Abstract
Rutin, a food derived-polyphenolic bioflavonoid, has been acknowledged for several health benefits. This study aims to explore the ameliorative effects of rutin against carbon tetrachloride (CCl4) toxicity in male rats. Adult male rats were given either CCl4 (30% in olive oil, 3 ml/kg b.w. intraperitoneally) alone or in combination with rutin (70 mg/kg intragastrically) twice a week for 4 weeks. Our data showed that rutin mitigated CCl4 hepatorenal damage, as indicated by diagnostic markers (i.e., transaminases, alkaline phosphatase, total bilirubin, total protein, albumin, urea, uric acid and creatinine), and histopathological findings. In addition, CCl4 induced profound elevation of free radical generation and oxidative stress, as evidenced by increasing lipid peroxidation and reducing catalase, superoxide dismutase and glutathione peroxidase activities in liver, kidney and testicular tissues; these effects were suppressed by coexposure with rutin. Moreover, the increase in the levels of serum triglycerides, cholesterol, low-density lipoprotein cholesterol, and very-low-density lipoprotein cholesterol induced by CCl4 was effectively counteracted by rutin. The decrease in the level of high-density lipoprotein cholesterol in the CCl4 group was also counteracted by rutin treatment. Interestingly, the decreased levels of hormonal mediators associated with sperm production, including serum testosterone, luteinizing hormone and follicle-stimulating hormone, and the impaired sperm quality induced by CCl4 were reversed by rutin. Data from the current study clearly demonstrated that rutin supplementation could at least partly overcome CCl4-induced hepatotoxicity, nephrotoxicity and reproductive toxicity by antioxidant and antidyslipidemic effects.
Keywords: Carbon tetrachloride, Hepatorenal failure, Rutin, Hypogonadism, Dyslipidemia, Oxidative stress
Introduction
Industrial progress has a poisonous nature. CCl4 is one of the most potent environmental contaminants (Hefnawy & Ramadan, 2013). Humans are exposed to CCl4 via oral, inhalation and dermal routes (Manibusan, Odin & Eastmond, 2007). CCl4 intoxication is associated with high free radical production in several organs, including the liver and kidney (Ozturk et al., 2003; Preethi & Kuttan, 2009). CCl4 binds to liver cytochrome P450 to form trichloromethyl (CCl3) free radicals, which initiate membrane lipid peroxidation (Abdel-Kader et al., 2018). Secondary metabolic radicals of CCl4, such as trichloromethylperoxy radical (CCl3O2), react with lipids or proteins. This alters the permeability of the mitochondria, endoplasmic reticulum, and plasma membrane, resulting in cell damage (Rahman et al., 2017). CCl4-induced damage also includes altering the endogenous antioxidants in tissues Alshammari, Balakrishnan & Chinnasamy (2017), which is manifested by histopathological lesions. Meanwhile, some researchers reported that the administration of CCl4 elevated cholesterol, triglycerides, and free fatty acids in the liver and kidney of rats (Marimuthu et al., 2013) and caused male genotoxic effects in mouse bone marrow and germ cells (Diab et al., 2018).
Antioxidants are vital substances that possess the ability to protect the body from damage caused by free radical-induced oxidative stress (Abdel-Moneim et al., 2015). Much attention is paid to the protective effects of natural antioxidants against chemically induced toxicities (Frei & Higdon, 2003). Among these compounds, flavonoids are plant-based polyphenols that exhibit a wide range of beneficial effects in a multitude of disease models (see Mahmoud et al., 2019 for review). Rutin (named vitamin P) is composed of flavonol quercetin and disaccharide rutinose (Ganeshpurkar & Saluja, 2017). It is an important constituent of food and beverages. Many studies have suggested that rutin exerts antioxidant, anti-inflammatory, anti-apoptotic, antihyperuricemic, and antihyperlipidemic properties (Hosseinzadeh & Nassiri-Asl, 2014; Gelen et al., 2017; Radwan & Abdel Fattah, 2017; Khajevand-Khazaei et al., 2018). It has been reported that rutin prevented methotrexate-induced hepatic injury in a rat model (Erdogan et al., 2015). Moreover, the use of rutin for the treatment of hyperglycemic complications has been documented (Ghorbani, 2017). Rutin also showed a promising effect against oxidative insult associated with male infertility (Mehfooz et al., 2018). Therefore, the present work investigates the antioxidant and anti-hyperlipidemic effects of oral rutin administration as potential mechanisms driving its therapeutic ability against CCl4-induced hepatorenal and testicular toxicity.
Material and Methods
Chemicals and reagents
Carbon tetrachloride CCl4 was purchased from Sigma Chemicals (St. Louis, MO, USA). Rutin was purchased from Loba Chemie (Mumbai, India). All other reagents were of analytical grade.
Animals and experimental design
Male albino rats weighing 180–200 g were used in the present study. All experimental procedures were reviewed and approved by the research ethics committee at King Faisal University (Ref. No. KFU-REC/2019-03-01). Rats were maintained under standard laboratory conditions and had fed chow and water ad libitum. After 2 weeks of acclimatization, the animals were randomly divided into four groups (five rats in each). Group 1 served as the control and received only vehicles, i.e., 1 ml/kg b.w. saline intragastrically and olive oil (3 ml/kg b.w.) intraperitoneally twice a week for 4 weeks. Group 2 (CCl4) was administrated CCl4 (30% in olive oil) at a dose of 3 ml/kg b.w. intraperitoneally twice a week for 4 weeks. Group 3 (rutin) received rutin in normal saline at a dose of 70 mg/kg b.w. intragastrically twice a week for 4 weeks. Group 4 (rutin + CCl4) received rutin at a dose of 70 mg/kg b.w. intragastrically, in addition to CCl4 administration, twice a week for 4 weeks. Doses of CCl4 and rutin were selected as previously reported by Khan, Khan & Sahreen (2012). After 24 h of the last treatment, animals were sacrificed, and blood samples were drawn from the dorsal aorta into dry glass centrifuge tubes, left to clot and then centrifuged at 5,000 rpm for 10 min. Serum was separated and stored at −80 °C for further assays. Small parts of the liver, kidney and right testes were washed in ice-cold normal saline solution and frozen at −80 °C for future analysis. For histological studies, other portions of the liver and kidney were quickly removed and fixed in an appropriate fixative.
