Abstract
Background
Observational reports suggest that supplementation that increases citric acid cycle intermediates via anaplerosis may have therapeutic advantages over traditional medium-chain triglyceride (MCT) treatment of long-chain fatty acid oxidation disorders (LC-FAODs) but controlled trials have not been reported. The goal of our study was to compare the effects of triheptanoin (C7), an anaplerotic seven-carbon fatty acid triglyceride, to trioctanoin (C8), an eight-carbon fatty acid triglyceride, in patients with LC-FAODs.
Methods
A double blinded, randomized controlled trial of 32 subjects with LC-FAODs (carnitine palmitoyltransferase-2, very long-chain acylCoA dehydrogenase, trifunctional protein or long-chain 3-hydroxy acylCoA dehydrogenase deficiencies) who were randomly assigned a diet containing 20% of their total daily energy from either C7 or C8 for 4 months was conducted. Primary outcomes included changes in total energy expenditure (TEE), cardiac function by echocardiogram, exercise tolerance, and phosphocreatine recovery following acute exercise. Secondary outcomes included body composition, blood biomarkers, and adverse events, including incidence of rhabdomyolysis.
Results
Patients in the C7 group increased left ventricular(LV) ejection fraction by 7.4% (p = 0.046) while experiencing a 20% (p = 0.041) decrease in LV wall mass on their resting echocardiogram. They also required a lower heart rate for the same amount of work during a moderate-intensity exercise stress test when compared to patients taking C8. There was no difference in TEE, phosphocreatine recovery, body composition, incidence of rhabdomyolysis, or any secondary outcome measures between the groups.
Conclusions
C7 improved LV ejection fraction and reduced LV mass at rest, as well as lowering heart rate during exercise among patients with LC-FAODs.
Introduction
Long-chain fatty acid oxidation disorders (LC-FAODs) are inherited defects in one of the transport or catalytic enzymes in the mitochondrial long-chain fatty acid beta- oxidation pathway. Collectively, the incidence of FAOD is approximately 1:9000 births (Lindner et al 2010; Bennett et al 2012). Patients with LC-FAODs can present with a wide range of symptoms varying from severe neonatal hypoglycemia, hepatomegaly, and cardiomyopathy to milder adolescent and adult phenotypes with recurrent rhabdomyolysis and exercise intolerance. The most common chronic manifestations of the disorders are frequent episodes of myalgia, recurrent rhabdomyolysis induced by exercise, fasting or illness, cardio- myopathy, and an associated decreased quality of life (Spiekerkoetter et al 2010; Rinaldo et al 2002; Wilcken 2010; Lindner et al 2011; Wang et al 2014).
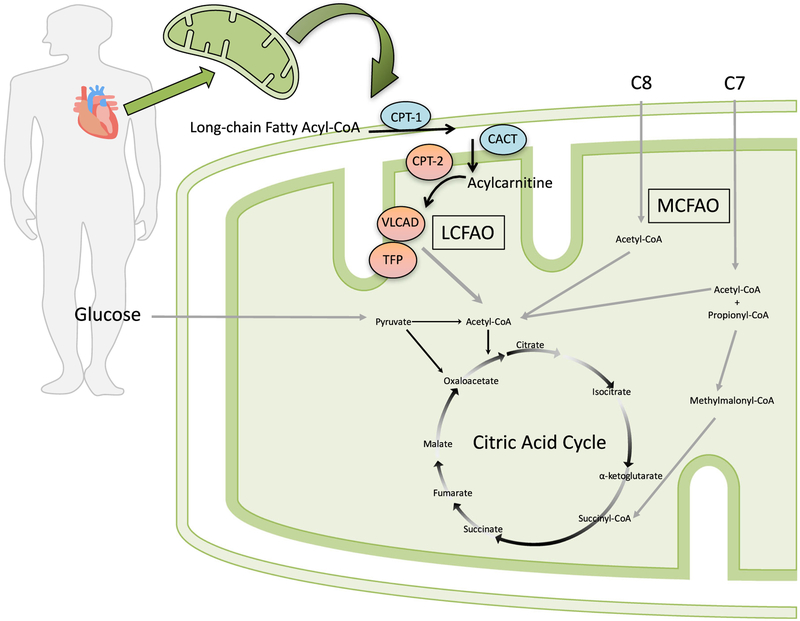
Current treatment for LC-FAODs includes avoidance of fasting, providing adequate energy intake, a long chain fat- restricted diet, and supplementation with medium-chain triglycerides (MCT), a fat source that does not require long chain FAO for its metabolism (Gillingham et al 2003; Gillingham et al 2006; Arnold et al 2009; Spiekerkoetter et al 2009). Commercially available MCT consists of a mix of octanoate (C8:0), decanoate (C10:0), and some dodecanoate (C12:0) fatty acids esterified to a glycerol backbone with the proportions of these fatty acids varying from lot to lot. Dietary treatment is designed to meet energy needs such that recurrent metabolic crises are reduced or eliminated, however, despite these efforts, patients continue to experience recurrent metabolic decompen- sation (Vockley et al 2015). It has been proposed that depletion of citric acid cycle (CAC) intermediates in the face of impaired long-chain FAO may further impair delivery of reducing equiv- alents for oxidative phosphorylation even from non-fat sub- strates such as glucose (Roe and Mochel 2006). Furthermore, an anaplerotic substrate, such as an odd chain medium length fatty acid, may replenish CAC intermediates, providing addi- tional clinical benefit over substrates that can supply only acetyl-CoA such as MCT oil (Fig. 1).
Fig. 1.
Model for the proposed benefit of triheptanoin (C7) compared to trioctanoate (C8) among patients with long-chain fatty acid oxidation disorders (LC-FAODs). Trioctanoate provides three 8 carbon fatty acids (C8) that, once imported into the mitochondria, are oxidized to 4 acetyl- CoA molecules. Triheptanoate provides three 7 carbon fatty acids (C7) that, once imported into the mitochondria, are oxidized to produce 2 acetyl-CoA and one propionyl-CoA molecule. Propionyl-CoA is converted to D-methylmalonyl-CoA by mitochondrial propionyl-CoA carboxylase followed by conversion to succinyl-CoA by D- methylmalonyl-CoA isomerase and L-methylmalonyl-CoA mutase. Succinyl-CoA is an intermediate of the citric acid cycle (CAC) and can increase intermediate pool size of carbon substrates. LCFAO = long-chain fatty acid oxidation; MCFAO = medium-chain fatty acid oxidation; CPT- 1 = carnitine palmitoyltransferase 1; CACT = carnitine acylcarnitine translocase; CPT-2 = carnitine palmitoyltransferase 2; VLCAD = very- long-chain acylCoA dehydrogenase; TFP = trifunctional protein. Patients enrolled in this clinical trial had CPT2, VLCAD or TFP deficiency, including long-chain 3-hydroxy acylCoA dehydrogenase (LCHAD) deficiency, as indicated by red enzyme color
Several open-label trials and retrospective chart reviews have reported positive clinical results following anaplerotic therapy with triheptanoin (C7) in patients with LC-FAODs (Roe et al 2002; Roe et al 2008; Roe and Brunengraber 2015; Vockley et al 2015; Vockley et al 2016). Triheptanoin is a triglyceride composed of three seven-carbon fatty acids, heptanoate, esterified to a glycerol backbone. Oxidation of one heptanoate yields two acetyl-CoA molecules and one of propionyl-CoA. Propionyl-CoA is converted to succinyl-CoA, which is an intermediate of the CAC cycle and subsequently increases the intermediate pool size. We conducted a double- blind randomized controlled trial (RCT) to compare the thera- peutic benefit of C7 to a pure trioctanoin (C8) comparator in patients with long-chain FAO disorders. The goal of the study was to test the hypothesis that an odd-chain triglyceride (C7) has a therapeutic advantage over an even-chain triglyceride (C8) in the treatment of LC-FAO disorders. We used a trioctanoin synthesized in a comparable manner to eliminate fatty acid variation in the composition of commercial MCT.
