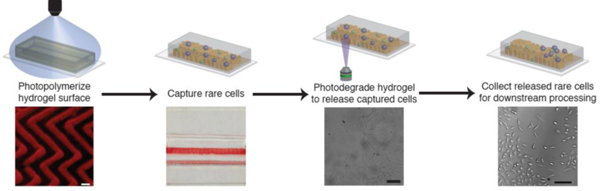
Abstract
Circulating tumor cells (CTCs) play a central role in cancer metastasis and represent a rich source of data for cancer prognostics and therapeutic guidance. Reliable CTC recovery from whole blood therefore promises a less invasive and more sensitive approach to cancer diagnosis and progression tracking. CTCs, however, are exceedingly rare in whole blood, making their quantitative recovery challenging. Several techniques capable of isolating these rare cells have been introduced and validated, yet most suffer from low CTC purity or viability, both of which are essential to develop a clinically viable CTC isolation platform. To address these limitations, we introduce a patterned, immunofunctional, photodegradable poly(ethylene glycol) (PEG) hydrogel capture surface for the isolation and selective release of rare cell populations. Flat and herringbone capture surfaces were successfully patterned via PDMS micromolding and photopolymerization of photolabile PEG hydrogels. Patterned herringbone surfaces, designed to convectively transport cells to the capture surface, exhibited improved capture density relative to flat surfaces for target cell capture from buffer, buffy coat, and whole blood. Uniquely, captured cells were released for collection by degrading the hydrogel capture surface with either bulk or targeted irradiation with cytocompatible doses of long wavelength UV light. Recovered cells remained viable following capture and release and exhibited similar growth rates as untreated control cells. The implementation of molded photodegradable PEG hydrogels as a CTC capture surface provides a micropatternable, cytocompatible platform that imparts the unique ability to recover pure, viable CTC samples by selectively releasing target cells.
Keywords: circulating tumor cell, microfluidics, biomaterials, immunocapture
Graphical abstract
1. Introduction
Rare cells circulating in the bloodstream are a potentially rich source of information that can inform disease characterization, diagnosis, and prognosis; yet, the isolation of these cells in a viable state remains a daunting technological challenge. Circulating tumor cells (CTCs) are a rare cell class of particularly widespread interest. CTCs are cancerous cells that have been shed from the primary tumor and intravasated into the bloodstream and are believed to be vectors and markers of metastasis.[1,2] Several studies have investigated the utility of CTCs as markers of disease progression in patients with various cancers including breast, non-small lung, and prostate cancer.[3–5] These studies revealed correlations between the number of CTCs found in a patient’s blood and the mean progression-free survival and overall survival, demonstrating the potential for these CTCs to be used as a prognostic indicator.
However, the isolation of CTCs has proven difficult due to their extraordinarily low abundance in whole blood, approximately one CTC per million to ten million leukocytes.[6] The challenge of efficient CTC isolation has been further exacerbated by technical limitations that affect sample throughput and captured cell purity and viability. A platform that addresses these issues may enable downstream cellular analysis that would exploit the potential of CTCs as a diagnostic or prognostic marker. Enhanced techniques for the isolation of these cells promises to help elucidate mechanisms of metastasis and enable improved genotyping and patient-specific drug screening, eventually leading to improved liquid biopsies for personalized therapies.
CTC isolation technologies have improved significantly following the development of microfluidic sample handling and processing technologies.[7] Of particular interest are microfluidic platforms that exploit unique or over-expressed cell surface antigens to create specific binding interactions between capture surfaces and target cells. Methods have been developed for affinity capture that utilize antibodies or aptamers to capture CTCs.[8–11] The most commonly used affinity marker for the capture of CTCs is anti-epithelial cell adhesion molecule (EpCAM) because many cancer cells have been found to over-express the EpCAM membrane protein.[12] In addition to the use of affinity interactions, the flow profile within microfluidic devices can be engineered to enhance the number of target cell-surface binding events and the purity of the captured population. By controlling the shear stress applied to the target cells within a microfluidic device improved capture efficiency and purity flow regimes can be achieved.[13–15] Building upon these results, recent studies have combined affinity and microfluidic-based capture technologies to improve the efficiency of CTC capture.
Physical interactions between target cells and the capture surface in microfluidic immunocapture devices are essential and have inspired the development of flow enhancements that increase the number of cell-surface interactions, and therefore the probability of successfully capturing specific cells. Post arrays within microfluidic devices have been designed to disrupt blood flow and to create size dependent flow trajectories toward improving the capture efficiency and purity of CTCs by minimizing the capture of smaller cells (i.e., red and white blood cells).[8,16–18] In a conceptually similar microfluidic device design, herringbone (HB) patterned roof topologies have been utilized to induce mircovortices within the flow profile, thus increasing the probability of cell-surface interactions.[19] The HB design has been optimized to enhance lateral recirculation within microfluidic devices and the HB roof topology has been applied over several different surfaces including polymer and nanowire coatings.[20–22] Additionally, recent work has highlighted the advantage of combining these techniques where post arrays were utilized to enrich CTCs and a HB patterned surface was used to enhance the subsequent capture of CTCs on an anti-EpCAM coated surface.[23] These platforms have improved the efficiency and throughput of target cell capture from complex media and emphasize the value of incorporating nanostructures within microfluidic devices for enhanced CTC capture.[24]
While several successful CTC capture platforms have been designed, the challenge of releasing captured cells in a viable state from the capture surface to facilitate in vitro CTC culture and downstream analysis that cannot be performed in situ with fixed or lysed cells still remains. Releasing cells from the capture surface requires addressing a new set of technological challenges to ensure cell viability, physiology, and purity are maintained off-chip. Numerous techniques have been proposed to release captured cells for downstream processing including enzymatic release (i.e., trypsin digestion[9,25], lysate induced surface degradation[26–28], and cleavage of anti-EpCAM surface linkages[29]), fluidic shear stress release (i.e., applying force beyond the collective antibody-antigen binding force)[10,30,31], and microenvironment changes (i.e., temperature[20,32] or pH[33] responsive surfaces). While efficient, these release strategies, especially enzymatic or shear stress release, can harm the cell membrane damaging the integrity of the released cells. Additionally, outside of applying localized shear stress from a frequency controlled microtip, these approaches largely represent bulk processing techniques that do not allow for targeted detachment of single cells which can be utilized to improve downstream cell purity.[20]
Light sensitive materials provide an alternative, cytocompatible capture and release strategy that enable spatiotemporal selectivity over targeted cells. Such materials incorporate a photocleavable linker, commonly an o-nitrobenzyl group, into their capture design.[34] This light responsive motif has led to the development of two different release mechanisms. One method employed a functionalized polydimethylsiloxane (PDMS) HB patterned surface with photocleavable ssDNA-conjugated anti-EpCAM from which captured cells could be released by surface irradiation with long wavelength UV light.[35] A limitation of this system is that the photocleavable molecule is small and forms a vanishingly thin barrier between the capture molecule and the surface often leading to secondary attachment and therefore incomplete release from the surface upon irradiation. The second method created photodegradable hydrogels with antibody spots to separate CD4+ from CD8+ T-cell populations following cell capture and subsequent targeted light irradiation.[36] This method demonstrated the ability to obtain pure cell populations by selective photodegradation of the hydrogel surface; however, it relied on complicated printing of antibody arrays to capture separate populations. Overall, light is a useful handle for regulating material properties in the presence of cells, and materials can be designed to respond to low doses of light appropriate for maintaining cell viability and function.[37–39] Further, the degradation and release can be achieved with single cell or even sub-cellular resolution at specific locations and at the desired time.[40] We hypothesized that the introduction of a HB pattern onto a photocleavable hydrogel surface within a microfluidic device would allow for efficient and robust capture and release of rare cell populations within complex media, including whole blood.