Assay of liver markers
Alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes and total protein were estimated by commercial kits (ALP; REF: ALP101050, AST; REF: GOT111120, ALT; REF: GPT1131001, total protein; REF: TP116150; BIOMED Diagnostics, Oberschleißheim, Germany). AST and ALT were measured by the decrease in absorbance at 340 nm, which is directly proportional to the oxidation of NADH to NAD. ALP activity was determined by measuring the per time absorption increase at 405 nm. Total bilirubin was measured using a Diamond Diagnostics kit, Germany (REF: BIL099100). Albumin was determined by the modified bromocresol green colorimetric method using its relevant kit (REF: 210 002, SPECTRUM, Egypt).
Assay of kidney markers
Urea and creatinine acid were estimated using commercial kits (urea; REF: URE118200, creatinine; REF: CRE106240; BIOMED Diagnostics, Oberschleißheim, Germany). Briefly, serum urea was enzymatically determined and colorimetrically measured, where the conversion of urea in the sample by urease enzyme provided a colored complex that can be measured by spectrophotometry at 578 nm. Creatinine was determined by standard alkaline picric acid method, where creatinine reacts with picric acid in an alkaline medium to give a yellow-orange color complex, which was measured at 492 nm. The intensity of the color is proportional to the concentration of the creatinine concentration. The uric acid kit was obtained from SPECTRUM, Egypt (REF: 323001). Uric acid level was estimated colorimetrically by the dual action of uricase and peroxidase enzymes in the presence of 4-amino-antipyrine. The formed red-color quinoneimine dye was recorded at 546 nm.
Hepatic and renal histopathology
Liver and kidney tissues were fixed in 10% neutral formalin solution for 24 h, dehydrated in ascending series of ethanol, cleared in xylene then embedded in paraffin wax. Sections were stained with conventional haematoxylin and eosin (H&E) dye and examined using a Nikon 80i light microscope (Nikon Corporation, Tokyo, Japan).
Estimation of tissue malondialdehyde (MDA) and antioxidant enzymes
For homogenate preparation, the desired tissues were quickly removed, cleaned and washed in ice-cold saline. They were finely minced and homogenized in 0.1 M phosphate buffer pH 7.4 using glass Teflon homogenizer. The homogenate was centrifuged at 6,000 rpm for 30 min at 40 °C then the supernatant was used for biochemical estimations.
MDA levels and superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) enzyme activities were assayed according to the manufacturer’s protocol (MDA; CAT No.: MD 2528, SOD; CAT. No.: SA 2520, CAT; CAT. No.: CA 2516; GPx; CAT. No.: GP 2524; Biodiagnostic, Dokki, Egypt). Principally, the colorimetric assay of MDA involves the reaction of MDA with thiobarbituric acid (TBA) in acidic medium at 95 °C to form thiobarbituric acid reactive product, and the absorbance of the resultant pink product can be measured at 534 nm. SOD assay relies on the ability of the SOD to inhibit the phenazine methosulphate-mediated reduction of nitro-blue tetrazolium dye. CAT assay colorimetric method depends on the measurement of the hydrogen peroxide (H2O2) substrate remaining after the action of CAT present in the sample. The rest of H2O2 reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone to yield a colored chromophore with a color intensity that is inversely proportional to the amount of CAT in the sample. The activity of GPx was measured indirectly, based on the principle that oxidized glutathione (GSSG), produced upon reduction of an organic peroxide by GPx, is immediately recycled to its reduced form (GSH) by glutathione reductase (GR). This is accompanied by oxidation of NADPH (GR coenzyme) to NADP+, which was monitored by the decrease in absorbance at 340 nm. The protein contents of the tissue samples (i.e., liver, kidney and testis) were determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Estimation of serum lipid profile
Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels were determined by using commercial kits (TC; CAT. No.: CH 1220, TG; CAT. No.: TR 2030, HDL-C; CAT. No.: CH 1230; Biodiagnostic, Dikka, Egypt). Very-low-density lipoprotein cholesterol (VLDL-C) and low-density lipoprotein cholesterol (LDL-C) were calculated using Friedewald’s formula (Friedewald, Levy & Fredrickson, 1972).
Estimation of reproductive hormones
Serum testosterone was measured using a rat testosterone ELISA kit (CAT. No. KT-29533) purchased from K-assay, WA, USA. The levels of luteinizing hormone (LH) were determined by using Shibayagi’s rat LH ELISA kit, while the levels of follicle stimulating hormone (FSH) were estimated by using a rat FSH ELISA kit obtained from Biovendor, Tokyo, Japan. All hormonal assays employ the competitive inhibition enzyme immunoassay technique. Briefly, a monoclonal antibody specific for the estimated hormone has been pre-coated onto a microplate. A competitive inhibition reaction was started between biotin labeled hormone and the unlabeled one in our samples with the pre-coated antibody specific for the hormone. After incubation, the unbound conjugate was washed off. Then, avidin conjugated to horseradish peroxidase (HRP) was added to each well and incubated. The amount of bound HRP conjugate was reversely proportional to hormone concentration in the samples. After addition of the substrate solution, the intensity of color developed was reversely proportional to the concentration of hormone in the sample. The plate was read at 450 nm using a BioTek Instruments EL800 Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Assessment of sperm concentration, motility and abnormality
Sperm suspension was obtained by mincing the left testes in a prewarmed saline (37 °C). The percentage of total and progressive motility was estimated microscopically according to the method of Morrissey et al. (1988). A total of at least 200 sperm cells were evaluated from four different fields in each sample. The sperm count was determined using a hemocytometer (Freud & Carol, 1964). Sperm abnormality was recorded by using one or two drops of previously warmed (37 °C) eosin-nigrosin stain (Evans & Maxwell, 1987).
