Abstract
The number of procedures performed with robotic surgery may exceed one million globally in 2018. The continual lack of haptic feedback, however, forces surgeons to rely on visual cues in order to avoid breaking sutures due to excessive applied force. To mitigate this problem, the authors developed and validated a novel grasper-integrated system with biaxial shear sensing and haptic feedback to warn the operator prior to anticipated suture breakage. Furthermore, the design enables facile suture manipulation without a degradation in efficacy, as determined via measured tightness of resulting suture knots. Biaxial shear sensors were integrated with a da Vinci robotic surgical system. Novice subjects (n =17) were instructed to tighten 10 knots, five times with the Haptic Feedback System (HFS) enabled, five times with the system disabled. Seven suture failures occurred in trials with HFS enabled while seventeen occurred in trials without feedback. The biaxial shear sensing system reduced the incidence of suture failure by 59% (p = 0.0371). It also resulted in 25% lower average applied force in comparison to trials without feedback (p = 0.00034), which is relevant because average force was observed to play a role in suture breakage (p = 0.03925). An observed 55% decrease in standard deviation of knot quality when using the HFS also indicates an improvement in consistency when using the feedback system. These results suggest this system may improve outcomes related to knot tying tasks in robotic surgery and reduce instances of suture failure while not degrading the quality of knots produced.
Keywords: Haptic feedback, Shear sensor, Robotic surgery, Force sensor, Haptics, Da Vinci
1. Introduction
Since FDA approved in 2000, robot-assisted minimally invasive surgery (RMIS) has been favorably adopted and even preferred for many surgical procedures. RMIS improves both the accuracy and dexterity of the operating surgeon, thus resulting in perceived superiority in comparison with minimally invasive and open surgical techniques (Barbash et al. 2014). Over 18 years later, however, RMIS still does not provide an effective form of tactile or haptic feedback to the surgeon. The absence of haptic feedback may negatively contribute to surgical outcome and become a defining weakness of RMIS systems if not corrected (Puangmali et al. 2008).
Several studies have demonstrated the mechanical superiority of knots tied manually (i.e., with hands) in comparison to knots tied using either the da Vinci robot or laparoscopically (Kitagawa et al. 2005; Livermore et al. 2010). Moreover, suture breakages are frequent during the use of contemporary robotic systems. This result is largely attributed to the combination of large forces concentrated at the tips of the instrument graspers and a limited depth perception afforded to the user (Cartmill et al. 1999; Marucci et al. 2000; Bhatia and Tandon 2005; De et al. 2016). Intraoperatively, suture failure may be dangerous, complicate the procedure, and even necessitate execution of corrective measures (LeBlanc 2004). In the postoperative setting, the patient may encounter additional harmful from failed sutures due to resulting complications (e.g., peritonitis due to bowel anastomosis disruption (Anup and Balasubramanian 2000) or hemorrhage from vascular anastomosis (Melinek et al. 2004)). On the other hand, a use of minimal force during suture tying may be just as harmful. For example, insufficient knot tension increases the possibility of post-operative knot slippage and similarly results in patient harm. Finally, this need for an assistive technology that warns surgeons of impending suture failure is increasing due to the burgeoning popularity and widespread adoption of RMIS (Hirano et al. 2010; Diks et al. 2007).
Previous attempts for predicting suture breakage have measured suture strain through visual cues and image analysis, but these approaches are limited due to their dependence on image quality and the presence of these specific cues (Martell et al. 2011). Other approaches utilized a visual overlay to relay metrics of force and met resistance due to the increased cognitive load required of the user. Moreover, in certain applications, a visual representation is not as effective as force feedback for reducing the amount of applied force (Vitense et al. 2003; Reiley et al. 2008; Gwilliam et al. 2009).
In prior work, our group designed and validated a vibrotactile feedback system using an uniaxial commercial piezoresistive sensor (Abiri et al. 2018). A limitation of the uniaxial system is that the surgeon rarely pulls the suture in only one direction. More specifically, the actual tension in the suture is a combination of bi-axial shear and normal forces applied to the sensors. In this work, the authors seek to improve their prior design and develop a bi-axial shear sensor. This system is designed for integration onto the end effectors of robotic surgical instruments and detects the applied force to sutures. Thus, a suture breakage warning system is constructed to reduce occurrences of suture failure by providing vibrotactile feedback when the measured tension approaches the suture’s failure load. We hypothesize that such a system will reduce the occurrence of suture failures without negatively affecting the quality of knot produced during robot-assisted surgery.