Here, we have created a patterned, antibody-conjugated, photopolymerizable and photodegradable poly(ethylene glycol) (PEG) hydrogel cell capture surface that combines the advantages of immunoaffinity capture with reliable, cytocompatible, and on-demand cell release. Our method utilizes a photodegradable PEG diacrylate that has been used in tissue engineering and drug delivery applications and shown to have minimal deleterious effects on cells or biologics.[41] Using this monomer, we developed a strategy for producing photodegradable hydrogel films polymerized using visible light and molded with a three dimensional (3D) HB microstructure. These responsive surfaces were utilized for the selective capture and release of model CTCs from complex media under continuous flow. After successful capture, target cells were released from the capture surface by irradiation with long wavelength UV light imparting exquisite spatiotemporal control over the hydrogel properties. Using light as the release modality allows either bulk or individually targeted release of cells from the surface. The approach established here to utilize light for both patterning hydrogel cell capture surfaces and degrading the hydrogel surface for on-demand cell release may prove to be broadly useful in developing a platform to isolate rare cell types.
2. Materials and Methods
2.1. Photodegradable PEG Synthesis
PEG-bis-amine monomer (Mn ~ 3,400 g/mol, Laysan Bio) was modified with an o-nitrobenzyl acrylate moiety using a previously described procedure.[38] Briefly, the small molecule photodegradable o-nitrobenzyl monomer 4-(4-(1-(acryloyloxy)ethyl)-2-methoxy-5-nitrophenoxy)butanoic acid was synthesized through a multistep reaction starting with acetovanillone. This o-nitrobenzyl monomer (2.2 eq per amine on PEG) subsequently was coupled to amine-end functionalized PEG (1 eq) with the activating agent 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU, 2.2 eq per amine on PEG) in the presence of N,N-diisopropylethylamine (DIPEA, 4 eq per amine on PEG) in dimethylformamide (DMF). The resulting functionalized polymer was obtained by precipitation in ethyl ether, centrifugation, and removal of residual solvent under reduced pressure. The polymer was purified further by dialysis against DI water (MWCO 1000 g/mol) and then lyophilized to obtain an orange solid. The photolabile polymer product, PEGdiPDA, was characterized by 1H NMR spectroscopy (Bruker Daltonics, Billerica, MA, 600 Hz, 128 scans using deuterated dimethyl sulfoxide as a solvent) using protons associated with amide appearing at 7.91 ppm and protons associated with acrylate at 6.35 ppm relative to PEG backbone at 3.5 ppm.
2.2. LAP Synthesis
The photoinitiator lithium phenyl-2,4,6-trimethylbenzolphosphinate (LAP) was synthesized based on a published procedure.[42,43] Briefly, 3.2 g (18 mmol) of 2,4,6-trimethylbenzoyl chloride was added slowly to 3.0 g (18 mmol, 1 eq.) of dimethyl phenylphosphonite under stirring at 22 °C and under argon. The mixture was reacted for 18 h after which 6.1 g (72 mmol, 4 eq.) of lithium bromide in 100 mL of 2-butanone was added to the mixture. The solution was then heated to 50 °C. After approximately 10 min, a solid precipitate formed and the reaction was cooled to 22 °C for 4 h and filtered. The filtrate was rinsed 3 times with 100 mL of 2-butanone to remove unreacted lithium bromide and excess solvent was removed under vacuum. The final product was characterized by 1H NMR spectroscopy (Bruker Daltonics, Billerica, MA, 600 Hz, 16 scans using deuterated water).
2.3. Microfluidic Device Fabrication
Poly(dimethylsiloxane) (PDMS) microchannels were formed using conventional soft lithography techniques as previously describe.[44] Briefly, Sylgard 184 (Dow Corning) part A (viscous base) and part B (curing agent) were mixed by hand in a 1:10 weight ratio. The mixed solution was poured over a patterned photoresist-on-silicon master, degassed under vacuum, and placed in a 70 °C oven for 12 h to cure. The PDMS mold was removed from the silicon master, trimmed to the desired size, and inlet and outlet orifices were punched with a sharpened 20G blunt syringe tip. If bonding of the PDMS mold was desired, then the prepared PDMS channel and a clean glass slide were placed in a plasma chamber (Harrick Plasma Cleaner model PDC-001). The pressure of the chamber was brought to 500 mTorr of pure oxygen and a plasma was struck using the medium power setting (10.2 W) for 90 sec. After plasma exposure, the PDMS device was carefully pressed onto the glass surface to form an irreversible covalent bond. This bond was fully established by incubation of the device in a 70 °C oven for a minimum of 5 min. Bonded devices were cooled to 22 °C before use.
2.4. Fabrication of Acrylate Modified Glass Surfaces
To ensure that the PEG hydrogel capture surfaces remained attached to the glass, slides were first modified to present acrylate groups via a silane coupling chemistry. Glass surfaces were flamed and placed in a solution containing 190 proof ethanol (Sigma Aldrich) and 3-(Acryloyloxy) propyltrimethoxysilane (APTS, Alfa Aesar). The glass slides were removed after 5 minutes, rinsed with 190 proof ethanol, and placed in an oven to dry (T = 70°C). PDMS channels were placed in contact with acrylate-modified glass prior to hydrogel molding, where natural adhesion forces formed a reversible conformal seal.