Data analysis
Statistical calculations were performed using SPSS for Windows (version 17; SPSS Inc., Chicago, IL, USA). The results were expressed as the mean ± S.E. (standard error) and statistical significance (P ≤ 0.05) was evaluated by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post hoc test.
Results
Effects of rutin on CCl4-induced hepatotoxicity
As shown in Table 1, CCl4 administration induced significant increase in serum ALP (157.6%), AST (191.8%) and ALT (160.4%) as compared to control values. Rutin treatment with CCl4 significantly reduced ALP by −28.7%, AST by −55.3% and ALT by -39.1% when compared to CCl4 group. In addition, total bilirubin significantly increased (100%) while protein and albumin levels significantly decreased (−37.8% and −55.9%, respectively) in CCl4 group as compared to control. However, rats in the Rutin + CCl4 group had significantly lower levels of total bilirubin (−25%), and higher levels of total protein (41.3%) and albumin (80%) than those in the CCl4 group.
Table 1. Effect of rutin on serum liver markers in male rats intoxicated with CCl4.
| ALT (U/L) | AST (U/L) | ALP (U/L) | Bilirubin (gm/dl) | Protein (gm/dl) | Albumin (gm/dl) | |
|---|---|---|---|---|---|---|
| Control | 32.8a± 1.50 | 56.4a± 1.50 | 36.8a± 2.40 | 0.4a± 0.01 | 7.4a± 0.30 | 3.4a± 0.30 |
| CCl4 | 85.4b± 4.30 | 164.6b± 3.40 | 94.8b± 2.60 | 0.8b± 0.03 | 4.6b± 0.20 | 1.5b± 0.30 |
| Rutin | 30.2a± 2.30 | 56.0a± 2.90 | 38.4a± 2.70 | 0.5a± 0.01 | 7.6a± 0.20 | 3.5a± 0.20 |
| Rutin + CCl4 | 52.0c± 2.90 | 73.6c± 1.10 | 67.6c± 3.00 | 0.6c± 0.02 | 6.5c± 0.30 | 2.7c± 0.20 |
Data are expressed as mean ± SE of n = 5 rats/group. Different superscripts within the same column indicate significant differences between the groups (p ≤ 0.05). Treatments: control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
- AST
- aspirate transaminase
- ALT
- alanine transaminase
- ALP
- alkaline phosphatase
Effects of rutin on CCl4-induced changes in the levels of serum renal function test parameters
The levels of urea, creatinine and uric acid significantly increased in CCl4 group (122.2%, 350%, 70.4%, respectively) as compared with the control group values (Fig. 1). Conversely, Rutin + CCl4 group manifested significant declines in urea (−45%), creatinine (−44.4%) and uric acid (−41.3%) as compared to the CCl4 group.
Figure 1. Serum kidney markers in male rats after administration of CCl4 and/or rutin.
(A) Urea, (B) creatinine and (C) uric acid. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both Rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Effects of rutin on CCl4-induced liver histopathology
Liver tissue from control rats (Fig. 2A) presented a normal histological image and hepatocyte structure. In the group treated with CCl4, we noted massive centrilobular necrosis with hepatocyte degeneration, vacuolar fatty change and mild mononuclear cell infiltration (Fig. 2B). In the rutin group, the histological appearances of liver lobules and hepatocytes were normal (Fig. 2C). The livers of rats cotreated with rutin showed minimal hepatocellular necrosis and maintained lobular architecture and hepatocyte structure with no evidence of inflammatory cell infiltration or fatty change (Fig. 2D).
Figure 2. Liver histopathology in male rats after administration of CCl4 and/or rutin.
(A) Control; (B) CCl4; (C) rutin; and (D) Rutin + CCl4. Note centrilobular necrosis (ne), fatty degeneration (arrows) and inflammation (arrowhead) in the liver parenchyma. Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Effects of rutin on CCl4-induced renal histopathology
Normal histological architecture of the kidney was observed in the control group (Fig. 3A). CCl4 administration caused distinct morphologic changes, especially in the renal cortex (Fig. 3B). Glomerular changes were focal and included mild dilation of Bowman’s space with an adhesion between the visceral and parietal layers of Bowman’s capsule and glomerular atrophy. In addition, renal tubules were severely dilated, and their epithelial cells tended to be flattened. Moreover, inflammatory cell infiltration was also detected in the renal interstitium. The kidneys of rats treated with rutin alone showed no histological evidence of nephrotoxicity (Fig. 3C). The group of rats cotreated with rutin reduced the CCl4-induced renal lesions (Fig. 3D).
Figure 3. Renal histopathology in male rats after administration of CCl4 and/or rutin.
(A) Control; (B) CCl4; (C) rutin; and (D) Rutin + CCl4. Note dilatation of Bowman’s space (*), glomerular atrophy (arrowhead), tubular dilatation (short arrows) and inflammatory cell infiltration (long arrow). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both Rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Effects of rutin on CCl4-induced changes in oxidant-antioxidant system parameters
Data presented for liver, kidney and testis showed that CCl4 administration significantly increased the lipid peroxidation marker, MDA (135.4%, 171.2% and 181.8%, respectively) (Fig. 4) and significantly decreased the activities of SOD (−55.5%, −45.91% and −59.41%, respectively) (Fig. 5), CAT (−70.6%, −52.3% and −56.8%, respectively) (Fig. 6) and GPx (−59%, −59.1% and −47.1%, respectively) (Fig. 7) when compared to control corresponding values. The combination of rutin with CCl4 significantly inhibited MDA levels in liver (−34.7%), kidney (−51.8%), and testis (−40.7%) compared with those of CCl4-treated rats. In contrast, co-treatment by rutin resulted in significant enhancement of hepatic, renal and testicular enzymatic activities, i.e., SOD (58.5%, 47.1%, and 63%, respectively), CAT (142.4%, 61.7%, 75.9%, respectively) and GPx (67.7%, 75.2%, and 50.6%, respectively) versus the CCl4-treated group values.