Methods
Study design
This was a randomized double-blind parallel-design RCT con- ducted at two sites, Oregon Health and Science University (OHSU) and the University of Pittsburgh, between November 2011 and February 2015. C7, an odd chain triglyceride, and C8, an even chain triglyceride, were compared in patients with LC- FAODs. Patients aged ≥7 years with a confirmed diagnosis of carnitine palmitoyltransferase-2 (CPT-2; OMIM#255110, or #600649), very long-chain acylCoA dehydrogenase (VLCAD; OMIM# 201475), trifunctional protein (TFP; OMIM#609015) or long-chain 3-hydroxyacylCoA dehydrogenase (LCHAD; OMIM#609016) deficiencies were enrolled. The diagnosis was confirmed by review of medical records documenting at least one significant episode of rhabdomyolysis and at least two of the following diagnostic criteria: 1) disease specific eleva- tions of acylcarnitines on a newborn blood spot or in plasma,2) low enzyme activity in cultured fibroblasts, or 3) one or more known pathogenic mutations in the CPT2 (OMIM #600650), ACADVL (OMIM #609575), HADHA (OMIM#600890), or HADHB (OMIM#142450) genes. Exclusion criteria included anemia (hemoglobin <10 g/dL), peripheral neuropathy limiting the ability to walk, pregnancy, breastfeeding, or a history of myocardial infarction.
Study approvals
The institutional review board at each site approved the trial protocol (supplement). Written informed consent and assent was obtained from all patients and their legal guardians. The trial was registered at clinicaltrials.gov (NCT01379625).
Randomization
After baseline assessments, participants were assigned to either C7 or C8 supplementation in a 1:1 ratio stratified by diagnosis (CPT2, VLCAD or TFP/LCHAD). In-patient evaluation at baseline and study completion was conducted in the respective institutional Clinical and Translational Research Center. Primary outcomes included changes in total energy expenditure (TEE), cardiac function by echocardiogram, exercise tolerance, and phosphocreatine recovery following acute exercise. Secondary outcomes included body composition, blood biomarkers, and adverse events. Outcomes were measured at baseline and after 4 months of treatment with either C7 or C8. The study statistician generated a randomization table and the treatment assignment remained unknown to all study personal until data collection was complete, except for the research pharmacy dispensing the oil (OHSU), the bionutrition kitchen preparing meals, and the primary study coordinator (JM). Power analysis using TEE data adjusted for age from a previous trial in patients with LC-FAODs indicated 32 subjects, 16 subjects per treatment arm, were sufficient to achieve >80% power; the study stopped when 32 subjects enrolled.
Intervention
Participants were already following a diet low in long-chain fats, supplemented with commercial MCT at baseline. Subjects were instructed to consume 20% of their estimated total energy needs from the study oil during the study. Study oils were synthesized by Stepan, Inc. as a C7 triglyceride (triheptanoin) or a C8 triglyceride (trioctanoin) and shipped to the OHSU research pharmacy, which dispensed all study oil to the participant’s home (IND #113386). During the 4-months of treatment, subjects were contacted weekly to assess for com- pliance and adverse events. Compliance with prescribed study oil was assessed by multiple 3-day diet records and by measuring the amount of unconsumed oil at the end of study.
Body composition
Weight and height and body composition by dual-energy X- ray absorptiometry were measured after an overnight 10-h fast (Gillingham et al 2013). Liver and soleus muscle lipid deposition was measured by 1H magnetic resonance spectroscopy (MRS) as previously described (Gillingham et al 2013).
31P spectroscopy
Approximately 3 h after lunch, the subject was positioned in the MRI and instructed to perform a leg-flexion exercise protocol against the resistance of a latex band with three 2 min phases: i) baseline (rest), ii) exercise, and iii) recovery (rest). At the Pittsburgh site, the coil was placed on the vastus lateralis and the subject extended the lower leg in a repeat flexion exercise. At OHSU site, the coil was placed on the tibialis anterior and the subject performed ankle-flexion exercise. The 31P spectra were acquired continuously every 1.1 s during the three phases of the protocol (Diekman et al 2016). Not all subjects completed the PCr recovery measurement and children under age 16 did not complete this outcome measure. Data was available for eight subjects randomized to the C7 group and seven subjects randomized to the C8 group.
Energy expenditure
Resting energy expenditure was measured after an overnight fast by indirect calorimetry and substrate oxidation was estimated as previously described (Gillingham et al 2013; DeLany et al 2014). Total energy expenditure was measured using a doubly labeled water method (DLW; 2H2 O and H218O purchased from Cambridge Isotope Laboratories, Tewksbury, MA) (DeLany et al 2013). Ingestion of the DLW and subse- quent collection of urine samples for analysis took place after returning home from the baseline assessment and prior to returning to the clinical site for end-of-study assessment in order to control for differences in ground water isotope enrich- ment among the physical locations of the subjects’ homes.
Resting echocardiogram
A comprehensive echocardiographic evaluation of myocardial mechanics was performed at baseline and again after 4 months of treatment with either C7 or C8. In each case, echocardiogram was performed at rest approximately one hour after lunch. The echocardiograms were subsequently analyzed using a vendor-independent software package (Epsilon Imaging, EchoInsight®, Madison WI, USA); complete data was available for 10 subjects randomized to the C7 group and 11 subjects randomized to the C8 group. Intra-observer concordance correlation (correlation for repeated ECHO analysis by SH) was r = 0.75 (95% CI: 0.09—0.95, p = 0.03) inter- observer concordance correlation (between SH and LJB) was r = 0.89 (95% CI: 0.51—0.98, p = 0.003). Analysis of echo- cardiographic variables was completely blinded.