2.5. Hydrogel Formation
The hydrogel-forming polymer solution was prepared by combining PEGdiPDA (8.2 wt%), PEG monoacrylate (PEGMA, MW= 375 Da, 5 wt%), LAP (3 wt%), and acryl-biotin (acrylate PEG biotin 5000 Da, Nanocs Inc., 0.9 mg/mL) in PBS. The hydrogel-forming solution was flowed into prepared PDMS devices and photopolymerized by irradiation of the surface with collimated light (Omnicure 2000, Lumen Dynamics) passed through a 405 nm long-pass filter (Semrock) for 150 s (I0 = 10 mW cm−2). By initiating the polymerization with a wavelength of light distinct from that of the major o-nitrobenzyl absorbance peak, PEGdiPDA hydrogels could be fabricated with minimal cleavage of the o-nitrobenzyl groups allowing for subsequent, on-demand photodegradation with 365 nm light.[45] The hydrogel was flushed with PBS following polymerization and refrigerated in PBS until use. Hydrogels were used within 48 hours of formation.
Two hydrogel surface geometries were created: 1.) long, flat and 2.) HB patterned surfaces. To create long, flat capture surfaces the PDMS mold (5 cm long, 4 mm wide, and 50 µm deep) was bonded to a glass slide and the hydrogel forming solution polymerized within it. Oxygen inhibition at the PDMS-hydrogel forming solution interface was exploited to ensure that the hydrogel did not polymerize to the top of the PDMS channel, thus leaving a significant gap to accommodate fluid flow. A different fabrication motif was used for HB-patterned hydrogels, in order to provide a uniform flow conduit with a flat boundary above the capture surface. A plasma-cleaned HB PDMS mold (2.1 cm long, 2.3 mm wide, and 60 µm in depth) was soaked in water for 10 minutes then reversibly attached to an acrylate modified glass slide by surface energy. The channel was filled with the hydrogel forming solution, polymerized, and the mold carefully removed, leaving behind the patterned hydrogel capture pad. HB features were designed to be approximately 60 µm in height, supported by a 10 µm film.
A straight microchannel (2 cm long, 2.3 mm wide, and 55 µm deep) was bonded over the top of the HB hydrogel surface. A carbon black PDMS channel was formed as described above, but with carbon black powder mixed into the uncured PDMS before patterning. The carbon black PDMS channel was placed over the hydrogel surface before being placed in the plasma chamber to protect the surface from both the oxygen plasma and the UV radiation emitted by the plasma. The bonded device was maintained at 22 °C for 10 min before being rehydrated and placed in a 70 °C oven for 5 mins. Once bonding was complete the hydrogel was flushed with PBS and refrigerated until use.
Fluorescent confocal microscopy was used to characterize the height of hydrogel surfaces. The hydrogel-forming monomer solution was modified for imaging purposes to contain 1% by volume acryl-Rhodamine in place of the acryl-biotin. Confocal microscopy (Zeiss 710) Z-plane imaging was utilized to determine the hydrated hydrogel height. Z-plane image stacks were reconstructed into 3D images of the surface using ImageJ ‘Volume Viewer’.
2.6. Surface Functionalization
Surface functionalization of hydrogel films was achieved by avidin-biotin coupling chemistry. Channel surfaces were blocked with 1% BSA for 30 min followed by functionalization with NeutrAvidin (NA, 50 µg/mL in 1% BSA) for 45 min. Channels were subsequently rinsed with PBS before being incubated with biotinylated anti-EpCAM (20 µg/mL in 1% BSA) for 45 min. The channels were flushed with PBS and stored at 4 °C. All biomolecules were added to the hydrogel surface at a flow rate of 20 µL min−1 (NeMESYS syringe pump) and used the same day as functionalization.
2.7. Cell Handling
Human lung carcinoma cells (A549 cells) were cultured in 75 mL tissue culture flasks with Dulbecco’s modified eagle medium (DMEM) supplemented with 1% fetal bovine serum (FBS) and streptomycin/fungizone. Cells were passaged when 80% confluence was reached by addition of trypsin. For cell capture experiments, cells were removed from the flask surface using trypsin, dyed with CMTPX celltracker (Life Technologies) following manufacture specifications, and suspended in the desired media.
2.8. Cell Line Capture from Buffer
Fluid flow was introduced to devices via Tygon microbore tubing (ID = 0.010”, OD = 0.030”) attached to a 30G blunt syringe tip, connected to a 3 mL plastic syringe (BD). To limit cell sedimentation within the syringe a magnetic stir bar was placed in the syringe and the cell suspension was gently and continuously agitated. The prepared syringes were mounted in NeMESYS syringe pumps and NeMESYS software was used to control flow conditions. For flow experiments, the syringe pump was set horizontally, and short tubing was used to avoid cell sedimentation within the tubing. 90 µL of cell suspension was flowed through the device at a rate of 3 µL min−1. The surface was subsequently rinsed with 100 µL PBS at 10 µL min−1 followed by imaging to determine the number of cells attached to the surface.[46]
Initial capture efficiency experiments were performed with CMTPX celltracker (Life Technologies) dyed A549 cells suspended in PBS buffer at 5 × 104 cells mL−1. The gel surface was imaged under a 4x objective using a TXRED microscope filter cube to illuminate cells. Cells were counted using the ‘Analyze Particles’ feature on ImageJ (NIH). The capture efficiency was defined as the number of cells captured divided by the total number of cells passed over the surface. Capture density, the number of cells captured per square millimeter of surface area, was also calculated. The surface area for both geometries was calculated by assuming a rectangular geometry of the surface. A total number of three surfaces were analyzed for both the flat and HB surface geometries.
2.9. Cancer Cell Line Capture from Complex Solution
Blood samples were collected from healthy donors according to IRB protocol. Blood samples were stored in EDTA-coated tubes and used within 24 hours of collection to ensure minimal coagulation of the sample. The ability to capture A549 cells from buffy coat and whole blood was investigated. The buffy coat was collected from a whole blood sample using previously described methods. Briefly, a whole blood sample was mixed with PBS containing 2% FBS in a 1:1 ratio. The mixed sample was centrifuged (200 g) at 22 °C for 10 min. After centrifugation the buffy coat was carefully removed from the other blood components using a pipette. The collected buffy coat was mixed with A549 cells in a 3:1 ratio with final cell concentrations of 9 × 104 WBCs mL−1 and 3 × 104 A549 cells mL−1. The purity, defined as the number of target cells captured divided by the total number of cells captured, was determined along with the capture efficiency of both A549 cells and WBCs. A total number of three surfaces were analyzed for both the flat and HB surface geometries. Further characterization was performed by suspending prepared A549 cells in a whole blood sample at 105 cells mL−1. Once the artificial CTC sample was prepared, the sample was immediately flowed over the two surface geometries following the procedure outlined above (Section 2.8). Capture experiments with both the flat and HB surface geometries were run in triplicate.