Figure 4. Tissue malondialdehyde (MDA) levels in male rats after administration of CCl4 and/or rutin.
(A) Liver; (B) Kidney; and (C) Testis. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Figure 5. Tissue superoxide dismutase (SOD) levels in male rats after administration of CCl4 and/or rutin.
(A) Liver; (B) Kidney; and (C) Testis. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Figure 6. Tissue catalase (CAT) level in male rats after administration of CCl4 and/or rutin.
(A) Liver; (B) Kidney; and (C) Testis. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Figure 7. Tissue glutathione peroxidase (GPx) levels in male rats after administration of CCl4 and/or rutin.
(A) Liver; (B) Kidney; and (C) Testis. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both Rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
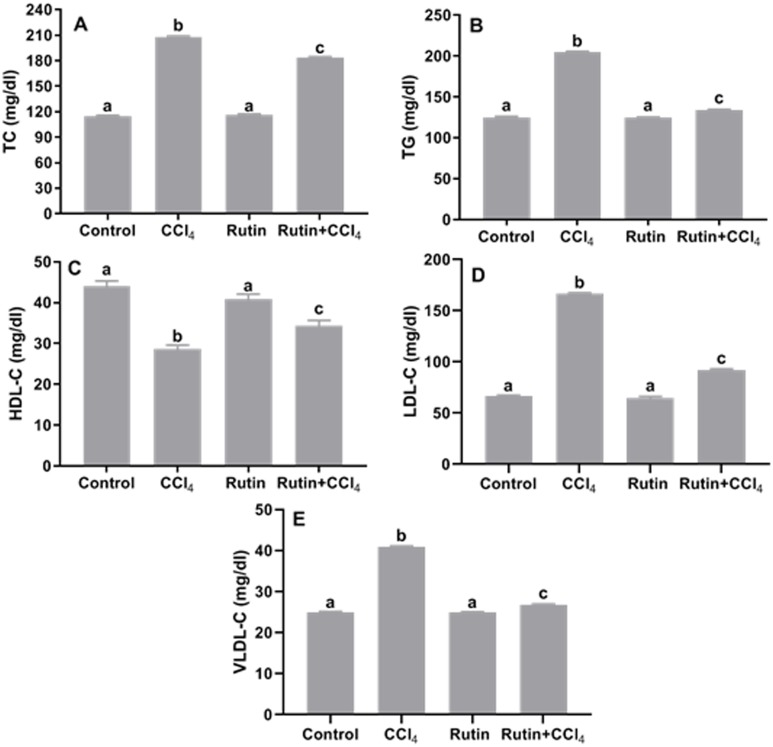
Effects of rutin on CCl4-induced changes in serum lipid content
The lipid profile presented in Fig. 8 showed significant increase in the levels of TC (81.2%), TG (63.4%), LDL-C (150.5%) and VLDL-C (63.6%) and significant decrease in HDL-C (−35%) in the CCl4 group as compared to the corresponding control values. However, treatment with rutin remarkably decreased TC (−11.5%), TG (−34.5%), LDL-C (−44.7%) and VLDL-C (−34.5%) levels and increased HDL-C (20.3%) as compared to CCl4 treated rats.
Figure 8. Serum lipid profile in male rats after administration of CCl4 and/or rutin.
(A) TC: total cholesterol, (B) TG: triglycerides, (C) HDL-C: high-density lipoprotein-cholesterol, (D) low-density lipoprotein-cholesterol and (E) VLDL-C: very-low-density lipoprotein-cholesterol. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Effects of rutin on CCl4-induced changes in serum testosterone, LH and FSH
Treatment of rats with CCl4 led to a significant decrease in serum levels of testosterone (−61.5%), LH (−50%) and FSH (−40%) when compared to the corresponding control levels. While, these hormones were restored in Rutin + CCl4 group through significant increasing of testosterone (133.3%; normalization), LH (28.6%) and FSH (33.3%) relative to their corresponding values in CCl4 treated group (Fig. 9).
Figure 9. Serum reproductive hormones in male rats after administration of CCl4 and/or rutin.
(A) Testosterone, (B) LH and (C) FSH. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Effects of rutin on CCl4-induced changes in sperm parameters
Relative to control, CCl4 administration caused significant decrease in sperm count (−36.3%) and sperm motility (−59.2%) and significant increase in the percentage of sperm abnormality (197.3%) (Fig. 10). However, simultaneous supplementation of CCl4 plus rutin significantly increased sperm count (28.4%) and sperm motility (56.1%), and decreased sperm abnormality (−45.2%) compared to rats treated with CCl4 alone.
Figure 10. Sperm parameters in male rats after administration of CCl4 and/or rutin.
(A) Sperm counts, (B) sperm motility and (C) sperm abnormality. Data are expressed as the mean ± SE of n = 5 rats/group. Bars with different letters show significant differences between the groups (p ≤ 0.05). Treatments: Control (vehicles); CCl4 (3 ml/kg b.w., by intraperitoneal route); rutin (70 mg/kg b.w., by gavage); both rutin + CCl4 co-treated group received CCl4 and rutin at the same doses twice a week for 4 weeks.
