Abstract
Background:
As long-term use of medicinal and recreational cannabis becomes more common and concentrations of delta-9-tetrahydrocannabinol (THC) in cannabis increase, it is timely to identify strategies to counteract the cognitive effects of cannabinoids.
Objective:
Galantamine is an acetylcholinesterase inhibitor approved for the treatment of Alzheimer’s disease and other dementias. This study aimed to investigate the feasibility of galantamine administration to individuals with cannabis use disorder (CUD), and the effects of galantamine on cognition. We hypothesized galantamine would be well tolerated and would not have procognitive effects in the absence of acute cannabis intoxication.
Methods:
Thirty individuals with CUD (73.5% male, 26.5% female) participated in a randomized, double-blind, parallel-group trial. Participants completed a baseline session followed by a 10-day outpatient treatment period, during which they received either 8 mg/day of galantamine orally or placebo. Cognitive assessments were conducted at three time points and self-reported measures that may impact cognitive performance (cannabis withdrawal, craving, and mood) were completed at six time points.
Results:
There were no significant differences in demographic and baseline variables between groups (galantamine vs. placebo). There were no significant adverse effects from galantamine. Cannabis withdrawal and craving continuously decreased over the study. We saw evidence of a modest improvement in cognitive outcomes during the 10-day period, exemplified by a statistically significant increase in measures of response inhibition (increased median reaction time on the Stop Signal Task), and a trend for improvement in measures of attention (increased RVP A’), for both groups. Analyses did not show, however, a significant main effect for treatment or treatment-by-time interactions.
Conclusions:
The findings of this pilot study support the feasibility of the administration of galantamine for individuals with CUD. Adequately powered, randomized, placebo-controlled trials are required to investigate the potential of galantamine to improve cognitive deficits associated with CUD.
Keywords: cannabis, galantamine, marijuana, THC, addiction, cognition
1. Introduction
Cannabis is one of the most widely used substances in the United States. According to the 2017 National Survey on Drug Use and Health (NSDUH), 26 million individuals – 9.6% of Americans aged 12 years or older – used cannabis in the past month (1). Approximately 41 million individuals used in the past year, and 3 million used for the first time, which amounts to approximately 8,300 new users each day (1). From 1992 until 2017, the proportion of Americans regularly using cannabis increased by approximately 60% (1). Several factors may account for this increase, such as the significant momentum gained by changes in medicinal and recreational cannabis legislations, with several states moving toward legalization, and the increased perception of cannabis use as “benign” and socially acceptable (2).
As long-term use of medicinal and recreational cannabis becomes more common and concentrations of delta-9-tetrahydrocannabinol (THC) in cannabis increase (3), it is timely to identify strategies to reduce the cognitive effects of cannabinoids. Cognitive effects of cannabinoids may be a particular challenge for treatment-seeking individuals with cannabis use disorder (CUD), as cognitive impairments may be a predictor of higher treatment dropout (4). These findings are consistent with studies of individuals with other substance use disorders (e.g., alcohol and cocaine use disorders), which also found negative effects of cognitive impairments on treatment retention (5–7). Among the cognitive domains impaired by cannabis are sustained attention, response inhibition, verbal learning, and memory (8, 9). THC, the main psychoactive constituent of cannabis, is partial agonist at the cannabinoid receptors 1 (CB1-R), which is densely expressed in the hippocampus and the prefrontal cortex, neuroanatomical structures markedly implicated in cognitive processes such as sustained attention, response inhibition and verbal learning and memory (8, 9). Preclinical studies indicate that CB1-R are particularly concentrated on presynaptic cholinergic terminals (10, 11), which are involved in long-term potentiation (LTP), widely regarded as a neurophysiological substrate of learning and memory (12, 13). By binding at the presynaptic CB1-R located at the cholinergic nerve terminals, cannabinoids may cause inhibition of acetylcholine release, contributing to acute cognitive deficits (14–16). This is also exemplified by reports of cognitive effects of cannabinoids as manifesting clinically akin to the cognitive impairment produced by cholinergic antagonists (17, 18). Collectively, these data suggest cannabinoids act on the cholinergic system and raise the question whether increasing acetylcholine levels pharmacologically can counteract the cognitive effects of cannabinoids, via the administration of an acetylcholinesterase inhibitor (AChEI) (19–21).