2. Methods and procedures
A triaxial, miniaturized, high-resolution, cost-effective capacitive sensor with minimal wiring was constructed to meet the existing constraints for tactile sensing in a minimal invasive surgical system (Dahiya and Valle 2013; Yousef et al. 2011). The sensor system can resolve forces as small as 275 mN or better in both x- and y- shear directions for forces ranging from 0 to 20 N, and the system can transmit this data with a latency under 42 ms. The sensor system is coupled to a pneumatic actuator at the users’ fingertips, with the total system latency less than 110 ms.
The single-sided capacitive sensor is designed for facile integration with the surgical system. The sensor comprises polydimethylsiloxane (PDMS) as an elastic dielectric material embedded between two layers of gold (Au) conducting plates and is manufactured using batch fabrication processes that can simultaneously produce hundreds of sensors with high precision (Fig. 1). The key component of our material design is the microstructuring of the thin film of the dielectric elastomer PDMS, which is well known for its outstanding elasticity and biomedical compliance with both human tissue (Mi et al. 2006) and living cells (Balaban et al. 2001).
Fig. 1.
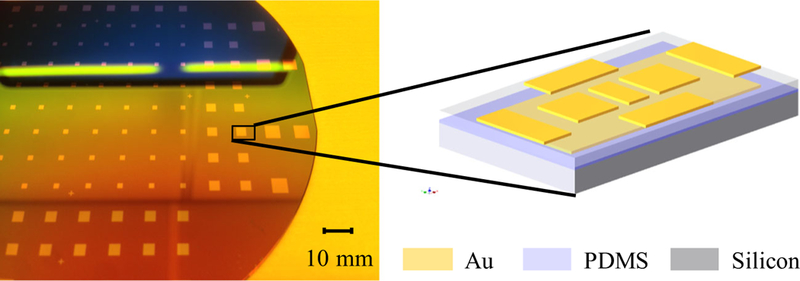
Batch-fabricated sensors on a silicon wafer. (inset) a 3D graphic of the fabricated sensor (bottom be the silicon substrate, then Au as electrodes and PDMS as the elastic polymer). The proposed capacitive sensor has a differential shear design
The differential capacitance operation principle yields a high-precision measurement for capacitive sensors with increased noise immunity. Thus, this differential design minimizes errors caused by parasitic capacitance and stray capacitance of the sensor pads, which are shortcomings of the capacitive sensor. Furthermore, benefits resulting from the differential shear design include: tilt compensation, temperature, humidity, and pressure variance tolerance.
Capacitance change due to an applied shear force is exemplified in Fig. 2. When a lateral force is applied to the sensor surface a difference in overlap area cause a change in capacitance. In the design, electrodes corresponding to the shear force are used for excitation signal input and differential capacitance input.
Fig. 2.

Applying a shear force will cause a change in overlap of two parallel electrodes and a corresponding change in capacitance between the electrodes
Utilizing these shear sensors, a biaxial shear feedback system was created that outputted vibrotactile feedback to the user when force applied to sutures neared the breakage force. The configuration of the feedback system for this study is illustrated below (Fig. 3).
Fig. 3.

The shear force sensing feedback system flow chart
The vibratory feedback system, described in greater detail in prior publication (Abiri et al. 2018), was setup to provide two levels of vibratory feedback of increasing intensity. These vibrotactile feedback levels were attained by controlling the input voltage to an Eccentric Rotating Mass (ERM) vibration motor. This motor was mounted on the master controls of the da Vinci system in a manner permitting contact of the operator’s fingertips with the motor (Fig. 4). The influence of other types of sensory feedback, including skin stretch, augmented reality, audio feedback, requires comprehensive further evaluation.
Fig. 4.