Discussion
CCl4 is known for its hepatotoxicity and nephrotoxicity. The degree of toxicity depends on the overproduction of reactive oxygen species (ROS), oxidative injury and the inflammatory process. CCl4 intoxication generates free radicals such as nitric oxide and peroxynitrite that trigger a cascade of events resulting in liver and kidney damage in rats (Khan & Zehra, 2013; Jan & Khan, 2016). Accordingly, we found significant elevation of serum ALP, AST, ALT, and bilirubin levels, while protein and albumin levels were significantly decreased in the CCl4-treated group. These results are indicators of hepatocyte dysfunction, cellular leakage and loss of functional integrity of the cell membrane in the liver, as previously reported (Khan, Khan & Sahreen, 2012). Rutin administration remarkably prevented the hepatic injury caused by CCl4. Rutin maintains liver enzyme homeostasis by acting as a membrane-stabilizing agent that inhibits leakage of enzymes due to its polyphenolic natural effects (Khan, Khan & Sahreen, 2012; Gelen et al., 2017). In addition, rutin enhances antioxidant gene expression and decreases hepatic DNA damage in the oxidative stress pathway initiated by CCl4 administration (Hafez et al., 2014). Recently, rutin treatment was proven to have hepatoprotective effects against STZ-induced type 1 diabetic conditions in mice (Li et al., 2018). Reddy et al. (2017) showed the superiority of rutin over silymarin in restoring the pathological alterations in paracetamol-induced hepatotoxicity in Wistar albino rats.
Administration of CCl4 causes nephrotoxicity as indicated by elevation in serum levels of urea, creatinine and uric acid. The high levels of creatinine and urea are indicators of severe damage to the structural integrity of nephrons. Coadministration of rutin significantly reduced these diagnostic markers. Several researchers have reported that rutin can modulate nephrotoxicity through its regulatory effect on apoptotic pathways including inhibition of several activation of caspases (Alhoshani et al., 2017; Khajevand-Khazaei et al., 2018). Radwan & Abdel Fattah (2017) indicated that the nephroprotective effect of rutin might be valuable in improving the therapeutic index of cisplatin.
Oxidative stress is a term that refers to altered cellular redox balance. Our results revealed a significant increase in the LPO marker (i.e., MDA) and a significant decrease in SOD, CAT and GPx enzyme activities in rat tissues following CCl4 application. This is in agreement with previous studies (Szymonik-Lesiuk et al., 2003; Alqasoumi & Abdel-Kader, 2012; Jan & Khan, 2016; Noureen et al., 2017). Treatment with rutin significantly decreased MDA and increased SOD, CAT and GPx activities. Rutin acts as a master redox switch through Nrf2 activation and iNOS suppression (Singh et al., 2018). The antioxidant activities of rutin have been found to be due to (1) its chemical structure, which can directly scavenge ROS (Hanasaki, Ogawa & Fukui, 1994); (2) its ability to increase the production of GSH and the cellular defense system (Shenbagam & Nalini, 2011); and (3) its inhibitory effect on xanthine oxidase, which is involved in generating ROS (Kostić et al., 2015).
The current results implied that CCl4 affected testicular spermatogenic function. We found a significant decrease in serum levels of testosterone, LH, and FSH compared with control values. LPO induced by CCl4 may affect the testis response to FSH and LH and subsequently reduce testosterone secretion (Rafiee et al., 2016). The oxidative stress of the testicular germinal layer is a central cause of the intrinsic apoptotic cascade, which can trigger mitochondrial ROS generation and provoke hypogonadism (Aitken et al., 2014). In addition, CCl4 reduced the secretion of testosterone, FSH and LH in rats through direct effects on the central nervous system and Leydig cells and/or indirectly by affecting the hypothalamic-pituitary estrogen receptor axis (Okolo, Siminialayi & Orisakwe, 2016; Noureen et al., 2017). CCl4 enhanced cellular degeneration by increasing the production of H2O2 and nitrite, which resulted in infertility/testicular dysfunction (Sahreen, Khan & Khan, 2013; Ojo et al., 2016). In turn, H2O2 might diffuse across the membrane and affect the sperm vital enzymes, thereby resulting in decreased sperm motility (Gautam et al., 2006; Khan & Ahmed, 2009; Sun et al., 2017). According to previous studies, antioxidants can help prevent and repair cell damage, so they may have an impact on the semen quality parameters and fertility potential (e.g., Dang et al., 2017). In this context, Aksu et al. (2017) showed that the antioxidant potential of rutin mitigated sperm damage, testicular degeneration and apoptosis induced by cisplatin treatment in adult male rats. Further research with rutin has shown the capacity to prevent cadmium-induced reproductive toxicity in rats, maintaining both antioxidants and testicular androgenic enzymes (Abarikwu, Iserhienrhien & Badejo, 2013).
For the lipid profile, the serum levels of total cholesterol, TG, LDL-C, and VLDL-C showed remarkable increases in CCl4-treated rats. In fact, high serum levels of cholesterol are associated with testicular dysfunction (Kalender et al., 2013) and decreased sperm quality (Lassoued et al., 2018). Diets with low cholesterol and high antioxidative agents can help to improve spermatogenesis and consequently promote male fertility (Khorrami et al., 2015). Previous studies have indicated that increased LDL plasma concentration is an independent risk factor for spermatogenesis (Chen et al., 2011). The oxidative conversion of pure LDL to oxidized LDL by free radicals is considered to be involved in cell injury and DNA damage, especially in tissues with a high rate of cell division, such as testis, epididymis and seminal vesicles (Kosola et al., 2013). Furthermore, experimental studies have found that longstanding hyperlipidemia leads to increased lipid oxidation and progression of liver and kidney pathologies (Gyebi, Soltani & Reisin, 2012; Gou et al., 2016). Herein, we found that rutin treatment alleviated the severity of hyperlipidemia in CCl4-treated rats. Rutin has hypolipidemic action due to its ability to inhibit lipogenesis and activate the metabolism of fatty acids (Liu et al., 2017). It was suggested that supplementation of quercetin, one of the metabolites of rutin, promotes an increase in faecal sterols, which in turn leads to a decreased absorption of dietary cholesterol as well as lowered plasma and hepatic cholesterol (Bok et al., 2002).