Galantamine is an AChEI used to treat major neurocognitive disorder associated with Alzheimer’s disease and other disorders characterized by cognitive impairment (22). AChEIs have also been shown to enhance cognitive functioning in healthy volunteers and individuals with mild neurocognitive disorder (22–29). Further, evidence exists that populations with substance use disorders can derive procognitive effects from galantamine, as exemplified by findings of galantamine-induced increases in sustained attention among individuals with cocaine use disorder (30). On the other hand, other clinical studies suggest that galantamine’s effects in reducing cocaine (31) or tobacco use (32) is not mediated by its procognitive effects. Preclinical studies suggest that by increasing ACh levels in basal ganglia, especially in the nucleus accumbens, galantamine may reduce the reinforcing effects of drugs of abuse (33).
This study had two goals: (1) to examine the feasibility of galantamine administration for individuals with CUD; and (2) to examine the effect of galantamine on cognition among individuals with CUD. We hypothesized that galantamine would be well-tolerated. Further, as previous studies with another AChEI, rivastigmine, have only shown procognitive effects in humans acutely intoxicated with cannabis (34, 35), we hypothesized the effect of galantamine for counteracting the cognitive effects of cannabinoids would not be observed in the absence of acute cannabis intoxication.
2. Materials and Methods
2.1. Participants
Participants were aged between 18 and 55. The inclusion criteria were: (a) a DSM-IV diagnosis of cannabis abuse or dependence (i.e., DSM-5 cannabis use disorder); (b) a history of cannabis use on the average of at least twice a week over a one-month period; (c) recent exposure to cannabis confirmed by positive urine toxicology for cannabinoids; (d) absence of current medical problems and a normal ECG. Exclusion criteria were: (a) current major psychiatric disorders (i.e., mood, psychotic, or anxiety disorders); (b) current alcohol or other substance use disorders (except cannabis and tobacco); (c) history of major medical illnesses; (d) use of other medications; (e) known allergy to galantamine, and (e) current pregnancy or breastfeeding. Participants were asked to refrain from using alcohol and drugs during study participation. Those who were non-compliant were discharged from the study. Although participants were asked to refrain from using cannabis, the extreme lipid solubility and long half-life of cannabinoid metabolites precludes accurate estimation of recent exposure. Urine toxicology tests for cannabinoids may be positive for as long as two months in chronic users. To minimize nicotine withdrawal effects on cognitive performance, participants who used nicotine were advised to continue smoking as usual.
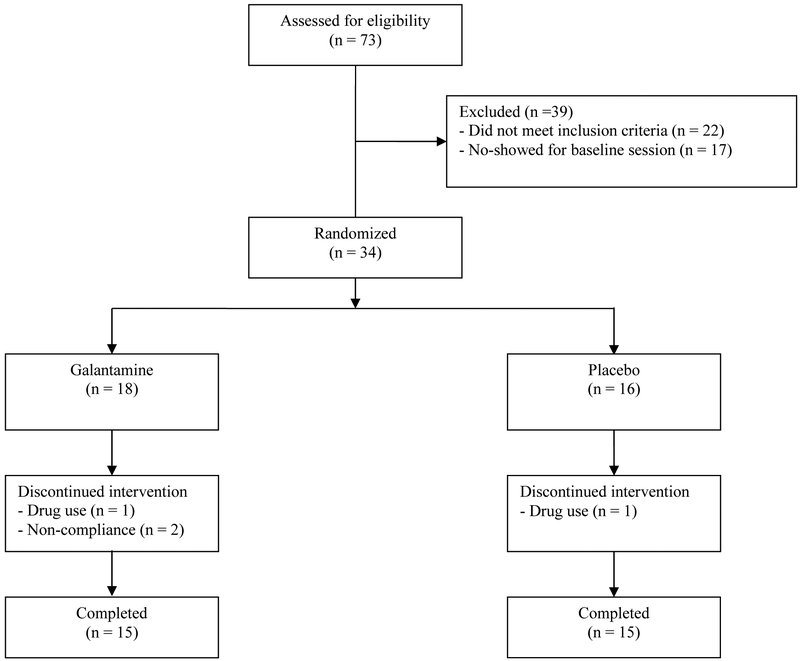
Seventy-three individuals were screened to participate in the study. Thirty-four participants were randomized, and 30 completed all study visits (Figure 1). Of the four participants that did not complete the study, two were discharged for drug use and two for non-compliance with study procedures (1 participant attended the day 1 visit and did not return, and 1 participant missed a dose of medication and was discharged). There were no differences between study completers and non-completers on any demographic variables, or by group assignment. All participants provided informed consent prior to study entry and were paid for participation. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
Figure 1.