Metabolic response to a meal
Subjects consumed a mixed breakfast meal containing 20% C8 at baseline and either 20% C7 or C8 at the end of study. Blood samples were drawn immediately before consuming the meal (fasting) and again 1, 2, and 4 h post-prandial. Samples were analyzed for creatine kinase (CK) by the local clinical lab, insulin, and free fatty acids by colorimetric assay, acylcarnitines by electrospray tandem mass spectrometry (Smith and Matern 2010), and glucose, lactate, pyruvate, ß- hydroxybutyrate, acetoacetic acid, and the five carbon ke- tones, ß-ketopentanoate, ß-hydroxypentanoate, by stable iso- tope dilution gas chromatography-mass spectrometry (GC/MS) (Behrend et al 2012).
In vivo fatty acid oxidation
Subjects consumed 17 mg/kg body weight of a 1-13C-Oleic acid tracer (purchased from Cambridge Isotope Laboratories, Tewksbury, MA) with the mixed test meal. Breath samples were collected hourly for 12 h and analyzed for 13CO2 by isotope ratio mass spectrometry (IRMS) (DeLany et al 2000). Recovery of the label in breath represents one complete cycle of fatty acid oxidation, and further oxidation of the acetyl-CoA to release 13CO2.
Exercise test
Patients began treadmill ergometry 2 h after a standardized lunch. At baseline, all patients received C8 in their lunch and again as an oral bolus 20 min prior to exercise testing. The bolus dose was 0.3 g oil/kg lean body mass (LBM). At 4-months, patients repeated treadmill ergometry and received either C8 or C7 prior to exercise based upon treatment randomization. Treadmill testing was performed as previously described; speed, grade, and duration were held constant between the two assessments (Gillingham et al 2006; Behrend et al 2012).
Statistics
Mixed-effect models were used to analyze all primary end- points. All models treated subject as a random effect, while treatment (C7 vs C8), time (4-month vs baseline), and the interaction between these dichotomous factors were held as fixed effects. Models also included medical diagnosis and site as additional fixed effects. The interaction assessed whether change over time (4-month vs baseline) differs by treatment and serves as the effect of interest. In some instances, the endpoint being investigated exhibited pronounced (positive) skewness or extreme range (> 3-fold separation between maximum and minimum observation), leading us to log-transform the response prior to fitting the above models. In these cases, differences in the mean response on the log scale represent multiplicative (i.e., relative) changes on the original scale. Endpoints that comprised a series of measurements over time (as with treadmill tests) had mixed-effect models fit to the observed change between follow-up. All models were fit using Stata (v.14.1; StataCorp, College Station, TX) and uti- lized Satterthwaite’s method to compute approximate degrees of freedom for purposes of estimation and testing. All p-values are two-sided and significance set to 0.05 for each endpoint considered.
Results
Study population characteristics
Sixteen patients were randomized to receive C7, and 16 received C8 (CONSORT diagram supplemental Figure 1). There were five subjects with CPT-2, four with VLCAD, and seven with LCHAD/TFP deficiency in the C7 group and six subjects with CPT-2, five with VLCAD, and five with LCHAD/TFP deficiency in the C8 group. Individual participant gene mutations and characteristics are given in Table 1. Subjects with one or fewer identified pathogenic mutations had low enzyme activity or accumulation of dis- ease specific acylcarnitines in cultured skin fibroblasts. Of the 12 subjects with LCHAD/TFP, all had a history of multiple rhabdomyolysis episodes requiring hospitalization. Seven were diagnosed by newborn screening and had no cardiac complications, while five presented symptomatically in infancy, and three of these had significant cardiac manifestations at the time of presentation that re- solved with diagnosis and treatment. One young adult had a recent history of sudden cardiac arrest with resuscitation. All subjects with LCHAD/TFP had normal cardiac function at the time of the study. Of the nine subjects with VLCAD, all had a history of recurrent rhabdomyolysis episodes requiring hospitalization, one was diagnosed by newborn screening, while seven subjects presented in childhood or adulthood with exercise intolerance and there was no history of cardiac complications. One subject with VLCAD presented in infancy with severe cardiac complications that resolved after diagnosis and treatment but cardiac function had subsequently deteriorated and was ab- normal at the time of enrollment into this study. Of the 11 subjects with CPT2 deficiency, all had a history of re- current rhabdomyolysis episodes requiring hospitalization, one was diagnosed by newborn screening, and the remaining subjects presented symptomatically in childhood or adulthood with exercise intolerance. None of the patients with CPT2 had a history of cardiac disease. All 32 subjects completed the protocol. Based on 3-day diet record analysis, subjects consumed approximately 16% and 14% of total reported energy intake from C7 and C8 respectively during the 4-month treatment period. Based on oil dispensed–oil returned, subjects consumed 74% and 76% of prescribed study oil from the C7 and C8 respectively.
Table 1.