Conclusions
In the present work, it appears that the administration of rutin contributes to its protective effect against CCl4-induced hepatorenal injuries and testicular/fertility disturbances not only through recovery of ROS-mediated oxidative stress but also through marked hypolipidemic activity. Therefore, in the future, oral rutin intake as an adjunct natural therapy may be advisable for male subjects to protect against liver/kidney failure-mediated inhibitory effects on their reproductive function.
Supplemental Information
Funding Statement
This work was financially supported by the Deanship of Scientific Research at King Faisal University (Saudi Arabia) under grant no. 17122008. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Hany Elsawy and Azza Sedky conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Gehan M. Badr conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Basem M. Abdallah analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Abdullah M. Alzahrani analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Ashraf M. Abdel-Moneim analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All experimental procedures were reviewed and approved by the research ethics committee at King Faisal University (Ref. No. KFU-REC/2019-03-01).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File and were used for statistical analysis.
References
- Abarikwu, Iserhienrhien & Badejo (2013).Abarikwu SO, Iserhienrhien BO, Badejo TA. Rutin- and selenium-attenuated cadmium-induced testicular pathophysiology in rats. Human and Experimental Toxicology. 2013;32(4):395–406. doi: 10.1177/0960327112472995. [DOI] [PubMed] [Google Scholar]
- Abdel-Kader et al. (2018).Abdel-Kader MS, Abulhamd AT, Hamad AM, Alanazi AH, Ali R, Alqasoumi SI. Evaluation of the hepatoprotective effect of combination between hinokiflavone and Glycyrrhizin against CCl4 induced toxicity in rats. Saudi Pharmaceutical Journal. 2018;26(4):496–503. doi: 10.1016/j.jsps.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim et al. (2015).Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLOS ONE. 2015;10(12):e0144509. doi: 10.1371/journal.pone.0144509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken et al. (2014).Aitken R, Smith T, Jobling M, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian Journal of Andrology. 2014;16(1):31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu et al. (2017).Aksu EH, Kandemir FM, Özkaraca M, Omur AD, Kucukler S, Comakli S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49(1):e12593. doi: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- Alhoshani et al. (2017).Alhoshani AR, Hafez MM, Husain S, Al-Sheikh AM, Alotaibi MR, Al Rejaie SS, Alshammari MA, Almutairi MM, Al-Shabanah OA. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrology. 2017;18(1):194. doi: 10.1186/s12882-017-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqasoumi & Abdel-Kader (2012).Alqasoumi SI, Abdel-Kader MS. Terpenoids from Juniperus procera with hepatoprotective activity. Pakistan Journal of Pharmaceutical Sciences. 2012;25(2):315–322. [PubMed] [Google Scholar]
- Alshammari, Balakrishnan & Chinnasamy (2017).Alshammari GM, Balakrishnan A, Chinnasamy T. 2-Hydroxy-4-methoxy benzoic acid attenuates the carbon tetra chloride-induced hepatotoxicity and its lipid abnormalities in rats via anti-inflammatory and antioxidant mechanism. Inflammation Research. 2017;66(9):753–763. doi: 10.1007/s00011-017-1054-2. [DOI] [PubMed] [Google Scholar]
- Bok et al. (2002).Bok SH, Park SY, Yong BP, Lee MK, Jeon SM, Jeong TS, Choi MS. Quercetin dihydrate and gallate supplements lower plasma and hepatic lipids and change activities of hepatic antioxidant enzymes in high cholesterol-fed rats. International Journal for Vitamin and Nutrition Research. 2002;72(3):161–169. doi: 10.1024/0300-9831.72.3.161. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2011).Chen MM, Lan XX, Li CY, Tian ZM, Chen KF. Diet-induced obesity increases the apoptosis of testicular spermatogenic cells in pubertal male rats. Zhonghua Nan Ke Xue. 2011;17(4):342–347. [PubMed] [Google Scholar]
- Dang et al. (2017).Dang Y, Li Z, Luo B, Pan L, Wei Q, Zhang Y. Protective effects of apigenin against acrylonitrile-induced subchronic sperm injury in rats. Food and Chemical Toxicology. 2017;109(Pt 1):517–525. doi: 10.1016/j.fct.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Diab et al. (2018).Diab KA, Fahmy MA, Hassan ZM, Hassan EM, Salama AB, Omara EA. Genotoxicity of carbon tetrachloride and the protective role of essential oil of Salvia officinalis L. in mice using chromosomal aberration, micronuclei formation, and comet assay. Environmental Science and Pollution Research. 2018;25(2):1621–1636. doi: 10.1007/s11356-017-0601-2. [DOI] [PubMed] [Google Scholar]
- Erdogan et al. (2015).Erdogan E, Ilgaz Y, Gurgor PN, Oztas Y, Topal T, Oztas E. Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cirurgica Brasileira. 2015;30(11):778–784. doi: 10.1590/s0102-865020150110000009. [DOI] [PubMed] [Google Scholar]
- Evans & Maxwell (1987).Evans G, Maxwell WMC. Salamon’s artificial insemination of sheep and goat. In: Maxwell WMC, editor. Handling and examination semen. Butterworths; Sydney: 1987. pp. 93–106. [Google Scholar]
- Frei & Higdon (2003).Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Journal of Nutrition. 2003;133(10):3275S–284S. doi: 10.3945/ajcn.113.060186. [DOI] [PubMed] [Google Scholar]
- Freud & Carol (1964).Freud M, Carol B. Factors affecting haemocytometer count of sperm concentration in human semen. Journal of Reproduction and Fertility. 1964;8:149–155. doi: 10.1530/jrf.0.0080149. [DOI] [PubMed] [Google Scholar]