2.10. Cell Release and Viability
Cells were released from hydrogel surfaces by either bulk or targeted light exposure. Throughout the release procedure, PBS was flowed over the surface at 10 µL min−1 to collect released cells. The entire capture surface was degraded by flood exposure to long wavelength UV light (365 nm at 10 mW cm−2) for 90 s. Individual release was accomplished by closing the field aperture of an inverted microscope (Olympus IX81) and focusing the long wavelength UV light (365 nm at 6.97 mW cm−2) on the region of the gel to be degraded through a 20x microscope objective (Olympus, NA = 0.45) and irradiating for 210 s.
The viability of cells after capture from buffer and after release was determined using a live/dead viability assay performed using manufacturer’s guidelines (Life Technologies). Additionally, cells captured and released from the surface were seeded into a 96 well plate at approximately 350 cells mL−1. Cell attachment, proliferation, and growth was monitored until 80% confluence was reached. A control cell population, cells not captured or released from the device, was also seeded into a 96-well plate at 350 cells mL−1 and monitored. The time to small colony formation, large colony formation, and 80% confluence was recorded for the two cell populations.
2.11. Statistical Analysis
All values presented in the paper are expressed as sample mean plus or minus the sample standard deviation. All data was presented for three replicates. Equal variance was determined using a F-test. All samples found to have equal variance were subjected to a two-tailed student’s T-test to determine statistical significance between groups. A p-value of 0.05 was used to determine significance.
3. Results
3.1. Capture Surface Formation and Characterization
PEG was chosen as the capture surface base material because it can be readily formed into bulk hydrogels, is easily modifiable, and resists nonspecific protein adsorption.[47] The latter advantage is particularly significant in enabling the capture of pure and viable cell populations. Photodegradable PEG-diacrylate (PEGdiPDA) was successfully synthesized through the addition of an acrylate o-nitrobenzyl group to an amine functionalized linear PEG (Figure 1a). Polymerization of PEGdiPDA hydrogels was achieved by exposure to light wavelengths of 405 nm or greater in the presence of a LAP photoinitiator, whereas degradation of the network was achieved using long wavelength UV light at 365 nm. The ability to use light to both polymerize and degrade the hydrogel network is a unique feature of this system that allows for photopatterning, as well as targeted formation or degradation of hydrogel structures. The photopolymerization conditions were informed by a statistical-kinetic model of photodegradation in PEGdiPDA networks to ensure minimal cleavage of the o-nitrobenzyl groups during network formation, where the degradation of PEGdiPDA networks is approximately 20 times slower at 405 nm than at 365 nm due to the decreased absorbance of the photocleavable group at that longer wavelengths.[45]
Figure 1. Photodegradable hydrogel capture surface formation.
(a) The chemical structure of photodegradable PEG diacrylate (PEGdiPDA) where green indicates the acrylate groups, blue the o-nitrobenzyl photodegradable groups, and black the PEG backbone. In the presence of a photoinitiator and 405 nm light the polymer undergoes a free radical initiated polymerization reaction to form a hydrogel network. The hydrogel network can then be degraded by irradiation with 365 nm light to cleave the o-nitrobenzyl groups. (b) Schematic of the fabrication and degradation process of patterned photodegradable hydrogel films. To form the hydrogel thin films by micromolding, a PDMS master is filled with the hydrogel forming polymer solution and irradiated with 405 nm light. A flow channel is then bonded over the top of the hydrogel film which can then be degraded under flow conditions by irradiation with 365 nm light. (c) The PDMS molds used to pattern the hydrogel capture surface demonstrating the difference in size between the flat and herringbone (HB) patterned surfaces. Scale bar 1 cm. (d) Representative images of flat (top) and HB (bottom) micromolded hydrogel surfaces. Scale bar 1 mm. (e) A three-dimensional confocal image of a photodegradable hydrogel HB patterned surface to show the replication of the HB pattern using the micromolding process. Scale bar 100 µm.
Micromolding, a facile technique that offers the ability to create a wide range of 3D surface geometries based on PDMS mold geometry (Figure 1b), was utilized to create two different surface textures: flat and HB patterned (Figure 1c-d). Typically, microfluidic-integrated hydrogels are long flat surfaces that permit only low sample throughput and exhibit poor capture yields.[26] To address the poor efficiency of flat surfaces, we also produced the HB-patterned photodegradable hydrogel. The HB design was implemented to form fluidic microvortices within the microfluidic channel to enhance the probability of cell-surface interactions and thereby improve capture efficiency. Introducing a flux of cells to the capture surface, significantly greater than their sedimentation velocity, also serves to increase cell interactions per linear flow distance, and thus sample throughput.
The long flat hydrogel surfaces were fabricated with an average height of 25.4 ± 2.7 µm. The hydrogel height was approximately 25 µm shorter than the depth of the PDMS mold due to the persistence of an unpolymerized zone near the PDMS surface caused by oxygen inhibition of the radical-initiated photopolymerization of PEGdiPDA.[48] The continuous flux of oxygen from the gas permeable PDMS mold into the PEG-hydrogel forming monomer solution isotopically reduced feature dimensions. The resulting unpolymerized gaps between the PDMS and hydrogel surface were predictable and consistent in height. Importantly, these gaps were of the appropriate scale for whole blood flow and therefore were used directly as flow channels, eliminating the need to remove the mold and bond a new flow channel over the flat capture surface after hydrogel formation.
The HB-patterned surface was found to have a maximum height of 28.75 ± 2.5 µm, which was again approximately 25 µm less than the PDMS mold depth due to the oxygen inhibition zone near the PDMS-hydrogel forming monomer solution interface. In the case of the HB-patterned surfaces, the non-crosslinked layer allowed for facile removal of the HB patterned PDMS mold so that a flat PDMS channel could be bonded over the patterned surface to develop the desired flow profile. Evidence of oxygen inhibition affecting HB patterning can also be observed in the rounded peaks formed upon polymerization of the hydrogel forming solution (Figure 1e). The fidelity of mold replication achieved here is significant in the HB patterned case because deviations from mold geometry can lead to non-optimal flow profile development. Investigation into the flow profile developed on HB patterned surfaces indicated good mixing within the chamber, suggesting that pattern transfer was adequate to generate the desired recirculating flow (Figure S1).