Subject Characteristics
| Age (Years) | Sex | Diagnosis | Mutations | FAO probe in fibroblasts | Body Wt (kg) | BMI (kg/m2) | % of kcals from triheptanoin | % prescribed dose consumed | age at presentation | Cardiac complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Triheptanoin (C7) treatment group (N = 16) | ||||||||||
| 7 | F | LCHAD/TFP | c.1528G > C/c.1528G > C | 24.2 | 16.97 | 16.2 | 92.6% | NBS | N | |
| 7 | M | LCHAD/TFP | c.1528G > C/c.703C > T | 18.6 | 14.21 | 13.0 | 108.6% | NBS | N | |
| 7 | F | VLCAD | c.1619 T > C/c.1707_1716dupGACGGGGCC | 26.4 | 17.42 | 15.7 | 112.8% | NBS | N | |
| 11 | M | LCHAD/TFP | c.1528G > C/ c.1528G > C | 64.5 | 23.86 | 15.0 | 73.1% | I | Y | |
| 16 | M | LCHAD/TFP | c.1150–1G > T/ c.208T > C | 80.7 | 24.36 | 17.4 | 71.0% | C | N | |
| 21 | F | CPT2 | Common mutations not detected | 7% CPT2 | 63.2 | 23.70 | 11.3 | 73.7% | C | N |
| 23 | M | LCHAD/TFP | c.1528G > C/? | ↑OHACs | 65.5 | 22.09 | 19.3 | 52.3% | I | Y |
| 24 | F | LCHAD/TFP | c.1528G > C/c.479–482 T AGC > AATA | 55.2 | 21.56 | 16.8 | 78.7% | I | Y | |
| 29 | F | LCHAD/TFP | c.1528G > C/ c.1528G > C | 63.8 | 23.55 | 19.6 | 41.4% | NBS | N | |
| 33 | M | VLCAD | c.1322G > A/ c.1837C > T | 92.1 | 31.31 | 18.0 | 71.5% | 1 | Y | |
| 36 | F | VLCAD | No DNA analysis available | ↑LCACs | 68.1 | 24.21 | 15.8 | 37.8% | A | N |
| 39 | F | CPT2 | No DNA analysis available | 20% CPT2 | 93.8 | 32.65 | 19.3 | 74.5% | A | N |
| 39 | M | VLCAD | c.343delG/c.1244C > T | 88.0 | 27.50 | 22.0 | 82.8% | A | N | |
| 41 | F | CPT2 | c.338C > U/c.1238_1239delAG | 63.5 | 26.50 | 14.7 | 43.0% | A | N | |
| 41 | F | CPT2 | c.338C > T/c.1511C > T | 81.4 | 28.17 | 16.5 | 69.5% | A | N | |
| 64 | F | CPT2 | c.338C > T/ c.338C > T | 45.1 | 18.53 | 15.3 | 99.8% | A | N | |
| 27 | 62 | 24 | 16.62 | 73.94% | ||||||
| 16 | 24 | 5 | 2.66 | 22.59% | ||||||
| Trioctanoin (C8) treatment group (N = 16) | ||||||||||
| 8 | F | LCHAD/TFP | c.1528G > C/? | ↑OHACs | 21.9 | 14.91 | 15.20 | 79.7% | NBS | N |
| 9 | M | LCHAD/TFP | c.1528G > C/ c.1528G > C | 34.3 | 18.68 | 15.20 | 107.3% | NBS | N | |
| 9 | M | CPT2 | c.338C > T/c.340 + 3A > T | 2% CPT2 | 29.8 | 16.57 | 10.50 | NA | I | N |
| 11 | F | LCHAD/TFP | c.1528G>C/ c.1528G>C | 49.0 | 23.18 | 15.30 | 92.1% | NBS | N | |
| 15 | M | CPT2 | c.338C > T / c.1239_1240delGA /c.1342 T > C | 72.8 | 26.42 | NA | NA | C | N | |
| 16 | F | CPT2 | c.338C > T/ c.1666 1667delTT | 69.3 | 24.70 | 16.50 | 84.3% | C | N | |
| 17 | F | LCHAD/TFP | c.1528G > C/ c.1528G > C | 65.5 | 23.07 | 17.80 | 84.9% | I | N | |
| 17 | F | LCHAD/TFP | c.901G > A/? | 15% LCHAD in sibling | 44.7 | 18.49 | 16.50 | 30.7% | NBS | N |
| 19 | M | CPT2 | c.338C > T and c.1666_1667delTT | 91.7 | 29.60 | 5.60 | 86.5% | A | N | |
| 24 | F | VLCAD | c.1500_1502delCTT / c.1500_1502delCTT | 54.4 | 21.65 | 18.00 | 58.4% | C | N | |
| 24 | F | CPT2 | c.338C > T/? | 16% CPT2 | 91.4 | 32.23 | 15.10 | 55.8% | C | N |
| 27 | F | VLCAD | c.898A > C/ c.1097G > A | ↑LCACs | 82.1 | 27.43 | 18.50 | 89.0% | C | N |
| 39 | F | VLCAD | c.637G > A/ c.1065_1067delCAT | 61.1 | 20.37 | 15.70 | 75.9% | A | N | |
| 42 | M | VLCAD | c.637G > A/ c.1065_1067delCAT | 113.0 | 31.47 | 15.40 | 61.8% | A | N | |
| 43 | M | VLCAD | c.694G > A/c.1388G > A | 94.8 | 30.23 | NA | 81.6% | A | N | |
| 43 | F | CPT2 | c.338C > T/c.1239 > 1240delGA | 97.1 | 34.53 | 12.30 | 70.3% | A | N | |
| 22.69 | 67.06 | 24.60 | 14.83 | 75.59% | ||||||
| 12.64 | 26.80 | 5.96 | 3.40 | 19.04% | ||||||
Individual subject characteristics for each participant include age in years, sex (Sex; F = female; M = male), long-chain FAO diagnosis (LCHAD/TFP = long-chain hydroxyacylCoA dehydrogenase/trifunctional protein, VLCAD = very long-chain acylCoA dehydrogenase, CPT2 = carnitine palmitoyltransferase 2 deficiencies), mutations note the change in the respective cDNA of the gene corresponding to the protein listed under diagnosis. Mutations:? = no 2nd mutation was identified after sequencing all the exons of the gene; for CPT2, common mutations not detected indicates sequencing for the six common mutations in CPT2 was completed but complete sequencing of the gene was not performed. FAO probe study in fibroblasts = results of FAO probe studies in cultured skin fibroblasts either as a calculated percent of enzymatic activity compared to normal (%) or the presence of disease specific aclycarnitines when cells were incubated with palmitate; LCACs = long-chain acylcarnitines, OHACs = long-chain hydroxyacylcarnitines. Body weight in kg, body mass index (BMI; kg/m2),%of kilocalories (kcals) consumed from the study oil based on 3-day diet diary analysis for each group, and % of prescribed dose consumed = oil returned/oil dispensed x100. Age at presentation is the age when the diagnosis was suspected; NBS = newborn screening; I = infancy between 0 and 2 years; C = childhood 2–10 years; A = adulthood >10 years. Cardiac complications = the subject had some cardiac manifestation of disease at presentation or anytime in their lifetime but not during the study described here; Y = yes; N = no. Only one subject had decreased cardiac function during the study. Mean for age, body weight, BMI, and % of kcals from study oil are provided below the respective column for each group and the standard deviation is listed below the mean
Left ventricular ejection fraction (LVEF) increased and left ventricular wall mass (LVWM) decreased from baseline among subjects treated with C7
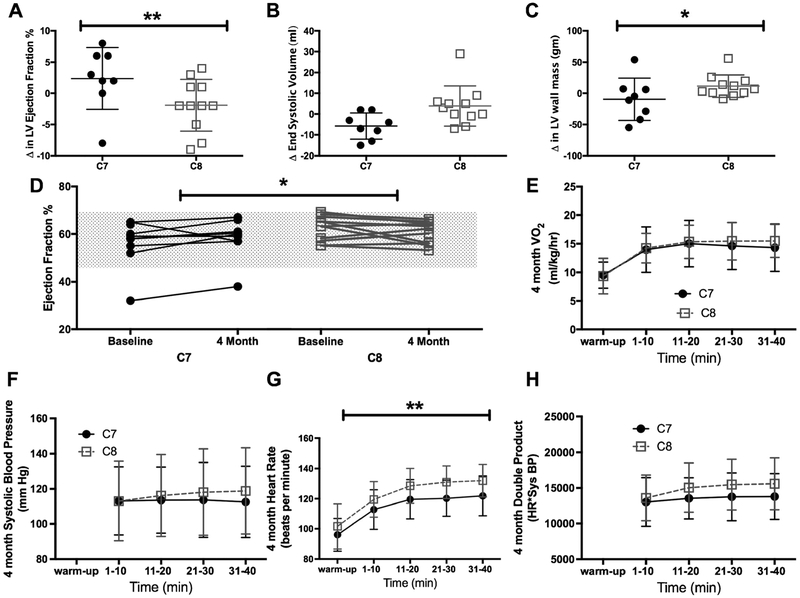
Echocardiographic data was only available for 10 subjects in the C7 and 11 subjects in the C8 group due to a logistic change in the protocol from cardiac MRI to echocardiogram after the first seven subjects were enrolled. Echocardiograms were uninterpretable in four subjects due to technical difficulties. Despite these limitations, relative change in left ventricular ejection fraction (LVEF; 4- month/baseline) differed significantly (Fig. 2a; p = 0.046) between groups, with relative LVEF being 7.4% greater for those given C7 compared to relative LVEF for those given C8 (95% of baseline CI: −0.1—15% greater). Left ventricular end systolic volume (LVESV) at 4-months was 7.0% lower than baseline for those given C7 and was 12% higher than baseline for those given C8, a marginal (Fig. 2b; p = 0.114) difference in relative change between groups. LV wall mass decreased after 4-months for those given C7 (Fig. 2c; 8% decrease, p = 0.270) but increased for those given C8 (15% increase; p = 0.059), with a significant difference in relative change of LV wall mass between groups (95% CI: 64.8—99.0%; p = 0.041). All but one subject had a normal EF% at baseline (Fig. 2d) and the majority of the observed changes occurred within the nor- mal range for LVEF. The analysis was run without the one participant with abnormal cardiac function at baseline and the relative change was not substantially different from the analysis using the full sample so the results of the full sample are presented.