- Friedewald, Levy & Fredrickson (1972).Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. doi: 10.1177/107424840501000106. [DOI] [PubMed] [Google Scholar]
- Ganeshpurkar & Saluja (2017).Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharmaceutical Journal. 2017;25(2):149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam et al. (2006).Gautam DK, Misro MM, Chaki SP, Sehgal N. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis. 2006;11(1):39–46. doi: 10.1007/s10495-005-3087-1. [DOI] [PubMed] [Google Scholar]
- Gelen et al. (2017).Gelen V, Şengül E, Gedikli S, Atila G, Uslu H, Makav M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pacific Journal of Tropical Biomedicine. 2017;7(7):647–653. doi: 10.1016/j.apjtb.2017.06.013. [DOI] [Google Scholar]
- Ghorbani (2017).Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomedicine & Pharmacotherapy. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Gou et al. (2016).Gou SH, Huang HF, Chen XY, Liu J, He M, Ma YY, Zhao XN, Zhang Y, Ni JM. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. Journal of the Chinese Medical Association. 2016;79(3):111–121. doi: 10.1016/j.jcma.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Gyebi, Soltani & Reisin (2012).Gyebi L, Soltani Z, Reisin E. Lipid nephrotoxicity: new concept for an old disease. Current Hypertension Reports. 2012;14(2):177–181. doi: 10.1007/s11906-012-0250-2. [DOI] [PubMed] [Google Scholar]
- Hafez et al. (2014).Hafez MM, Al-Shabanah OA, Al-Harbi NO, Al-Harbi MM, Al-Rejaie SS, Alsurayea SM, Sayed-Ahmed MM. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxidative Medicine and Cellular Longevity. 2014;2014 doi: 10.1155/2014/893212. Article 893212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanasaki, Ogawa & Fukui (1994).Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radical Biology Medicine. 1994;16(6):845–850. doi: 10.1016/0891-5849(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Hefnawy & Ramadan (2013).Hefnawy HTM, Ramadan MF. Protective effects of Lactuca sativa ethanolic extract on carbon tetrachloride induced oxidative damage in rats. Asian Pacific Journal of Tropical Disease. 2013;3(4):277–285. doi: 10.1016/S2222-1808(13)60070-5. [DOI] [Google Scholar]
- Hosseinzadeh & Nassiri-Asl (2014).Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. Journal Endocrinological Investigation. 2014;37(9):783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- Jan & Khan (2016).Jan S, Khan MR. Protective effects of Monotheca buxifolia fruit on renal toxicity induced by CCl4 in rats. BMC Complementary and Alternative Medicine. 2016;16(1):289. doi: 10.1186/s12906-016-1256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender et al. (2013).Kalender S, Uzun FG, Demir F, Uzunhisarcikli M, Aslanturk A. Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E. Food and Chemical Toxicology. 2013;55:456–462. doi: 10.1016/j.fct.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Khajevand-Khazaei et al. (2018).Khajevand-Khazaei MR, Mohseni-Moghaddam P, Hosseini M, Gholami L, Baluchnejadmojarad T, Roghani M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. European Journal of Pharmacology. 2018;833:307–313. doi: 10.1016/j.ejphar.2018.06.019. [DOI] [PubMed] [Google Scholar]
- Khan & Ahmed (2009).Khan MR, Ahmed D. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food and Chemical Toxicology. 2009;47(6):1393–1399. doi: 10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Khan & Zehra (2013).Khan MR, Zehra H. Amelioration of CCl4-induced nephrotoxicity by Oxalis corniculata in rat. Experimental and Toxicologic Pathology. 2013;65(3):327–334. doi: 10.1016/j.etp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Khan, Khan & Sahreen (2012).Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complementary and Alternative Medicine. 2012;12:178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorrami et al. (2015).Khorrami A, Ghanbarzadeh S, Ziaee M, Arami S, Vajdi R, Garjani A. Dietary cholesterol and oxidised cholesterol: effects on sperm characteristics, antioxidant status and hormonal profile in rats. Andrologia. 2015;47(3):310–317. doi: 10.1111/and.12262. [DOI] [PubMed] [Google Scholar]
- Kosola et al. (2013).Kosola J, Vaara JP, Ahotupa M, Kyröläinen H, Santtila M, Oksala N, Atalay M, Vasankari T. Elevated concentration of oxidized LDL together with poor cardiorespiratory and abdominal muscle fitness predicts metabolic syndrome in young men. Metabolism: Clinical and Experimental. 2013;62(7):992–999. doi: 10.1016/j.metabol.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Kostić et al. (2015).Kostić DA, Dimitrijević DS, Stojanović GS, Palić IR, Dordević AS, Ickovski JD. Xanthine oxidase: isolation, assays of activity, and inhibition. Journal of Chemistry. 2015;2015 doi: 10.1155/2015/294858. Article 294858. [DOI] [Google Scholar]
- Lassoued et al. (2018).Lassoued I, Mezghani M, Jridi M, Rahmouni F, Jamoussi K, Rebai T, El Feki A, Nasri M, Barkia A. Protective effects of thornback ray muscle protein hydrolysate against dyslipidemia, oxidative stress and reduced fertility induced by high cholesterol diet in adult male rats. RSC Advances. 2018;8:22303–22312. doi: 10.1039/c8ra00657a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018).Li CW, Li Q, Guo L, Wu AP, Hu L, Bai J. Effect of rutin on liver function and morphology in type 1 diabetes mice induced by streptozotocin. Journal of Sichuan University (Medical Science Edition) 2018;49:384–387. [PubMed] [Google Scholar]
- Liu et al. (2017).Liu Q, Pan R, Ding L, Zhang F, Hu L, Ding B, Zhu L, Xia Y, Dou X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. International Immunopharmacology. 2017;49:132–141. doi: 10.1016/j.intimp.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Lowry et al. (1951).Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193(1):265–275. doi: 10.1016/0304-3894(92)87011-4. [DOI] [PubMed] [Google Scholar]