Following fabrication, capture surfaces were functionalized via biotin-avidin coupling. The efficiency and coverage distribution of functionalization was characterized with a biotinylated rhodamine as a model ligand of biotinylated anti-EpCAM. Hydrogel surfaces were functionalized either one day or three days after fabrication and monitored for 24 hours using fluorescent microscopy. Hydrogel surfaces were imaged in a different location at each time point to limit photobleaching and artifacts. No statistical difference was observed among gels functionalized on different days. Also, there was no change in the normalized fluorescent intensity observed over a 24 h period, indicating stable functionalization (Figure S2). Based upon these results and to ensure consistency, all capture experiments were performed with hydrogels functionalized one day after formation and used after soaking in PBS for 1 hr.
3.2. Cell Line Capture from Buffer
To test the hypothesis that a HB patterned capture surface would display enhanced capture relative to a flat capture surface, both device styles were used to capture a human lung carcinoma cell line (A549) from PBS buffer under continuous flow. The capture efficiency, defined as the number of target cells captured normalized by the number of target cells passed through the device, was evaluated for both surfaces. To account for surface area differences between the two capture surfaces, the cell capture density, a measure of the number of cells captured per square millimeter, was calculated. The area of capture was determined to be approximately 200 mm2 for the flat hydrogel and 42 mm2 for the HB hydrogel. A549 cells were successfully captured on both capture surface geometries (Figure 2a-b). Cells were primarily captured toward the entrance of flat capture surface devices, whereas cells captured on the HB surface were distributed along the entire channel length. These results highlight the difference in flow across each surface, with flat and HB surfaces displaying unidirectional and recirculatory flows, respectively. The laminar flow profile developed along the flat surface caused cells that were close to the surface upon entering the device to be captured quickly, but the remaining cells did not have sufficient time to approach the capture surface by sedimentation. Conversely, recirculating flow within the HB devices produced cell-hydrogel contact along the entire channel length.
Figure 2. Capture of lung cancer cell line from buffer.

Representative images of A549 cells captured from a PBS solution on a flat (a) and a HB patterned (b) photodegradable hydrogel surface. The edges of the HB channel are outlined with dashed lines and the edges of the flat channel are outside of the field of view because of the larger size of the channel used. Scale bars are 100 µm. (c) The capture efficiency was higher on the flat capture surface than the HB surface. (d) However, the capture density was found to be approximately three times greater on the HB patterned hydrogel surface compared to the flat hydrogel surface. Error bars are for n = 3 and * is for a p < 0.05.
Gross capture efficiency was observed to be greater for the flat surface, 44.8 ± 12.9 %, than the HB surface, 29.9 ± 4.7 % (Figure 2c); however, capture densities were 11.3 ± 4.2 and 32.0 ± 5 cells mm−2 for the flat and HB hydrogel surfaces, respectively (Figure 2d). Consequently, an almost threefold increase in the number of cells captured per equivalent surface area was observed for the HB hydrogel surfaces. The higher capture density for HB surfaces indicates that cells were captured with higher proficiency on these surfaces in comparison to flat surfaces. Further improvements in the HB surface capture efficiency and density could be realized through increasing the total available capture surface area by, for instance, increasing the channel length, width, or running multiple capture surfaces in parallel.
3.3. Cell Line Capture from Buffer in the Presence of White Blood Cells
Nonspecific capture was evaluated by isolating A549 cells from buffy coat obtained from whole blood. Isolated white blood cells (WBCs) were immediately mixed with fluorescently labeled A549 cells in a 3:1 ratio and the cell solution was pumped through devices containing capture surfaces of both types (Figure 3a). The capture efficiency of the flat surface was 20.4 ± 3.0 % for A549 cells and 14.4 ± 0.01 % for WBCs. For the HB surface, it was 32.1 ± 8.4 % and 8.5 ± 2.8 % for A549 cells and WBCs, respectively (Figure 3b). Again, a significant difference between the capture density of the target cell upon the flat and HB surfaces was observed, with an approximately 10-fold higher density achieved on the HB surface (Figure 3c). Notably, the capture efficiency of HB patterned surfaces increased in the presence of WBCs relative to buffer experiments, whereas the capture efficiency of flat surfaces decreased. We hypothesize that this is due to both the flow profile and the contact time of cells with the capture surfaces. For the flat capture surface, only a limited number of cells will be exposed to the surface, and those cells will roll along the surface yielding more opportunities to interact with the antibody functionalized surface for adhesion, both specifically and non-specifically. However, in the HB-patterned device, there is a more uniform dispersion of cells and interaction times over the device length increasing the likelihood that a cell will stick to the surface specifically as opposed to non-specifically.
Figure 3. Capture of lung cancer cell line from buffer containing white blood cells.

(a) Shown are representative images of both cell types captured on the flat (top) and HB patterned (bottom) photodegradable hydrogel surfaces. Red circles represent A549 cells and orange circles represent WBCs which were differentiated by the presence of a fluorescent signal. Scale bars are 50 µm. (b) The capture efficiency was found to be approximately 1.5-fold greater on HB patterned capture surfaces when compared to flat capture surfaces. (c) The capture density was found to be approximately 10-fold greater on the HB patterned capture surfaces than the flat capture surface. (d) Additionally, the purity of the cell population captured on the HB patterned capture surface was approximately 1.5-fold higher than the purity of the cell population captured on the flat capture surface. Error bars are for n = 3 and * is for a p < 0.05.
The purity of cell populations captured from complex suspensions was calculated as the number of target cells normalized by the total number of cells captured. Purities of 32.0 ± 3.2 % and 55.9 ± 10.3 % were obtained for the flat and HB patterned surfaces, respectively (Figure 3d). The observed purity values for both surfaces are higher than those reported for PDMS functionalized capture surfaces, highlighting the ability of PEGdiPDA hydrogel capture surfaces to resist nonspecific binding. Specifically, in seminal work describing HB patterned PDMS surfaces, 14% purity of cancer cells captured from buffer containing leukocytes was reported.[19] The purity observed here for A549 cell capture with HB-patterned hydrogel surfaces is approximately four times greater than reported previously.