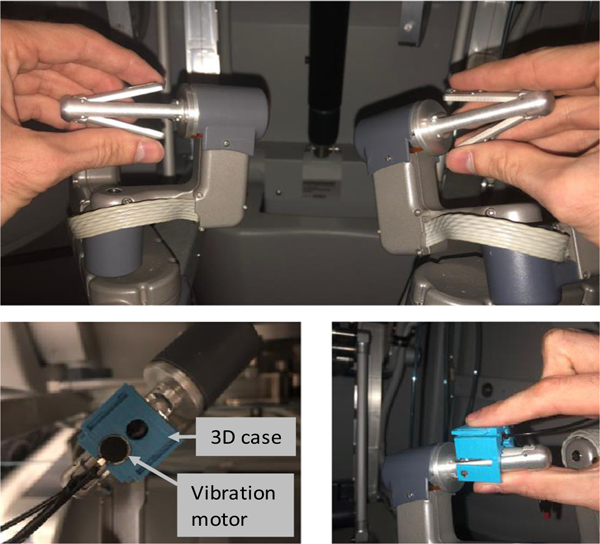
The mounted vibration motor on the da Vinci master controller (operator’s hand in direct contact with the motor)
Notably, the ultimate tensile strength of a suture can be affected by many factors (e.g., material, gauge, orientation, rate of applied tension, and potential material defect (Abiri et al. 2017)). Silk 3–0 suture was used in this study. Suture breakage force values were consistent with our prior experimental results (Abiri et al. 2017) for Silk 3–0 sutures.
Based on user feedback during preliminary testing, feedback levels were varied in order to maximize the user experience (i.e., setting the timing of both the first feedback level to avoid unnecessary distraction and also the second level to provide enough time to respond before suture failure). Thus, shear force feedback was configured to provide vibration feedback to the fingertips of the operator at two levels of intensity for this study. The first level of vibratory feedback was activated at ~ 30% below the failure load of the suture (15 N in (Abiri et al. 2017)), and while the second level of higher intensity was activated at ~ 5% below the failure load.
In order to assess the efficacy of our haptic feedback system for suture tying in robotic surgery, we measured the performance of 17 subjects with 5 trials of HFS enabled and 5 trials of HFS disabled for a total of 10 trials for each subject, resulting in a total of 170 trials, 85 of which utilized vibrotactile feedback and 85 of which received no feedback. Work with human subjects was approved by the Institutional Review Board (IRB) under protocol #11–000077. A total of six statistical analyses were performed on the data:
-
(1)
Population analysis of faults (suture breakage) that occurred during the trials.
-
(2)
Population analysis of suture quality (quantified by the slippage amount).
-
(3)
Population analysis of average force data during each trial.
-
(4)
Population analysis of a peak force value during each trial.
-
(5)
Population analysis of time for completing one trial.
-
(6)
Analysis of the learning process.
For each of the 6 analyses, we evaluated the difference using a paired sample t-test when the population was normally distributed or a Wilcoxon signed-rank test in the absence of assumed normal population distribution.
3. Results and discussion
A total of 17 novice subjects were asked to tighten a knot by pulling on both free ends of the suture (Fig. 5). Each subject was provided with a setup containing ten knots, and HFS was enabled in an alternating fashion with the order of the trials randomized to avoid bias for HFS or no feedback trials. For each subject, five of the trials were tightened while HFS was enabled and five while HFS was disabled. A proctor set up the experiment by tying two knots in each suture in order to maintain consistency of tightness around smooth, solid, cylindrical object, and then starting a third knot without tightening it. The subject was asked to tighten the third knot to replicate a surgeon tying multiple knots at the same location to ensure anastomoses stability. This approach also enabled the knots to easily slide out after the completion of the trial.
Fig. 5.

Suture tying procedure with haptic feedback system integrated with the da Vinci surgical robot. a the sensor surface (b) the front side and backside of the circuit board (c) demonstration of x, y, z reading with ensuing suture failure
Removal of the knots was necessary for testing knot quality and was measured as the amount of knot slippage (in mm) that occurred when pulling on both ends of the knot following removal from the solid cylinder. The number of suture breakages was counted with each trial having at maximum two possible breakages, one on each arm. The applied shear force was also recorded throughout the trial. Data were grouped by trial type (i.e. HFS vs. No Feedback), followed by appropriate statistical analysis.