Fig. 2.
Echocardiogram and treadmill results. Data are presented as individual change over 4 months, mean and 95% confidence intervals. a) Left ventricular (LV) ejection fraction increased after 4 months of triheptanoin (C7) supplementation but did not change in the trioctanoate (C8) group. b) End systolic volume decreased and c) there was a decrease in LV wall mass in the C7 treated group compared to the C8 group. d) All but one participant had normal cardiac function at baseline and at the end of 4 months of treatment (gray shaded area indicates the normal range). Individual participant EF at baseline and 4 months of treatment is shown. More participants in the C7 group had an increase in EF compared to the random increase and decrease in EF observed in the C8 group within the normal test/retest variability of resting echocardiograms. Data are presented as mean ± standard deviation e) Ventilation during the moderate intensity treadmill was similar between treatment groups suggesting that workload was similar. f) Systolic blood pressure was not different but g) Heart rate was significantly lower in subjects treated with C7 compared to subjects in the C8 group H) double product was not significantly different between groups
Maximum heart rate during a moderate intensity treadmill exercise test decreased among subjects treated with C7 (Fig. 2g; change from baseline = 0.04)
Each subject underwent two moderate intensity exercise trials, one at baseline and again after four months treatment. Total work performed, and therefore oxygen consumption, was kept constant in both trials. Maximum heart rate after 4 months treatment was on average 6.98 beats per minute (95% CI: 0.34—13.63 bpm, p = 0.040) lower in those subjects treated with C7 compared with the C8 group. Systolic blood pressure remained constant over the 45 min of the exercise trial, with no statistically significant effects attributable to time, treatment, or the combination of these factors (p = 0.501; omnibus test) (Fig. 2f). The peak double product (systolic blood pressure multiplied by heart rate), a marker of cardiac workload, was not significantly different between groups during the 4-month exercise test (Fig. 2h; time X treatment interaction p = 0.12).
There was no significant difference between treatment groups in musculoskeletal symptoms of a LC-FAO disorder over the 4-month treatment period
Eleven of 16 subjects (69%) in the C7 group, and 10 of 16 subjects (63%) in the C8 group reported intermittent muscle pain, or elevated creatine kinase (CK) during the treatment consistent with a LC-FAOD (Table 2). In addition, there were seven hospitalizations for acute rhabdomyolysis in both the C7 and the C8 treatment groups (Table 2) and there was no difference in length of stay or peak CK concentration during those admissions (data not shown).
Table 2.
Adverse events
| Randomization | ||||
|---|---|---|---|---|
| C7 | C8 | |||
| # of events | # of subjects | # of events | # of subjects | |
| Expected adverse events | ||||
| Diarrhea/loose stools/steatorrhea | 9 | 5 | 12 | 6 |
| Gastrointestinal upset | 24 | 11 | 38 | 12 |
| Emesis/vomiting | 7 | 6 | 0 | 0 |
| Musculoskeletal pain/cramping/elevated CPK | 16 | 11 | 18 | 10 |
| Rhabdomyolysis | 7 | 5 | 7 | 4 |
| Fatigue/lethargy | 3 | 3 | 2 | 2 |
| Unexpected adverse events | ||||
| Headache | 17 | 5 | 7 | 3 |
| Viral illness | 22 | 15 | 17 | 11 |
| Localized pain not associated with rhabdomyolysis | 5 | 4 | 2 | 2 |
| Dermatitis | 1 | 1 | 4 | 4 |
| Hemorrhoids | 1 | 1 | 0 | 0 |
| Depression | 1 | 1 | 0 | 0 |
| Constipation | 1 | 1 | 0 | 0 |
| Avulsion of nail bed on left thumb | 1 | 1 | 0 | 0 |
| Vertigo | 1 | 1 | 0 | 0 |
| Broken front tooth | 1 | 1 | 0 | 0 |
Expected and unexpected adverse events are reported by randomization group (C7 and C8), as the number of each event (# of events) and the number of subjects (# of subjects) who experienced that adverse event
There was no difference in the rate of phosphocreatine recovery, a measure of ATP synthesis, after a bout of acute phosphocreatine depleting exercise in the lower leg
Eight adult subjects in the C7 group and seven adults in the C8 group completed a defined exercise protocol involving repetitive right leg (Pittsburgh) or ankle (OHSU) flexion/extension against resistance while monitoring 31P spectra of the isolated working muscle, an indirect measure of muscle ATP content. Isolated exercise produced a significant decrease in phosphocreatine but the magnitude of decrease, and the rate of recovery with rest were not different between treatment groups (PCr, Suppl. Fig. 2A). The rate constants of phosphocreatine synthesis (kPCr) measured during the recovery phase for each subject at baseline and after 4 months treatment are shown in Suppl. Fig. 2B; the change in kPCr from baseline to end of study was not different between treatment groups.