CONSORT diagram
2.2. Design and Procedures
This was a randomized, double-blind, parallel-group study. Participants were randomized to receive oral galantamine extended release (8 mg/day) or placebo treatment for 10 days. All participants had a baseline session (day 0), where they were familiarized with study procedures and baseline measures (e.g., cognitive performance and mood assessments) were collected. On day 1, participants received the first dose of the study medication in the clinic. Participants returned to the clinic for outpatient visits on days 2, 4, 7, and 9 or 10 to receive medication. Participants received take-home bottles to self-administer study medication for days 3, 5, 6, and 8. If participants completed their outpatient visit on day 10, they also received take-home medication for day 9. Pill counts were conducted to ensure adherence to study medication. Cognitive test sessions took place at baseline and days 4, 9 or 10.
2.3. Measures
2.3.1. Physiological measures
Blood pressure and heart rate were measured at each visit. Urine toxicology screening tests were performed before each visit to rule out exposure to drugs other than cannabis. Breath alcohol was measured prior to each visit to rule out acute alcohol intoxication.
2.3.2. Adverse effects
Adverse events were monitored using a locally developed comprehensive symptom checklist that included side effects of galantamine.
2.3.3. Cannabis use, withdrawal, craving, and mood
Cannabis use was collected from the medical interview screening notes. The Marijuana Withdrawal Checklist (MWC; 36, 37) was used to assess withdrawal symptoms in the last 24 hours and the 12-item Marijuana Craving Questionnaire (MCQ; 38); was used to assess cannabis craving. The 65-item Profile of Mood States (POMS), composed of six subscales – tension, depression, anger, vigor, fatigue, and confusion – was used to measure mood (39). Withdrawal, craving, and mood measures were collected as they can influence cognitive outcomes (30).
2.3.4. Cognitive battery
The cognitive battery used in this study included the Hopkins Verbal Learning Test Revised (HVLT-R; 40) and two modules from the Cambridge Neuropsychological Test Automated Battery (CANTAB; 41, 42) the Rapid Visual Information Processing (RVIP) and the Stop Signal Task (SST). Practice effects were minimized by the use of alternate versions of the HVLT-R and parallel versions of the CANTAB modules across all testing sessions.
The HVLT-R is a brief verbal learning and memory test with six alternate forms. Aside from verbal memory and learning being one of the cognitive domains most affected by cannabinoids (15), previous studies indicate that HVLT-R performance is sensitive to the effects of cannabinoids on verbal memory (43). In this study, the HVLT-R outcomes were total recall, delayed recall, percent retention, and recognition discrimination index scores.
The RVIP is a measure of sustained attention with a working memory component and is also sensitive to the cognitive effects of cannabinoids (43, 44). The RVIP has three key outcome measures: 1) A’ (target sensitivity, a measure of the ability to detect sequences); 2) B” (response bias, a measure of the tendency to respond regardless of whether a target is present) and; 3) mean latency.
The SST is a test of response inhibition. Cannabis-using individuals have previously shown impairment in the SST (43, 44). The SST has four main outcome measures: 1) direction errors; 2) proportion of successful stops; 3) median correct response time on ‘Go’ trials and; 4) the stop signal delay, at which the subject is able to stop 50% of the time (SSD 50%).
2.4. Data Analysis
Pearson chi-square and t tests were used to compare groups on baseline characteristics. The primary outcomes were analyzed with repeated measures of variance. For these analyses, effects of treatment (galantamine or placebo) and time (baseline, test session 1, test session 2) and the interaction between treatment and time were included.
3. Results
3.1. Demographics and baseline assessments
Demographic information for all randomized participants is presented in Table 1. There were no statistically significant differences between groups on frequency of cannabis use or any of the demographic variables (p-values > .05). Moreover, there were also no significant group differences on any of the baseline physiological, self-report or cognitive measures (p-values > .05), presented in Table 2. At baseline, compared to normative cognitive performance, participants in this study showed evidence of cognitive deficits on measures of sustained attention (RVP A’, Z-score = −0.6, and RVP B’ Z-score = −0.51, or 73nd, and 70th percentiles, respectively). Further, participants demonstrated evidence of verbal memory deficits indexed by HVLT measures (total recall delayed recall, percent retention and recognition discrimination index), both compared to normative data (45) and also compared to previously conducted studies of healthy individuals and individuals with CUD (46).
Table 1.