3.4. Cell Line Capture from Whole Blood
The clinical utility of photodegradable capture surfaces was evaluated by spiking a known number of A549 cells (105 A549 per milliliter of blood) into whole blood obtained from a healthy patient to create a controlled “liquid biopsy” (Figure 4a-b). A relatively high concentration of A549 cells was used to quantitatively characterize the platform’s ability to capture a target cell from whole blood. The capture efficiency for the flat and HB patterned surfaces was nearly identical, 13.4% on average for both surfaces (Figure 4c). However, the capture density on the HB surface, 9.6 ± 2.0 cells mm−2, was 9 times greater than that of the flat surface, 1.0 ± 0.4 cells mm−2 (Figure 4d). Additionally, the purity of captured cells was 21.6 ± 18.7 % and 23.8 ± 13.3 % for the flat and HB surfaces, respectively (Figure 4e). While experimental variability was observed, the capture purity from whole blood was consistently lower than that from buffy coat, but at significantly higher target cell dilutions. This observation again highlights the ability of PEGdiPDA hydrogels to resist non-specific capture when compared to PDMS surfaces; the purity observed here for A549 cell capture onto HB-patterned hydrogel surfaces from a whole blood sample was approximately 10% greater than that observed with cancer cells captured from buffer containing leukocytes on a PDMS HB patterned surface.[19]
Figure 4. Capture of lung cancer cell line spiked into whole blood.
(a) Flat (left) and HB patterned (right) photodegradable hydrogel capture surfaces being exposed to a whole blood sample containing A549 cells. Scale bars 1 cm. (b) A representative image of A549 cells, white blood cells, and red blood cells captured on the HB patterned hydrogel capture surface. Scale bar 100 µm. (c) The capture efficiency for A549 cell capture was approximately the same for both the flat and HB patterned hydrogel capture surfaces. (d) In contrast, the capture density was approximately 9-fold greater for the HB patterned capture surface when compared to the flat capture surface. (e) The purity for both surfaces was statistically the same when A549 cells were captured from whole blood. Error bars are for n = 3 and * is for a p < 0.05.
3.5. Cell Release and Viability
Releasing viable cells from capture surfaces is necessary for characterization of cancer type, progression, and other diagnostic and prognostic factors. Additionally, the selective, individually addressable release of cells provides a mechanism by which to increase the purity of recovered cells beyond that dictated by the capture surface. The viability of captured cells on each captured surface was determined directly after capture before cells were released from the surface and found to be approximately 92%, on both capture surfaces (Figure 6a). These results indicate that the capture method itself is passive to cells.
Figure 6. Viability and activity of captured and released lung cancer cells.

The viability of A549 cells captured on both surfaces remained above 90% after both the capture and release process. Representative images of the viability of A549 cells captured (a) and released (b) from PBS buffer on a HB hydrogel capture surface are shown. Live cells are green and dead cells red. Cells released from the capture surface (c) were cultured to assess their function compared to control cells (pristine cells cultured in growth medium) (d). Shown are representative images from after cell seeding, when small colonies formed, when large colonies formed, and when 80% confluence was reached. Cell proliferation rates and population growth were maintained in cells that were captured and released from the hydrogel capture surface with 80% confluency being reached by both experimental and control cell populations in 168 h (7 days) post seeding. Scale bars are 100 µm (a, b) and 20 µm (c, d).
Next, the release of viable cells from the capture surface was investigated. Cells captured from buffer were successfully released from the capture surface by irradiation of the hydrogel surface with low doses of long wavelength UV light (10 mW cm−2 at 365 nm for ≤ 90 s). The dose of light (900 mJ cm−2) was kept within documented cytocompatible limits.[39,41,49,50] PBS was continuously flowed over the surface during the entire release process to collect cells. Two different release motifs were investigated: bulk and targeted release. For bulk release, the entire capture surface was irradiated while under flow conditions to simultaneously release all captured cells for collection downstream (Figure 5a).
Figure 5. Release of captured cells through photodegradation of the hydrogel surface.

(a) Bulk film degradation by flood irradiation with long wavelength UV resulted in the release of all captured cells from the hydrogel capture surface simultaneously. Scale bars 100 µm. (b) Targeted release of individual cells from the hydrogel film was achieved using focused long wavelength UV light. Red and orange circles are used for a visual guide. The cell in the red circle is selectively released from the surface while the cell in the orange circle remains. Scale bars = 30 µm. For both release motifs, the image on the left represents t = 0 s; the middle image is at an intermediate time point; and the right image is when full degradation or cell release occurred.
To improve the purity of target cells captured from whole blood, targeted release was used to individually release clusters or single cells. Targeted cell release was achieved using focused long wavelength UV applied through a standard fluorescence microscope with a 20x objective. Cells were successfully released using targeted irradiation demonstrating the ability to improve cell yield via selective release (Figure 5b). The ability to selectively remove target cells from the capture surface is a powerful tool for improving downstream processing of CTC samples toward developing personalized therapies. An advantage of the selective cell isolation approach described is it can easily be implemented in any laboratory in possession of a standard fluorescence microscope equipped with a DAPI filter cube.
Cells released under a bulk degradation motif were collected, and their viability was assessed. Directly after release, the observed viability was approximately 94% (Figure 6b). To assess overall cellular health and function, released cells were seeded into a 96-well plate and observed until 80% confluence was reached. Cells released from the capture surface formed small colonies within 3 days, large colonies within 4 days, and reached 80% confluence after 7 days in culture (Figure 6c). Captured and released cells reached the indicated growth stages at the same rate as control cells (i.e., pristine cells cultured in growth medium) (Figure 6d). Additionally, the morphology of the two populations was visually similar, further indicating that cells were not affected significantly by capture and release from the hydrogel platform.
The release of cells by either a bulk or targeted degradation motif highlights the unique advantage of photodegradable capture surfaces to release cells using light. While all immunoaffinity surfaces suffer from nonspecific cell capture, photodegradable PEG hydrogels present a surface that not only resists non-specific capture, but also provides the unique ability to release only desired cells from the surface down to single cell resolution, therefore enabling samples that are 100% pure. Further, cells can be captured and released in a gentle manner that does not affect cell viability or their ability to be cultured in vitro following recovery.
4. Conclusion
A CTC capture and release platform utilizing a photopolymerizable, photodegradable hydrogel has been introduced to overcome the shortcomings of current immunoaffinity capture platforms by combining enhanced capture with selective release. This platform can be photopatterned with visible light and degraded with long wavelength UV light providing high-resolution controlled release over the hydrogel surface. The use of micromolding enables the production of complex three-dimensional geometries that can be used to alter flow properties within the device. The successful capture of target cells was demonstrated from complex media with improved capture density on HB surfaces compared to a traditional flat surface. Target cells were captured on PEGdiPDA hydrogel surfaces from whole blood with the ability to enhance the target cell purity by utilizing the targeted cellular release mechanism enabled by this photodegradable platform. These cells remained viable after release from the surface and were easily expanded, exhibiting similar growth rates and morphologies as control cells. Overall, the platform presented here is promising for the capture and release of pure and viable cells from a variety of mixed cell populations, including CTCs from whole blood, for use in both prognostic and diagnostic applications.