Body weight, body composition, and energy expenditure did not change from baseline nor were they different between groups
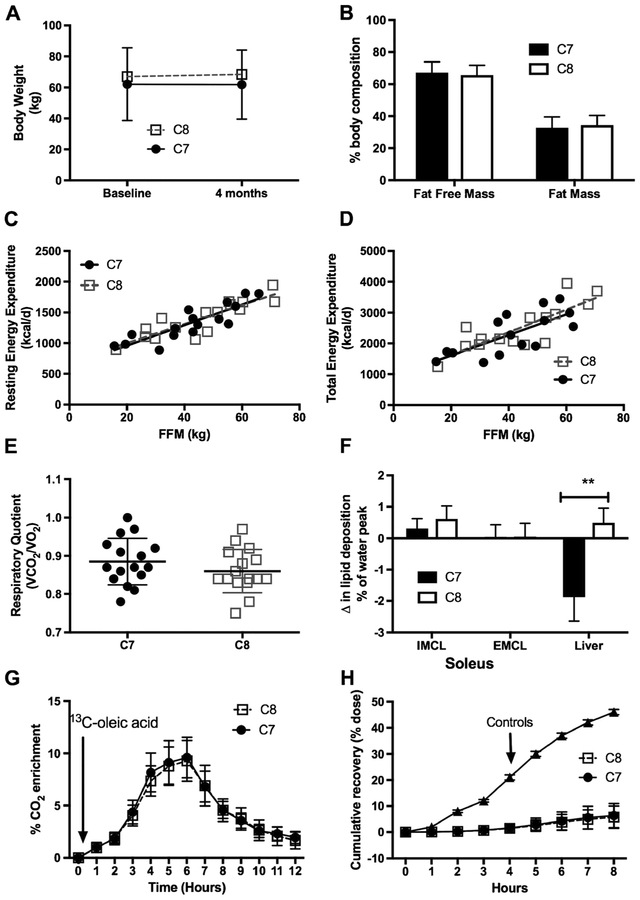
Subjects did not gain weight over the treatment period (Fig. 3a) and there was no difference in body weight or body composition between treatment groups (Fig. 3b). Resting energy expenditure and respiratory quotient were similar between groups at baseline (data not shown) and after 4 months (Fig. 3c and e) of either C7 or C8 treatment. There was no difference in total energy expenditure between groups (Fig. 3d) and no significant change from baseline (data not shown). Oxidation of a 1-13C-oleic acid was similar between groups of subjects with a LC-FAOD at baseline (data not shown) and at 4 months (Fig. 3g) but dramatically lower than values published in normal control subjects (Bergouignan et al 2013) (Fig. 3h), illustrating the effect of the disorder on overall substrate oxidation. Hepatic lipid deposition differed significantly between groups (p = 0.013, test of inter- action), with those given C7 averaging a 1.86 (95% CI: 0.56—3.16, p = 0.007) percent of the water peak decrease in hepatic lipid at 4 months compared to baseline (Fig. 3f), while those given C8 averaged a 0.49 percentage point increase compared to baseline (95% CI: 0.8 — 1.75 percentage point increase; p = 0.428).
Fig. 3.
Body composition and energy expenditure. Data are presented as mean ± standard deviation. Subjects were instructed to consume 20% of their estimated total energy needs from the assigned study oil over the 4-month treatment period. a) There was no difference between groups and no change in body weight over the course of the treatment period. Fat free mass (FFM) and fat mass (FM) are similar between groups at baseline (not shown) and at the end of study (b). c) Resting energy expenditure per kg of fat free mass (FFM) and d) Total energy expenditure per kg of FFM was not different between groups at the end of study. e) Respiratory quotient (VCO2/VO2) was similar between groups. RQ ranged from 0.8 to 1.0 after 10-h overnight fast suggesting participants were oxidizing a mix of carbohydrates and fat at rest. f) Lipid deposition of soleus intra-myocellular (IMCL) and extra-myocellular (EMCL) did not change over the course of the treatment period but liver lipid deposition significantly decreased in the C7 group compared to C8. g) There was no difference in oxidation of the 13C–oleic acid between groups. Label recovery peaked in all subjects about 4 to 6 h after the meal and returned toward baseline 12 h after the meal. There was no difference in RQ or oleic acid oxidation after the moderate intensity treadmill between treatment groups performed between 7 and 8 h after the oral load. h) Total fat oxidation was dramatically lower among subjects with an FAO disorder compared to published normal controls (18)
Blood markers of metabolism, metabolic control, and substrate oxidation were not different between groups
Glucose concentrations were similar between groups and varied with the normal range after a mixed meal and after exercise (Suppl. Fig. 3a); insulin concentrations were with- in expected ranges and not different between the two treatment groups (data not shown). Serum free fatty acid concentrations increased after an overnight fast, decreased with feeding, and rose with exercise as expected, but there was no difference between groups (Suppl. Fig. 3b). Lactate concentrations were within normal range and similar be- tween groups (Suppl. Fig. 3c). Total serum ketones (sum of 4 and 5 carbon ketones) rose minimally after meals but increased substantially after C7 or C8 pretreatment and 45 min of moderate intensity exercise (Suppl. Fig. 3d), but there was no significant difference between treatment groups. Five carbon ketones are produced only from the oxidation of odd-chain fatty acids such as heptanoate. There was very little rise in 4 or 5 carbon ketones after a mixed meal. Four-carbon ketones rose in the C8 group and 5-carbon ketones rose in the C7 group after exercise con- sistent with the pre-exercise supplement (Suppl. Fig. 3e & f). Free carnitine was similar between groups and stable over time (data not shown). Acetylcarnitine was similar between groups and rose after exercise; the change in acetylcarnitine correlated with changes in ketones (Suppl. Fig. 4a). Odd-chain acylcarnitines including C3, C5, and C7 rose after the meal and rose again after exercise in the participants treated with C7 (Suppl. Fig. 4b). The even chain acylcarnitines including C4, C6, C8, and C10 were similar between groups (Suppl. Fig. 4c). The long-chain acylcarnitines, including C14:0, C14:1, C16:0, C16:1, C18:0, C18:1, and C18:2, a marker of partial FAO and metabolic control, were similar between groups, decreased after a meal, and rose after exercise (Suppl. Fig. 4d).
Adverse effects related to C7 or C8 supplementation were minor and predominately consisted of gastrointestinal upset
There was no significant difference between treatment groups in the number of subjects who complained of GI adverse effects or in the number of events reported (Table 2). Participants also reported intermittent episodes of musculoskeletal pain, fatigue, and hospitalization for rhabdomyolysis consistent with the complications of a LC-FAODs; the incidence of these adverse events was not different between the two treatment groups.
Discussion
The heart has high energy demands and uses a variety of energy substrates including glucose, ketones, branched chain amino acids, and fatty acids but a healthy adult heart obtains 50–70% of its ATP from fatty acid oxidation (Lopaschuk et al 2010; Lopaschuk and Ussher 2016). Impaired energy production and in particular, fatty acid oxidation, is associated with heart failure, ischemic reperfusion injury, diabetic cardiomyopathy, and LC-FAODs such that the failing heart has been called “the engine without fuel” (Neubauer 2007; Brittain et al 2016; Trico et al 2016).