Shown in Fig. 6, a total of 7 instances of suture breakage occurred during the HFS trials, while without HFS, there were 17 instances of failure. In this case, the biaxial sensing feedback system helped to reduce the number of suture failures by 59% (p = 0.0371). All suture failures occurred at the point of contact with the grasper. This result is consistent with observations made in previous experiments conducted with the da Vinci microneedle driver.
Fig. 6.

The number of suture breakage and no breakage for trials with HFS enabled and no feedback involved
Knot quality was measured by taking the slippage value of the tied knot, as shown in Fig. 7. Results show 3.8% smaller slippage value with HFS enabled in comparison to no feedback. Though p = 0.37940 suggests the result of improved slippage with the haptic feedback system is not statistically significant, we see no evidence that the knot quality is degraded by the HFS. The standard deviation for HFS, however, was 55% smaller than without feedback (the test statistic F =0.199061, is not in the 95% critical value accepted range [0.3621:2.7614], meaning the difference between the sample standard deviation of HFS and NF populations is big enough to be statistically significant). This finding suggests higher consistency of quality knots tied with HFS enabled than without.
Fig. 7.
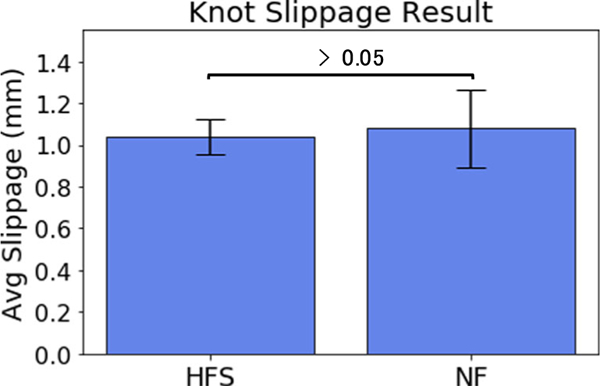
The average slippage value for 85 trials with HFS enabled and 85 trials with no feedback involved measured after each subject completed their full 10 trials
A 25.4% lower average force was observed for trials without feedback (p = 0.00034) when comparing the average force for trials with and without suture breakage (Fig. 8 left).
Fig. 8.
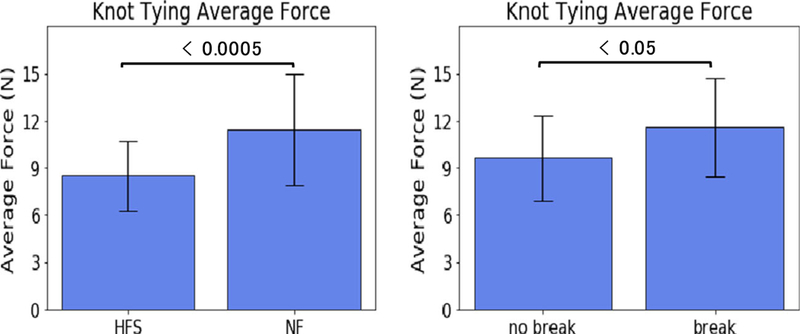
(left) The average combined bi-axial shear force used during each knot tying trial with HFS enabled and no feedback involved. (right) The average combined bi-axial shear force used during suture breakage trials and trials without suture breakage
We also found that the average force used during trials where suture failure occurred is 11.6 N, while the average force for trials without a suture breakage is 9.6 N (p = 0.03925) (Fig. 8 right), suggesting a relationship between average force and suture breakage events. This relationship is inline with our observations of reduced average force and reduced suture breakage when using the HFS.
The time required for completing a one knot-tying trail with HFS enabled and without feedback is depicted in Fig. 9. With HFS enabled, subjects took 13% longer than without feedback, but with a p = 0.328948, there is no evidence that this difference is significant.
Fig. 9.
