-effect duration evolves independently of fighting ability Dryad Digital Repository. ( 10.5061/dryad.34b1466) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Okada K, Okada Y, Dall SRX, Hosken DJ. 2019. Data from: Loser-effect duration evolves independently of fighting ability Dryad Digital Repository. ( 10.5061/dryad.34b1466) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data supporting this paper can be found in the Dryad Digital Repository: https://doi.org/10.5061/dryad.34b1466 [67].