Demographic information for randomized sample.
| Galantamine (n = 18) |
Placebo (n = 16) |
Total Sample (N = 34) |
|
|---|---|---|---|
| N (%) | |||
| Sex | |||
| Male | 15 (83.3) | 10 (62.5) | 25 (73.5) |
| Female | 3 (16.7) | 6 (37.5) | 9 (26.5) |
| Ethnicity | |||
| White | 3 (16.7) | 3 (18.8) | 6 (17.6) |
| Black | 14 (77.8) | 12 (75.0) | 26 (76.5) |
| Hispanic | 1 (5.6) | 1 (6.3) | 2 (5.9) |
| Current Smoker | 11 (61.1) | 11 (68.8) | 22 (64.7) |
| Mean (SD) | |||
| Age | 30.2 (8.0) | 29.8 (9.7) | 30 (8.8) |
| Cannabis use (joints per day* | 2.2 (1.3) | 1.7 (1.0) | 2.0 (1.2) |
| Years of cigarette smoking | 9.3 (9.8) | 7.4 (9.0) | 8.4 (9.3) |
| Years of education completed | 12.4 (1.0) | 12.6 (1.1) | 12.5 (1.0) |
There were no significant differences between conditions on any of the baseline assessment variables (p-values > 0.05).
Cannabis use data were missing for 2 participants in the placebo group.
Table 2.
Baseline assessment data for randomized sample.
| Galantamine (n = 18) |
Placebo (n = 16) |
Total Sample (N = 34) |
|
|---|---|---|---|
| Mean (SD) | |||
| Physiological | |||
| Systolic BP | 132.3 (16.5) | 133.0 (11.7) | 132.6 (14.3) |
| Diastolic BP | 73.4 (12.7) | 76.1 (10.4) | 74.7 (11.6) |
| Heart rate | 70.3 (11.6) | 73.3 (10.3) | 71.7 (10.9) |
| Marijuana Craving Questionnaire | |||
| Total score | 45.2(15.9) | 44.3 (19.7) | 44.8 (17.5) |
| Compulsivity subscale | 8.7 (4.5) | 8.8 (4.6) | 8.8 (4.5) |
| Emotionality subscale | 10.3 (4.9) | 11.6 (5.8) | 10.9 (5.3) |
| Expectancy subscale | 13.5 (5.0) | 11.9 (5.7) | 12.7 (5.4) |
| Purposefulness subscale | 12.7 (3.6) | 11.9 (5.5) | 12.3 (4.5) |
| Marijuana Withdrawal Checklist | |||
| Total Score | 6.7 (5.5) | 6.4 (5.6) | 6.5 (5.5) |
| Profile of Mood States | |||
| Tension subscale | 14.7 (3.6) | 16.2 (3.7) | 15.4 (3.7) |
| Depression subscale | 20.1 (4.5) | 21.1 (6.8) | 20.6 (5.6) |
| Anger subscale | 15.9 (4.5) | 16.1 (5.0) | 16.0 (4.7) |
| Vigor subscale | 21.7 (5.8) | 24.9 (3.7) | 23.2 (5.1) |
| Fatigue subscale | 9.7 (3.3) | 10.5 (4.1) | 10.0 (3.7) |
| Confusion subscale | 11.7 (3.4) | 11.8 (2.5) | 11.7 (2.9) |
| Cognition | |||
| Rapid Visual Information Processing (RVIP) | |||
| RVPA’ | 0.89 (0.06) | 0.89 (0.06) | 0.89 (0.06) |
| RVPB” | 0.81 (0.31) | 0.88 (0.19) | 0.84 (0.26) |
| Mean Latency | 412.40 (112.3) | 446.97 (132.40) | 428.67 (121.53) |
| Stop Signal Task (SST) | |||
| Direction errors | 1.72 (1.78) | 1.81 (2.37) | 1.76 (2.05) |
| Proportion of successful stops | 0.56 (0.14) | 0.57 (0.12) | 0.56 (0.13) |
| Median correct RT on Go trials | 520.14 (161.75) | 520.91 (178.78) | 520.50 (167.35) |
| SSD (50%) | 345.98 (173.93) | 355.72 (152.76) | 350.56 (161.91) |
| Hopkins Verbal Learning Test-Revised (HVLT-R) | |||
| Total recall | 19.0 (5.7) | 20.8 (6.2) | 19.9 (5.9) |
| Delayed recall | 6.7 (2.4) | 7.2 (2.7) | 6.9 (2.5) |
| Percent retention | 86.3 (25.4) | 82.5 (27.5) | 84.5 (26.1) |
| Recognition discrimination index | 9.8 (1.6) | 9.8 (1.7) | 9.8 (1.6) |
There were no significant differences between conditions on any of the baseline assessment variables (p-values > 0.05). SSD 50%: Stop signal delay, at which the subject is able to stop 50% of the time. RT: Reaction time.