As such, cardiomyopathy is a major life threatening complication in patients with LC-FAODs. We observed a statistically significant increase in cardiac efficiency, both at rest and during moderate exercise, following 4-months of C7 supplementation compared with C8 treatment. While the baseline resting LVEF was within the normal range for both treatment groups, we observed a significant increase in LVEF following 4 months of treatment with C7 as compared with C8. Moreover, with only 4 months of C7 supplementation, there was a significant decrease in the left ventricular wall mass, suggesting reverse cardiac remodeling. During exercise, C7 treated subjects were able to accomplish the same degree of aerobic work compared with baseline, but at a significantly lower maximal heart rate. Conversely, subjects in the C8 group experienced no decrement in heart rate. These data imply improved cardiorespiratory fit- ness after 4-months of C7 supplementation. The observed differences are remarkable given that changes in cardiac structure and function occurred over a relatively short treatment period (4-months), and with a very small difference in the form of the triglyceride consumed—one group consuming triheptanoin (C7) and the control group consuming trioctanoin (C8)—a difference of only one carbon per fatty acid.
Although statistically significant, the clinical relevance of changes in cardiorespiratory fitness within the normal range is minimal for the majority of participants who enrolled in the study who had normal cardiac function. However, previously published reports from open label and case review studies of C7 supplementation among patients with poor cardiac function corroborate our results and have reported improvement in cardiac function (Roe and Brunengraber 2015; Vockley et al 2015). In one recent uncontrolled study of 10 cardiomyopathy cases, young patients with a LC-FAOD showed dramatic improvements in cardiac function with C7 treatment (Vockley et al 2016). Although cardiac function was normal at baseline in our study population, a small portion of patients with LC- FAOD present in the newborn or infant period with severe cardiomyopathy, and are at high risk for significant morbidity and mortality (Brown-Harrison et al 1996; Martins et al 1996; Saudubray et al 1999). In addition, adolescents and young adults may also present with metabolic decompensation episodes, associated cardiomyopathy, and early demise (Parini et al 1998; Bonnet et al 1999). Given the positive alterations in cardiac function we report, it is possible that C7 treatment could be associated with even greater beneficial effects in patients with symptomatic cardiomyopathy. Further studies over a longer period of time are needed to evaluate long- term effects on cardiac metabolism.
We observed neither a decrease in the incidence of incident rhabdomyolysis nor improved post exercise muscle phosphocreatine recovery with C7 treatment, suggesting that there was no measurable short-term effect on skeletal muscle energy metabolism. Recurrent rhabdomyolysis is the most common and frequent complication of LC-FAODs and C7 has reportedly been beneficial for reducing rhabdomyolysis in some open labeled trials (Roe and Brunengraber 2015). However, a recent retrospective analysis also suggested that there may be no significant change in rhabdomyolysis rates with C7 treatment (Vockley et al 2015), and C7 alone may be insufficient to prevent musculoskeletal symptoms including rhabdomyolysis caused by LC-FAODs.
Administration of higher doses of C7 or MCT oil than we reported have been associated with substantial weight gain (Roe et al 2002; Roe et al 2008). In our trial, there was no observed weight gain or change in overall body composition during the course of treatment, indicating that the oil supple- mentation at 15–20% of total energy did not lead to excess energy intake. This finding is particularly important for long- term health outcomes in a patient population that has substantial barriers to losing weight due to limited capacity to oxidize stored body fat and impaired exercise tolerance.
We have previously reported lower total energy expenditure among subjects with LCHAD deficiency compared to healthy controls (Gillingham et al 2013) and had hypothesized that if C7 led to improved ATP production, subjects in this study treated with C7 would be more active and show an increase in their TEE in comparison to those receiving C8. However, we found no measurable effect upon TEE in either treatment group. Nor was there a difference in muscle phosphocreatine utilization or recovery during exercise following C8 or C7 supplementation. The 31P–phosphocreatine recovery study was designed to indirectly evaluate muscle ATP synthesis, but the acute nature of our testing protocol with resistance exercise for only 2 min may have been of insufficient duration or exercise type to fully engage muscle FAO and may have been inadequate to detect significant differences between treatment groups. Indeed, a recent published study evaluating PCr recovery rates among subjects with VLCAD deficiency and controls after 45 min of exercise observed that while PCr depletion was greater among subjects with VLCAD, the rate of recovery was similar to controls (Diekman et al 2016). These data suggest that the rate of PCr recovery is normal among subjects with a LC-FAOD and may not be expected to improve with treatment.
Limitations to this study include a relatively short follow up treatment time, as effects of C7 may require a longer duration to become fully manifest. Also, we had no method to measure any direct biochemical effect upon muscle anaplerosis. Finally, subjects consumed less than the pre- scribed amount of oil, averaging only about 15% of total energy from either C7 or C8. It is possible that other subtle differences in physiologic parameters would have been detected if the study had been a randomized cross-over design rather than an RCT. Cross-over designs can decrease variation and minimize sample size but they are also prone to inadequate wash-out and temporal exposure bias. Our statistical analysis looked at change from baseline within a subject to minimize the variation across a broad age spectrum and the RCT design is considered the most rigorous study design to address the hypothesis.
In conclusion, this is the largest double-blinded RCT con- ducted in patients with FAODs to date. The study provides a rigorous, comprehensive evaluation of the clinical effects of C7 compared to an isocaloric dose of C8, the current standard of care for treatment of LC-FAODs. We show that with only a four-month treatment period with C7, there is a significant improvement in cardiac structure and function, both at rest and during exercise. We further demonstrate advantageous cardiac modeling, and improved cardiorespiratory fitness. One should bear in mind that although the changes were sta- tistically significant, the clinical relevance in a patient population with baseline normal ejection, is not yet known. Potential beneficial effects of C7 treatment should also be explored in other models of cardiac dysfunction including adults with heart failure from various causes other than LC- FAODs (Nguyen et al 2015; Des Rosiers et al 2011; Taegtmeyer 2016).
Supplementary Material
Acknowledgements
This study was funded by the Food & Drug Administration, Orphan Drug Development Program, Grant FD038950. The authors sincerely thank all of the subjects who participated in this trial. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1TR000128 and UL1TR001857. JV was supported in part by NIH grant R01 DK78775. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding This study was funded by the Food & Drug Administration, Orphan Drug Development Program, Grant FD038950. Research report- ed in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1TR000128 and UL1TR001857. JV was sup- ported in part by NIH grant R01 DK78775.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10545-017-0085-8) contains supplementary material, which is available to authorized users.
Clinical Trial Registration:
Clinicaltrials.gov NCT01379625.