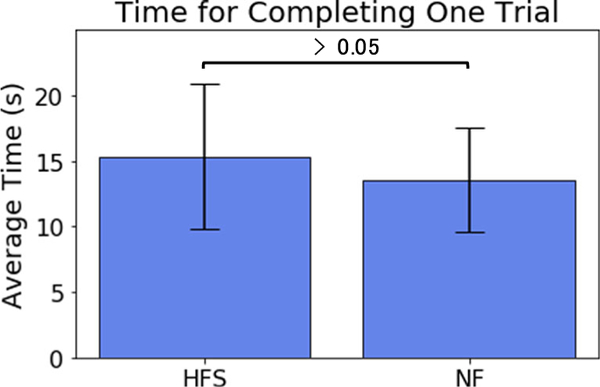
The average time for completing a knot tying task for each subject with HFS enabled and no feedback involved
Statistical analysis was performed to determine if subjects gained experience during the training (i.e., through the 10 trials they have performed). The knot quality of each subject’s 1st and 2nd trial was compared to the knot quality of the 9th and 10th trial (Fig. 10). The results indicate that generally users tied more quality knots during the later trials compared to the initial trials (p = 0.000129). A follow-on study could be performed to examine the role HFS played in improving knot quality over several trials by including a study where no haptic feedback is used.
Fig. 10.
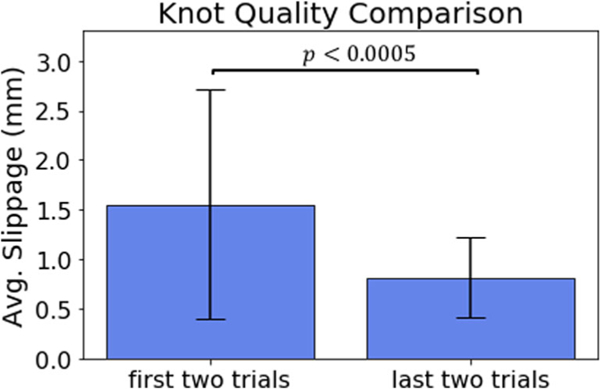
The knot quality comparison of the tightness between the first two knots tied and the last two knots tied by subjects
Our prior studies used 4 trials per subjects (Abiri et al. 2018), while in this study each subject performed 10 trials. In order to investigate the potential for improvement through training as compared to our prior studies, we consider the first 4 trials for each subject as an initial training, or break-in, period. As such, we have compared the suture failure rate between the first 4 trials and the remaining 6 trials for the HFS group and NF group (Fig. 11). For the HFS group, we have a significantly reduced breakage rate during the practice (p = 0.02355) while for the NF group, there is no significant difference (p = 0.45575). This result suggests that users required an adjustment period to acclimate to the feedback system, and significantly reduced the suture breakage rate, while subjects did not show improvement for trials without feedback. This result motivates further study in how users adapt to the feedback over multiple trials.
Fig. 11.

The breakage rate comparison of the HFS enabled trails and no feedback enabled trails between the first 4 trials and the later 6 trials
4. Conclusion
Knot tying is one of the more challenging tasks in robotic surgery not only because of the complexity of forming the knot, but also because the surgeon needs to strike a balance between pulling a suture to ensure the knot is sufficiently secure while not weakening the suture material. For the patient, postoperative suture failure may be the deciding factor between either a lengthened hospital stay due to associated complications or a quick recovery.
We have designed, fabricated, and evaluated a biaxial haptic feedback system that effectively warns the user of suture failure during use of a robotic surgical system. The drawback of a slightly longer procedure time is considered acceptable and may shorten with future iterations and developments. Our promising results from suture breakage experiments are consistent with our prior studies and demonstrate the utility of the improved system and provide valuable insight regarding the benefits of haptic feedback for robot-assisted suturing. These results warrant further validation to successfully translate this technology for clinical use with robot-assisted procedures.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abiri A, Paydar O, Tao A, LaRocca M, Liu K, Genovese B, et al. , Tensile strength and failure load of sutures for robotic surgery. Surg. Endosc. 31, 3258–3270 (August 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiri A, Askari SJ, Tao A, Juo YY, Dai Y, Pensa J, et al. , “Suture breakage warning system for robotic surgery,” IEEE Trans. Biomed. Eng. (September 2018). 10.1109/TBME.2018.2869417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anup R, Balasubramanian KA, Surgical stress and the gastrointestinal tract. J. Surg. Res. 92, 291–300 (August 2000) [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. , Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472 (May 2001) [DOI] [PubMed] [Google Scholar]