3.2. Adverse effects
There were no significant main effects and no significant group by time interactions for blood pressure and heart rate (p-values > .05). None of the participants reported adverse events during the study.
3.3. Cannabis withdrawal, craving, and mood
Though cannabis withdrawal total score decreased over time, we found no significant main effects and no significant group by time interactions for cannabis withdrawal scores (p-values > .05).
The total MCQ score [F (5, 140) = 11.72; p<0.001]) and total MWC score [F (5, 135) = 6.31; p<0.001] decreased over time, though there were no significant interactions by group. Three of the POMS subscale scores also decreased over time (Tension-Anxiety: [F (5, 135) = 6.61; p<0.001], Vigor-Activity: [F (5, 135) = 4.78; p<0.001], and Confusion-Bewilderment: [F (5, 135) = 3.64; p<0.01]), however there were no significant interactions by group.
3.4. Cognitive battery
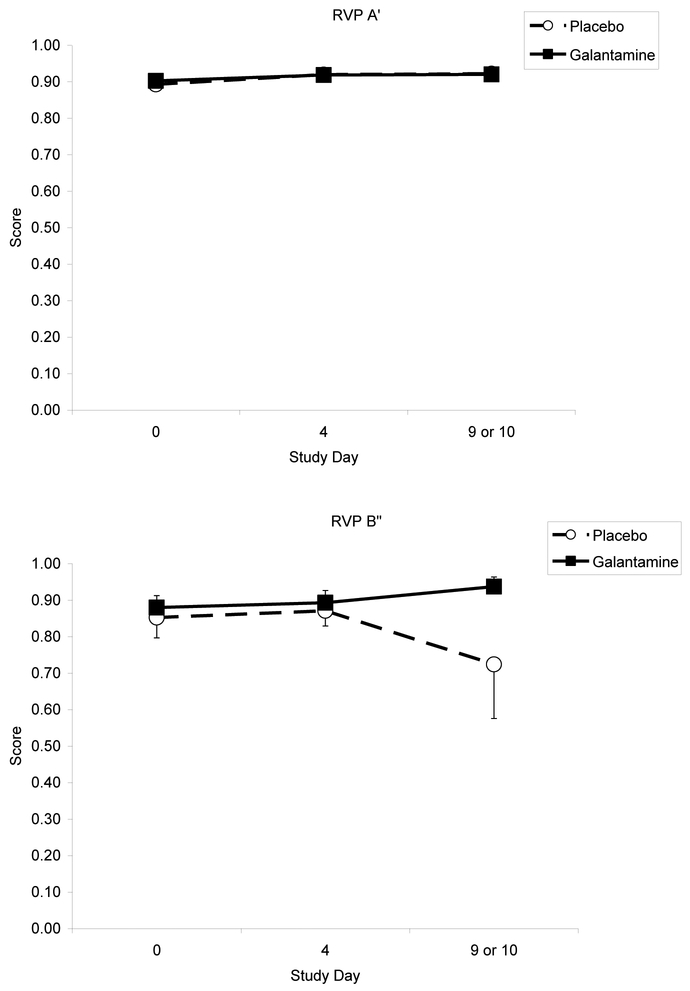
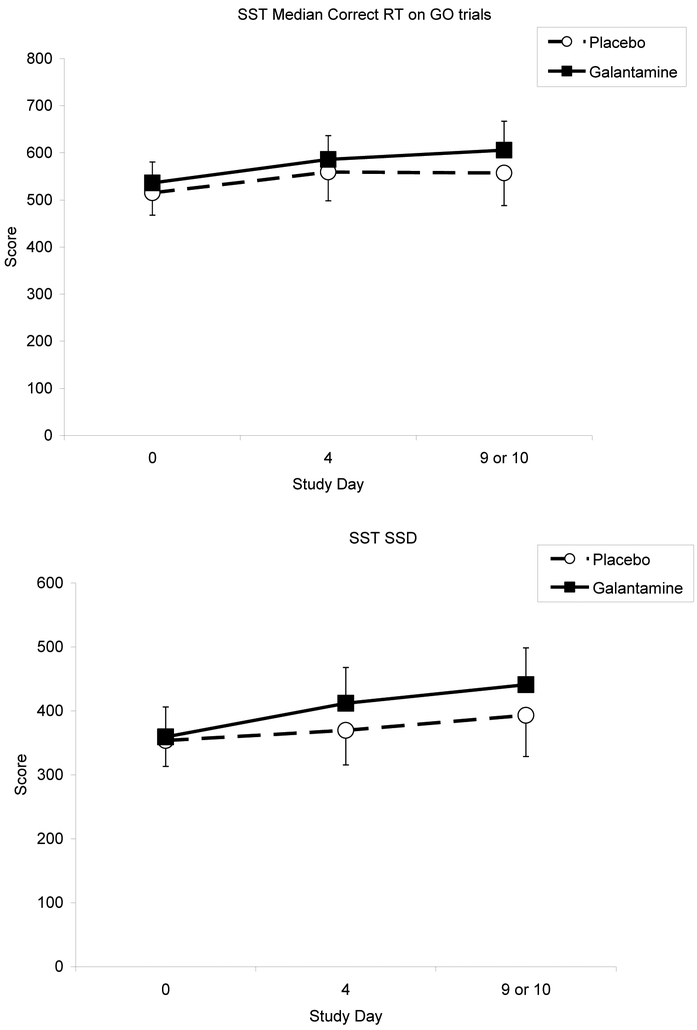
Table 3 shows the cognitive performance test score means by group, across the three testing sessions. There were no significant main effects and no significant group by time interactions for any of the RVIP measures (p-values > .05), though we found a trend for improvement in measures of attention (increased RVP A’) for both groups (Figure 2). For the SST, median correct reaction time on Go trials increased over time [F (2, 56) = 3.59; p <0.05], yet there was no significant interaction by group (Figure 3). For the HVLT-R, only delayed recall decreased over time [F (2, 56) = 4.48; p<0.05], but did not differ by group.
Table 3.
Cognitive testing scores for placebo vs. galantamine treatment.
| Baseline | Test Session 1 | Test Session 2 | |
|---|---|---|---|