Supplementary Material
Highlights:
Immunofunctionalized hydrogels successfully capture circulating tumor cells (CTCs)
Hydrogel micromolding allows microfluidic integration and optimal fluid/cell mixing
Photolabile hydrogels allow the facile release of viable CTCs for downstream analysis
Selective post-analysis cell release enables high CTC collection purity
Acknowledgements
This work was supported by the National Institutes of Health (NIH) (R15GM101636 to JO and R01GM102428 to JCG) and the NIH-funded Wyoming IDeA Networks of Biomedical Research Excellence program (P20GM103432). PJL gratefully acknowledges a graduate fellowship from the University of Wyoming Office of Academic Affairs. AMK acknowledges the Institutional Development Awards from NIH for COBREs (P20GM104316 and P30GM110758–01) for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplemental Information
Supplementary data can be found in the supplemental information file.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
- [1].Plaks V, Koopman CD, Werb Z, Circulating Tumor Cells, Science 341 (2013) 1186–1188. 10.1007/978-3-319-22909-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams SCP, Circulating Tumor Cells, PNAS 110 (2013) 4861 10.1007/978-3-319-22909-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, V Doyle G, Allard WJ, Terstappen LWMM, Hayes DF, Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer, N. Engl. J. Med 351 (2004) 781–791. 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- [4].Krebs MG, Sloane R, Priest L, Lancashire L, Hou J-M, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH, Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients With Non–Small-Cell Lung Cancer, J. Clin. Oncol 29 (2011) 1556–1563. 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- [5].De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, V Doyle G, Terstappen LWWM, Pienta KJ, Raghavan D, Cancer Therapy : Clinical Circulating T umor Cells Predict Survival Benefit from T reatment in Metastatic Castration-Resistant Prostate Cancer, 14 (2008) 6302–6309. 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- [6].Hong B, Zu Y, Detecting circulating tumor cells: Current challenges and new trends, Theranostics 3 (2013) 377–394. 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hyun K, Jung H-I, Advances and critical concerns with the microfluidic enrichments of circulating tumor cells., Lab Chip 14 (2014) 45–56. 10.1039/c3lc50582k. [DOI] [PubMed] [Google Scholar]
- [8].Nagrath S, V Sequist L, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M, Isolation of rare circulating tumour cells in cancer patients by microchip technology., Nature 450 (2007) 1235–9. 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Götten J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA, Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor, J. Am. Chem. Soc 130 (2008) 8633–8641. 10.1021/ja8015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu Y, Phillips JA, Yan J, Li Q, Fan ZH, Tan W, Box PO, Engineering B, Box PO, Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer cells, Anal. Chem 81 (2009) 7436–7442. 10.1021/ac9012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao L, Tang C, Xu L, Zhang Z, Li X, Hu H, Cheng S, Zhou W, Huang M, Fong A, Liu B, Tseng HR, Gao H, Liu Y, Fang X, Enhanced and Differential Capture of Circulating Tumor Cells from Lung Cancer Patients by Microfluidic Assays Using Aptamer Cocktail, Small 12 (2016) 1072–1081. 10.1002/smll.201503188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Munz M, Baeuerle PA, Gires O, The emerging role of EpCAM in cancer and stem cell signaling, Cancer Res 69 (2009) 5627–5629. 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- [13].Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF, Microfluidic Shear Devices for Quantitative Analysis of Cell Adhesion, Anal. Chem 76 (2004) 5257–5264. 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- [14].Cheng X, Irimia D, Dixon M, Ziperstein JC, Demirci U, Zamir L, Tompkins RG, Toner M, Rodriguez WR, A microchip approach for practical label-free CD4+ T-cell counting of HIV-infected subjects in resource-poor settings., J. Acquir. Immune Defic. Syndr 45 (2007) 257–261. 10.1097/QAI.0b013e3180500303. [DOI] [PubMed] [Google Scholar]
- [15].Sekine K, Revzin A, Tompkins RG, Toner M, Panning of multiple subsets of leukocytes on antibody-decorated poly(ethylene) glycol-coated glass slides, J. Immunol. Methods 313 (2006) 96–109. 10.1016/j.jim.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [16].Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, a Giannakakou P, Kirby BJ, Giannakakou A, Kirby BJ, Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody., Lab Chip 10 (2010) 27–29. 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang J, Zhao H, Shu W, Tian J, Huang Y, Song Y, Wang R, Li E, Slamon D, Hou D, Du X, Zhang L, Chen Y, Wang Q, An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells, Sci. Rep 7 (2017) 1–11. 10.1038/srep42612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murlidhar V, Zeinali M, Grabauskiene S, Ghannad-Rezaie M, Wicha MS, Simeone DM, Ramnath N, Reddy RM, Nagrath S, A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells, Small 10 (2014) 4895–4904. 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stott SL, Hsu C-HC-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman B. a., Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber D. a., Toner M, Isolation of circulating tumor cells using a microvortex-generating herringbone-chip, PNAS 107 (2010) 18392–7. 10.1073/pnas.1012539107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reátegui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, Bardia A, Sequist LV, Haber DA, Maheswaran S, Hammond PT, Toner M, Stott SL, Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells, Adv. Mater 27 (2015) 1593–1599. 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu YT, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, Song M, Xu X, Ouyang WHWWL, Ouyang WHWWL, Lichterman J, Luo Z, Xuan X, Huang J, Chung LWK, Rettig M, Tseng HR, Shao C, Posadas EM, NanoVelcro chip for CTC enumeration in prostate cancer patients, Methods 64 (2013) 144–152. 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang S, Liu K, Liu J, Yu ZTF, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee YK, Chung LWK, Huang J, Rettig M, Seligson D, Duraiswamy KN, Shen CKF, Tseng HR, Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers, Angew. Chemie - Int. Ed 50 (2011) 3084–3088. 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Z, Shu W, Chen R, Feng H, Hu Z, Wang Y, Tian G, Liu A, Chen Y, Integrated Microfluidic Chip for Rapid Capture and Drug Screening of Cancer Cells from Peripheral Blood, Integr. Cancer Biol. Res 2 (2018). [Google Scholar]