- Mahmoud et al. (2019).Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Medicine and Cellular Longevity. 2019;2019 doi: 10.1155/2019/5484138. Article 5484138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manibusan, Odin & Eastmond (2007).Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews. 2007;25(3):185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- Marimuthu et al. (2013).Marimuthu S, Adluri RS, Rajagopalan R, Menon VP. Protective role of ferulic acid on carbon tetrachloride-induced hyperlipidemia and histological alterations in experimental rats. Journal Basic and Clinical Physiology and Pharmacology. 2013;24(1):59–66. doi: 10.1515/jbcpp-2012-0053. [DOI] [PubMed] [Google Scholar]
- Mehfooz et al. (2018).Mehfooz A, Wei Q, Zheng K, Fadlalla MB, Maltasic G, Shi F. Protective roles of Rutin against restraint stress on spermatogenesis in testes of adult mice. Tissue and Cell. 2018;50:133–143. doi: 10.1016/j.tice.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Morrissey et al. (1988).Morrissey RE, Schwetz BA, Lamb JC, Ross MD, Teague JL, Morris RW. Evaluation of rodent sperm, vaginal cytology, and reproductive organ weight data from national toxicology program 13-week studies. Toxicological Sciences. 1988;11(2):343–358. doi: 10.1093/toxsci/11.1.343. [DOI] [PubMed] [Google Scholar]
- Noureen et al. (2017).Noureen F, Khan MR, Shah NA, Khan RA, Naz K, Sattar S. Pistacia chinensis: strong antioxidant and potent testicular toxicity amelioration agent. Asian Pacific Journal of Tropical Medicine. 2017;10(4):380–389. doi: 10.1016/j.apjtm.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Ojo et al. (2016).Ojo OA, Ojo AB, Ajiboye B, Fadaka A, Imiere OD, Adeyonu O, Olayide I. Protective influence of Ficus asperifolia Miq leaf extract on carbon tetrachloride (CCl4)-induced testicular toxicity in rat’s testes. Journal of Applied Pharmaceutical Sciences. 2016;6(6):037–041. doi: 10.7324/JAPS.2016.60607. [DOI] [Google Scholar]
- Okolo, Siminialayi & Orisakwe (2016).Okolo KO, Siminialayi IM, Orisakwe OE. Protective effects of Pleurotus tuber-regium on carbon-tetrachloride induced testicular injury in Sprague Dawley rats. Frontiers in Pharmacology. 2016;7 doi: 10.3389/fphar.2016.00480. Article 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk et al. (2003).Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology. 2003;62(2):353–356. doi: 10.1016/S0090-4295(03)00255-3. [DOI] [PubMed] [Google Scholar]
- Preethi & Kuttan (2009).Preethi KC, Kuttan R. Hepato and reno protective action of Calendula officinalis L. Flower extract. Indian Journal of Experimental Biology. 2009;47(3):163–168. [PubMed] [Google Scholar]
- Radwan & Abdel Fattah (2017).Radwan RR, Abdel Fattah SM. Mechanisms involved in the possible nephroprotective effect of rutin and low dose γ irradiation against cisplatin-induced nephropathy in rats. Journal of Photochemistry and Photobiology. B, Biology. 2017;169:56–62. doi: 10.1016/j.jphotobiol.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Rafiee et al. (2016).Rafiee F, Nejati V, Heidari R, Ashraf H. Protective effect of methanolic extract of Berberis integerrima Bunge. root on carbon tetrachloride-induced testicular injury in Wistar rats. International Journal of Reproductive Biomedicine. 2016;14(2):133–140. doi: 10.29252/ijrm.14.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman et al. (2017).Rahman MM, Muse AY, Khan DMIO, Ahmed IH, Subhan N, Reza HM, Alam MA, Nahar L, Sarker SD. Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomedicine and Pharmacotherapy. 2017;92:421–428. doi: 10.1016/j.biopha.2017.05.101. [DOI] [PubMed] [Google Scholar]
- Reddy et al. (2017).Reddy MK, Reddy AG, Kumar BK, Madhuri D, Boobalan G, Reddy MA. Protective effect of rutin in comparison to silymarin against induced hepatotoxicity in rats. Veterinary World. 2017;10(1):74–80. doi: 10.14202/vetworld.2017.74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahreen, Khan & Khan (2013).Sahreen S, Khan MR, Khan RA. Ameliorating effect of various fractions of Rumex hastatus roots against hepato- and testicular toxicity caused by CCl4. Oxidative Medicine and Cellular Longevity. 2013;2013 doi: 10.1016/j.spsy.2013.12.009. Article 325406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenbagam & Nalini (2011).Shenbagam M, Nalini N. Dose response effect of rutin a dietary antioxidant on alcohol-induced prooxidant and antioxidant imbalance—a histopathologic study. Fundamental and Clinical Pharmacology. 2011;25(4):493–502. doi: 10.1111/j.1472-8206.2010.00861. [DOI] [PubMed] [Google Scholar]
- Singh et al. (2018).Singh S, Dubey V, Meena A, Siddiqui L, Maurya AK, Luqman S. Rutin restricts hydrogen peroxide-induced alterations by up-regulating the redox-system: an in vitro and in vivo and in silico study. European Journal of Pharmacology. 2018;835:115–125. doi: 10.1016/j.ejphar.2018.07.055. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2017).Sun J, Wang H, Liu B, Shi W, Shi J, Zhang Z, Xing J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomedicine and Pharmacotherapy. 2017;88:500–506. doi: 10.1016/j.biopha.2017.01.066. [DOI] [PubMed] [Google Scholar]
- Szymonik-Lesiuk et al. (2003).Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Słomka M, Ma̧dro A, Celiński K, Wielosz M. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. Journal of Hepato-Biliary-Pancreatic Surgery. 2003;10(4):309–315. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File and were used for statistical analysis.