| Mean (SD) | |||
| Rapid Visual Information Processing (RVIP) | |||
| RVIPA’ | |||
| PLA | 0.89 (0.02) | 0.92 (0.02) | 0.92 (0.02) |
| GAL | 0.90 (0.02) | 0.92 (0.02) | 0.92 (0.01) |
| RVIP B” | |||
| PLA | 0.85 (0.06) | 0.87 (0.04) | 0.72 (0.15) |
| GAL | 0.88 (0.03) | 0.89 (0.03) | 0.94 (0.03) |
| RVIP Mean Latency | |||
| PLA | 449.15 (35.31) | 428.25 (39.34) | 436.26 (33.81) |
| GAL | 405.63 (31.21) | 398.37 (22.39) | 383.86 (18.65) |
| Stop Signal Task (SST) | |||
| Direction errors | |||
| PLA | 1.93 (0.62) | 1.13 (0.63) | 2.33 (1.09) |
| GAL | 1.73 (0.49) | 1.27 (0.49) | 1.73 (0.61) |
| Proportion of successful stops | |||
| PLA | 0.58 (0.03) | 0.57 (0.03) | 0.53 (0.04) |
| GAL | 0.56 (0.03) | 0.54 (0.03) | 0.57 (0.03) |
| Median correct RT on Go trials | |||
| PLA | 515.00 (47.36) | 559.03 (60.99) | 557.10 (69.41) |
| GAL | 536.00 (44.58) | 585.90 (50.55) | 605.63 (61.38) |
| SSD (50%) | |||
| PLA | 353.69 (40.77) | 369.40 (54.02) | 392.98 (64.42) |
| GAL | 359.18 (46.83) | 411.81 (54.02) | 441.02 (57.51) |
| Hopkins Verbal Learning Test-Revised (HVLT-R) | |||
| Total recall | |||
| PLA | 21.33 (1.57) | 21.67 (1.61) | 21.13 (1.14) |
| GAL | 19.80 (1.52) | 23.27 (1.14) | 21.07 (1.15) |
| Delayed recall | |||
| PLA | 7.27 (0.72) | 6.80 (0.63) | 6.07 (0.71) |
| GAL | 6.73 (0.64) | 7.73 (0.40) | 6.13 (0.64) |
| Percent retention | |||
| PLA | 82.27 (7.36) | 76.93 (4.99) | 72.40 (7.13) |
| GAL | 84.07 (5.51) | 82.73 (3.43) | 74.93 (6.60) |
| Recognition Discrimination Index | |||
| PLA | 9.73 (0.45) | 9.27 (0.49) | 9.87 (0.49) |
| GAL | 9.67 (0.43) | 9.67 (0.37) | 10.47 (0.35) |
Note: PLA = placebo; GAL = galantamine; n = 30 (15 PLA, 15 GAL). SSD 50%: Stop signal delay, at which the subject is able to stop 50% of the time. RT: Reaction time.