- [24].Sun D, Chen Z, Wu M, Zhang Y, Nanomaterial-based Microfluidic Chips for the Capture and Detection of Circulating Tumor Cells, Nanotheranostics 1 (2017) 389–402. 10.7150/ntno.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sheng W, Ogunwobi OO, Chen T, Zhang J, George TJ, Liu C, Fan ZH, Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip, Lab Chip 14 (2014) 89–98. 10.1039/C3LC51017D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shah AM, Yu M, Nakamura Z, Ciciliano J, Ulman M, Kotz K, Stott SL, Maheswaran S, Haber DA, Toner M, Biopolymer System for Cell Recovery from Microfluidic Cell Capture Devices, Anal. Chem 84 (2012) 3682–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xie M, Lu N-N, Cheng S-B, Wang X-Y, Wang M, Guo S, Wen C, Hu J, Pang D-W, Huang W-H, Engineered Decomposable Multifunctional Nanobioprobes for Capture and Release of Rare Cancer Cells, Anal. Chem 86 (2014) 4618–4626. [DOI] [PubMed] [Google Scholar]
- [28].Li W, Reátegui E, Park MH, Castleberry S, Deng JZ, Hsu B, Mayner S, Jensen AE, Sequist LV, Maheswaran S, Haber DA, Toner M, Stott SL, Hammond PT, Biodegradable nano-films for capture and non-invasive release of circulating tumor cells, Biomaterials 65 (2015) 93–102. 10.1016/j.biomaterials.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].V Nair S, Witek MA, Jackson JM, Lindell MAM, Hunsucker SA, Sapp T, Perry CE, Hupert ML, Bae-Jump V, Gehrig PA, Wysham WZ, Armistead PM, Voorhees P, Soper SA, Enzymatic cleavage of uracil-containing single-stranded DNA linkers for the efficient release of affinity-selected circulating tumor cells., Chem. Commun. (Camb) 51 (2015) 3266–9. 10.1039/c4cc09765c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheung LSL, Zheng X, Stopa A, Baygents JC, Guzman R, Schroeder JA, Heimark RL, Zohar Y, Siu L, Cheung LSL, Zheng X, Stopa A, Baygents JC, Guzman R, Schroeder JA, Heimark L, Zohar Y, Detachment of captured cancer cells under flow acceleration in a bio-functionalized microchannel., Lab Chip 9 (2009) 1721–1731. 10.1039/b822172c. [DOI] [PubMed] [Google Scholar]
- [31].Zheng X, Jiang L, Schroeder J, Stopeck A, Zohar Y, Isolation of viable cancer cells in antibody-functionalized microfluidic devices Isolation of viable cancer cells in antibody-functionalized microfluidic devices, Biomicrofluidics 8 (2014) 0–13. 10.1063/1.4873956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hou S, Zhao H, Zhao L, Shen Q, Wei KS, Suh DY, Nakao A, Garcia MA, Song M, Lee T, Xiong B, Luo SC, Tseng HR, Yu HH, Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures, Adv. Mater 25 (2013) 1547–1551. 10.1002/adma.201203185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu H, Sun K, Fan J, Zhang P, Meng J, Li Y, Wang S, Jiang L, Dual-Responsive Surfaces Modified with Phenylboronic\rAcid-Containing Polymer Brush To Reversibly Capture and\rRelease Cancer Cells, JACS 135 (2013) 7603–7609. 10.1021/ja401000m. [DOI] [PubMed] [Google Scholar]
- [34].Levalley PJ, Noren B, Kharkar PM, Kloxin AM, Gatlin JC, Oakey JS, Fabrication of Functional Biomaterial Microstructures by in Situ Photopolymerization and Photodegradation, ACS Biomater. Sci. Eng 4 (2018) 3078–3087. 10.1021/acsbiomaterials.8b00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deng Y, Zhang Y, Sun S, Wang Z, Wang M, Yu B, Czajkowsky DM, Liu B, Li Y, Wei W, Shi Q, An Integrated Microfluidic Chip System for Single-Cell Secretion Profiling of Rare Circulating Tumor Cells., Sci. Rep 4 (2014) 7499 10.1038/srep07499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shin DS, You J, Rahimian A, Vu T, Siltanen C, Ehsanipour A, Stybayeva G, Sutcliffe J, Revzin A, Photodegradable hydrogels for capture, detection, and release of live cells, Angew. Chemie - Int. Ed 53 (2014) 8221–8224. 10.1002/anie.201404323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kloxin AM, Benton JA, Anseth KS, In situ elasticity modulation with dynamic substrates to direct cell phenotype, Biomaterials 31 (2010) 1–8. 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kloxin AM, Tibbitt MW, Anseth KS, Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms., Nat. Protoc 5 (2010) 1867–1887. 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wong DY, Ranganath T, Kasko AM, Low-Dose, Long-Wave UV Light Does Not Affect Gene Expression of Human Mesenchymal Stem Cells, PLoS One 10 (2015) e0139307 10.1371/journal.pone.0139307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tibbitt MW, Kloxin AM, Dyamenahalli KU, Anseth KS, Controlled two-photon photodegradation of PEG hydrogels to study and manipulate subcellular interactions on soft materials, Soft Matter 6 (2010) 5100 10.1039/c0sm00174k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Photodegradable hydrogels for dynamic tuning of physical and chemical properties., Science (80-. ) 324 (2009) 59–63. 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS, Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility, Biomaterials 30 (2009) 6702–6707. 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sawicki LA, Kloxin AM, Light-mediated Formation and Patterning of Hydrogels for Cell Culture Applications, J. Vis. Exp (2016) e54462 10.3791/54462. [DOI] [PMC free article] [PubMed]
- [44].Xia Y, Whitesides GM, Soft lithography, Annu. Rev. Mater. Sci 28 (1998) 153–184. 10.1146/annurev.matsci.28.1.153. [DOI] [Google Scholar]
- [45].Tibbitt MW, Kloxin AM, Anseth KS, Modeling controlled photodegradation in optically thick hydrogels, J. Polym. Sci. Part A Polym. Chem 51 (2013) 1899–1911. 10.1002/pola.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu C-L, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S, Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer, Sci. Transl. Med 2 (2010) 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kharkar PM, Kiick KL, Kloxin AM, Designing degradable hydrogels for orthogonal control of cell microenvironments., Chem. Soc. Rev 42 (2013) 7335–72. 10.1039/c3cs60040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Krutkramelis K, Xia B, Oakey J, Monodisperse polyethylene glycol diacrylate hydrogel microsphere formation by oxygen-controlled photopolymerization in a microfluidic device, Lab Chip 16 (2016) 1457–1465. 10.1039/C6LC00254D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Griffin DR, Kasko AM, Photodegradable macromers and hydrogels for live cell encapsulation and release, J. Am. Chem. Soc 134 (2012) 13103–13107. 10.1021/ja305280w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xia B, Jiang Z, Debroy D, Li D, Oakey J, Cytocompatible cell encapsulation via hydrogel photopolymerization in microfluidic emulsion droplets, Biomicrofluidics 11 (2017) 044102 10.1063/1.4993122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