Conflict of interest Melanie B. Gillingham, PhD and Cary O Harding, MD received a research grant for a murine study from Ultragenyx Inc.
S. B. Heitner, J. Martin, S. Rose, A. Goldstein, A. H. El-Gharbawy, S. Deward, M. R. Lasarev, J. Pollaro, J. P. DeLany, L. J. Burchill, B. Goodpaster, J. Shoemaker, D. Matern, and J. Vockley declare that they have no conflict of interest.
Note: Ultragenyx Pharmaceutical was not involved with the design or execution of this study and did not provide triheptanoin for the trial.
References
- Arnold GL, Van Hove J, Freedenberg D et al. (2009) A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 96:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend AM, Harding CO, Shoemaker JD et al. (2012) Substrate oxida- tion and cardiac performance during exercise in disorders of long chain fatty acid oxidation. Mol Genet Metab 105:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Rinaldo P, Wilcken B, Pass KA, Watson MS, Wanders RJ (2012) Newborn screening for metabolic disorders: how are we doing, and where are we going? Clin Chem 58:324–331 [DOI] [PubMed] [Google Scholar]
- Bergouignan A, Momken I, Lefai E et al. (2013) Activity energy expen- diture is a major determinant of dietary fat oxidation and trafficking, but the deleterious effect of detraining is more marked than the beneficial effect of training at current recommendations. Am J Clin Nutr 98:648–658 [DOI] [PubMed] [Google Scholar]
- Bonnet D, Martin D, Pascale De L et al. (1999) Arrhythmias and conduc- tion defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 100:2248–2253 [DOI] [PubMed] [Google Scholar]
- Brittain EL, Talati M, Fessel JP et al. (2016) Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hy- pertension. Circulation 133:1936–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Harrison MC, Nada MA, Sprecher H et al. (1996) Very long chain acyl-CoA dehydrogenase deficiency: successful treatment of acute cardiomyopathy. Biochem Mol Med 58:59–65 [DOI] [PubMed] [Google Scholar]
- DeLany JP, Windhauser MM, Champagne CM, Bray GA (2000) Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 72:905–911 [DOI] [PubMed] [Google Scholar]
- DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH (2013) High energy expenditure masks low physical activity in obesity. Int J Obes 37:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLany JP, Dube JJ, Standley RA et al. (2014) Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the ab- sence of obesity. J Clin Endocrinol Metab 99:4307–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC (2011) Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res 90:210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman EF, Visser G, Schmitz JP et al. (2016) Altered energetics of exercise explain risk of rhabdomyolysis in very long-chain acyl- CoA dehydrogenase deficiency. PLoS One 11:e0147818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham MB, Connor WE, Matern D et al. (2003) Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficien- cy. Mol Genet Metab 79:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham MB, Scott B, Elliott D, Harding CO (2006) Metabolic control during exercise with and without medium-chain triglycerides (MCT) in children with long-chain 3-hydroxy acyl-CoA dehydro- genase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab 89:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham MB, Harding CO, Schoeller DA, Matern D, Purnell JQ (2013) Altered body composition and energy expenditure but nor- mal glucose tolerance among humans with a long-chain fatty acid oxidation disorder. Am J Physiol Endocrinol Metab 305:E1299–E1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M, Hoffmann GF, Matern D (2010) Newborn screening for dis- orders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 33:521–526 [DOI] [PubMed] [Google Scholar]
- Lindner M, Gramer G, Haege G et al. (2011) Efficacy and outcome of expanded newborn screening for metabolic diseases–report of 10 years from south-West Germany. Orphanet J Rare Dis 6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR (2016) Evolving concepts of myocardial en- ergy metabolism: more than just fats and carbohydrates. Circ Res 119:1173–1176 [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258 [DOI] [PubMed] [Google Scholar]
- Martins E, Costa A, Silva E et al. (1996) Lethal dilated cardiomyopathy due to long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 19:373–374 [DOI] [PubMed] [Google Scholar]
- Neubauer S (2007) The failing heart–an engine out of fuel. N Engl J Med 356:1140–1151 [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T (2015) Triheptanoin alleviates ventricular hypertrophy and improves myo- cardial glucose oxidation in rats with pressure overload. J Card Fail 21:906–915 [DOI] [PubMed] [Google Scholar]
- Parini R, Menni F, Garavaglia B et al. (1998) Acute, severe cardiomyop- athy as main symptom of late-onset very long-chain acyl-coenzyme a dehydrogenase deficiency. Eur J Pediatr 157:992–995 [DOI] [PubMed] [Google Scholar]
- Rinaldo P, Matern D, Bennett MJ (2002) Fatty acid oxidation disorders.Annu Rev Physiol 64:477–502 [DOI] [PubMed] [Google Scholar]
- Roe CR, Brunengraber H (2015) Anaplerotic treatment of long-chain fat oxidation disorders with triheptanoin: review of 15 years experience. Mol Genet Metab 116:260–8. doi: 10.1016/j.ymgme.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CR, Mochel F (2006) Anaplerotic diet therapy in inherited metabolic disease: therapeutic potential. J Inherit Metab Dis 29:332–340 [DOI] [PubMed] [Google Scholar]
- Roe CR, Sweetman L, Roe DS, David F, Brunengraber H (2002) Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest 110:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CR, Yang BZ, Brunengraber H, Roe DS, Wallace M, Garritson BK (2008) Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology 71:260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudubray JM, Martin D, de Lonlay P et al. (1999) Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis 22:488–502 [DOI] [PubMed] [Google Scholar]
- Smith EH, Matern D (2010) Acylcarnitine analysis by tandem mass spec- trometry. Curr Protoc Hum Genet 17(17.18):11–20. doi: 10.1002/0471142905.hg1708s64 [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Lindner M, Santer R et al. (2009) Treatment recommen- dations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis 32:498–505 [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Bastin J, Gillingham M, Morris A, Wijburg F, Wilcken B (2010) Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis 33:555–561 [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H (2016) Failing heart and starving brain: ketone bodies to the rescue. Circulation 134:265–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trico D, Baldi S, Frascerra S et al. (2016) Abnormal glucose tolerance is associated with a reduced myocardial metabolic flexibility in patients with dilated cardiomyopathy. J Diabetes Res 2016: 3906425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockley J, Marsden D, McCracken E et al. (2015) Long-term major clin- ical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment–a ret rospective chart review. Mol Genet Metab 116:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockley J, Charrow J, Ganesh J et al. (2016) Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain- fatty acid oxidation disorders. Mol Genet Metab 119:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sango-Jordan M, Caggana M (2014) Acute care utilization for inherited metabolic diseases among children identified through new- born screening in New York state. Genet Med 16:665–670 [DOI] [PubMed] [Google Scholar]
- Wilcken B (2010) Fatty acid oxidation disorders: outcome and long-term prognosis. J Inherit Metab Dis 33:501–506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.