Figure 2.
Effects of galantamine and placebo on sustained attention, indexed by Rapid Visual Processing (RVP) A’ and B’ scores, over time.
Figure 3.
Effects of galantamine and placebo on response inhibition performance over time. Response inhibition performance is indexed by stop signal delay, at which the subject is able to stop 50% of the time (SSD 50%), and reaction time (RT) scores.
4. Discussion
The current study investigated the feasibility and the potential efficacy of the centrally acting AChEI galantamine to improve cognitive function among individuals with cannabis use disorder. As hypothesized, galantamine was well tolerated by participants, such that there were no differences in physiological measures or adverse effects for participants who received galantamine, compared to those who received placebo.
The results of this study show that individuals with CUD demonstrated evidence of mild cognitive deficits at baseline, in accordance with previous studies demonstrating cognitive deficits among individuals with CUD (47, 48). As expected, the cognitive performance of participants who received galantamine in the absence of cannabis intoxication did not differ from those who received placebo, on any of the measures of sustained attention, response inhibition and verbal memory and learning. AChEI have been previously found to enhance cognition in acutely cognitively impaired individuals intoxicated with cannabis (34). In this study, the procognitive effects of galantamine were not demonstrated, likely due to the fact that our sample was composed of young, healthy individuals who did not demonstrate substantial cognitive deficits and were not acutely intoxicated with cannabis, unlike in a previous human laboratory study that found procognitive effects of rivastigmine in acutely intoxicated cannabis users (34). Some participants experienced mood changes during the course of the study, possibly in the context of early, mild cannabis withdrawal. However, on the Marijuana Withdrawal Checklist we did not see a pattern of scores initially increasing and then decreasing over time, which might be expected if participants were abstaining from cannabis. Moreover, because all participants were able to continue with full, uninterrupted sessions of the cognitive tests, we estimate the impact of any withdrawal symptoms on the study results was limited.
The absence of main effects of galantamine on cognition among individuals with CUD in this study is aligned with the conflicting preclinical data regarding the involvement of the brain acetylcholine (ACh) system in the neurobiology of the cognitive effects of cannabinoids. Some studies in rats have shown reduced choline uptake in the hypothalamus, reduced synthesis of ACh after THC administration (49), and a reduction of cannabis-induced working memory deficits by AChEI (50). In contrast, other studies have not confirmed the hypothesis that the cognitive effects of cannabinoids are explained by a reduction in ACh release. Nava et al. (2001) showed that the decrease in extracellular hippocampal ACh concentrations in rats was delayed in relation to the timing of the THC-induced cognitive deficits (51), thus not supporting a causal relationship between low ACh levels and cognitive deficits. Moreover, the AChEI physostigmine did not counteract THC-induced cognitive deficits in rats (52). Collectively, the preclinical human laboratory study and clinical trial data indicate the effects of AChEI on cognition may be time (i.e., may only be present during acute cannabis intoxication) and dose-dependent (i.e., be contingent on the cannabinoid and AChEI dose exposure). In this study, marijuana craving decreased over time for both groups, with no significant interactions by group. Thus, we did not find evidence that galantamine reduced the reinforcing effect of the drug.
The current study has several limitations that need to be addressed in further studies. First, although all participants met criteria for CUD and there were no significant differences in cannabis use histories, the effects of cannabis on cognition may depend on other factors such as the THC/CBD ratio of cannabis, with higher concentrations of CBD inducing less cognitive deficits (53). Further, the chronic cognitive effects of cannabinoids are more complex and challenging to interpret their acute effects, appearing to be related not only to the dose of exposure (frequency, duration, amount) but also to the age of onset of use (7, 54). Some individuals with CUD may have blunted responses to the cognitive deficits induced by cannabis, and in these populations abstinence from cannabis may, in fact, be associated with cognitive impairment (55). In addition, we were also unable to determine if individuals abstained from cannabis use during the study, due to the extended length of time that cannabis remains in the system. Future studies with a within-subject design may account for the potential confounding effects of individual differences in cannabis use histories.