- Barbash GI, Friedman B, Glied SA, Steiner CA, Factors associated with adoption of robotic surgical technology in US hospitals and relationship to radical prostatectomy procedure volume. Ann. Surg. 259, 1–6 (January 2014) [DOI] [PubMed] [Google Scholar]
- Bhatia V, Tandon RK, Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 20, 332–339 (2005) [DOI] [PubMed] [Google Scholar]
- Cartmill JA, Shakeshaft AJ, Walsh WR, Martin CJ, High pressures are generated at the tip of laparoscopic graspers. ANZ J. Surg. 69, 127–130 (1999) [DOI] [PubMed] [Google Scholar]
- Dahiya RS and Valle M, “Robotic Tactile Sensing Technologies and Systems,” 2013 [Google Scholar]
- De S, Rosen J, Dagan A, Hannaford B, Swanson P, Sinanan M, Assessment of tissue damage due to mechanical stresses. Int. J. Rob. Res. 26, 1159–1171 (2016) [Google Scholar]
- Diks J, Nio D, Linsen MA, Rauwerda JA, Wisselink W, Suture damage during robot-assisted vascular surgery: Is it an issue? Surg Laparosc Endosc Percutan Tech 17, 524–527 (December 2007) [DOI] [PubMed] [Google Scholar]
- Gwilliam JC, Mahvash M, Vagvolgyi B, Vacharat A, Yuh DD, and Okamura AM, “Effects of haptic and graphical force feedback on teleoperated palpation,” IEEE International Conference on Robotics and Automation (ICRA), Kobe, Japan (May 2009) [Google Scholar]
- Hirano Y, Ishikawa N, Watanabe G, Suture damage after grasping with EndoWrist of the da Vinci surgical system. Minim. Invasive Ther. Allied Technol. 19, 203–206 (August 2010) [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Dokko D, Okamura AM, Yuh DD, Effect of sensory substitution on suture-manipulation forces for robotic surgical systems. J. Thorac. Cardiovasc. Surg. 129, 151–158 (January 2005) [DOI] [PubMed] [Google Scholar]
- LeBlanc KA, Laparoscopic incisional and ventral hernia repair: Complications-how to avoid and handle. Hernia 8, 323–331 (December 2004) [DOI] [PubMed] [Google Scholar]
- Livermore RW, Chong ACM, Prohaska DJ, FCooke FW, TJones TL, Knot security, loop security, and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop (Belle Mead NJ) 39, 569–576 (2010) [PubMed] [Google Scholar]
- Martell J, Elmer T, Gopalsami N, Park YS, Visual measurement of suture strain for robotic surgery. Comput Math Methods Med 2011, 879086 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucci DD, Shakeshaft AJ, Cartmill JA, Cox MR, Adams SG, Martin CJ, Grasper trauma during laparoscopic cholecystectomy. Aust N Z J Surg 70, 578–581 (August 2000) [DOI] [PubMed] [Google Scholar]
- Melinek J, Lento P, Moalli J, Postmortem analysis ofanastomotic suture line disruption following carotid endarterectomy. J. Forensic Sci. 49, 1077–1081 (September 2004) [PubMed] [Google Scholar]
- Mi Y, Chan Y, Trau D, Huang P, Chen E, Micromolding of PDMS scaffolds and microwells for tissue culture and cell patterning: A new method of microfabrication by the self-assembled micropatterns of diblock copolymer micelles. Polymer 47, 5124–5130 (2006) [Google Scholar]
- Puangmali P, Althoefer K, Seneviratne LD, Murphy D, Dasgupta P, State-of-the-art in force and tactile sensing for minimally invasive surgery. IEEE Sensors J. 8, 371–381 (2008) [Google Scholar]
- Reiley CE, Akinbiyi T, Burschka D, Chang DC, Okamura AM, Yuh DD, Effects of visual force feedback on robot-assisted surgical task performance. J. Thorac. Cardiovasc. Surg. 135, 196–202 (January 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitense HS, Jacko JA, Emery VK, Multimodal feedback: An assessment of performance and mental workload. Ergonomics 46, 68–87 (January 15 2003) [DOI] [PubMed] [Google Scholar]
- Yousef H, Boukallel M, Althoefer K, Tactile sensing for dexterous in-hand manipulation in robotics—A review. Sensors Actuators A Phys. 167, 171–187 (2011) [Google Scholar]