Second, we only used one dose (8 mg/day) of galantamine in this study. Since this is the first study to assess galantamine on cognitive function in individuals with CUD, we established our dosage based on other studies of populations with substance use disorders – in particular, our previous study of the effects of galantamine on individuals with cocaine use disorder (30). As the recommended therapeutic target dose for galantamine for Alzheimer’s disease ranges from 8 to 24 mg, it is possible that higher doses of galantamine may have produced cognitive effects in our sample. This is supported by the fact that eptastigmine, a more potent AChEI than physostigmine, was effective for reducing the cognitive effects of cannabinoids in preclinical studies (50). Third, the short treatment duration and small sample size may not have been enough to capture procognitive effects of galantamine in individuals with CUD. A meta-analysis of neuropsychological studies found that only small magnitude effects are apparent in the first few weeks of abstinence from cannabis (of the order of d = 0.25 to 0.35), and these become smaller and non-significant with extended abstinence (to around d = 0.15) (43, 56). Future studies should test higher doses of galantamine for a longer period of time and with an adequately powered sample. Lastly, the small sample size prohibits us from examining differences in improvement between those with and without mild cognitive deficits. Larger studies with individuals of various levels of cognitive deficits are needed to help determine whether modulation of cholinergic neurotransmission with galantamine is a viable therapeutic strategy, especially for individuals with early onset CUD and severe CUD, who may have more substantial cognitive deficits.
In conclusion, this pilot study shows feasibility and tolerability of galantamine, for individuals with cannabis use disorder. This study has broad methodological implications for the emerging field of development of cognitive enhancers for addiction treatment (57). More specifically, it has significance for the design of future studies investigating the potential of galantamine for counteracting the cognitive effects of cannabinoids, a potential target for the treatment of CUD.
Highlights.
Galantamine was well-tolerated by individuals with cannabis use disorder
There were no group differences (galantamine v. placebo) in cognitive performance
Response inhibition increased over time for both galantamine and placebo groups
Cannabis withdrawal and craving decreased over time for both groups
Acknowledgements
We would like to thank Ellen Mitchell, R.N., Lance Barnes, and Stacy Minnix for their assistance.
Funding
This research was supported by the Veterans Administration Mental Illness Research, Education, and Clinical Center (MIRECC) and the National Institute on Drug Abuse (NIDA) grant K02-DA021304 (MS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
The authors report no conflicts of interest.
References
- 1.Abuse S. Mental Health Services Administration.(2018). Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report. Accessed October 2018; 19. [Google Scholar]
- 2.Blevins CE, Abrantes AM, Anderson BJ, Caviness CM, Herman DS, Stein MD. Identity as a cannabis user is related to problematic patterns of consumption among emerging adults. Addictive behaviors 2018; 79, 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol Psychiatry 2016; 79 (7), 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aharonovich E, Brooks AC, Nunes EV, Hasin DS. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug and alcohol dependence 2008; 95 (3), 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and alcohol dependence 2003; 71 (2), 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and alcohol dependence 2006; 81 (3), 313–322. [DOI] [PubMed] [Google Scholar]
- 7.Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)-Tetrahydrocannabinol. Neuropsychopharmacology 2018; 43 (1), 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya S, Atakan Z, Martin-Santos R, A Crippa J, K McGuire P. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: a critical review of neuroimaging studies. Current pharmaceutical design 2012; 18 (32), 5045–5054. [DOI] [PubMed] [Google Scholar]
- 9.Kucewicz MT, Tricklebank MD, Bogacz R, Jones MW. Dysfunctional prefrontal cortical network activity and interactions following cannabinoid receptor activation. Journal of Neuroscience 2011; 31 (43), 15560–15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtman A, Varvel S, Martin B. Endocannabinoids in cognition and dependence. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2002; 66 (2–3), 269–285. [DOI] [PubMed] [Google Scholar]
- 11.Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology 2002; 42 (8), 993–1007. [DOI] [PubMed] [Google Scholar]
- 12.Salavati B, Rajji TK, Price R, Sun Y, Graff-Guerrero A, Daskalakis ZJ. Imaging-based neurochemistry in schizophrenia: a systematic review and implications for dysfunctional long-term potentiation. Schizophrenia bulletin 2014; 41 (1), 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman AF, Lycas MD, Kaczmarzyk JR, Spivak CE, Baumann MH, Lupica CR. Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid ‘Spice’compounds: comparison with Δ9-tetrahydrocannabinol. Addiction biology 2017; 22 (2), 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav 1990; 37 (3), 561–565. [DOI] [PubMed] [Google Scholar]
- 15.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Lubman DI, Yücel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology 2011; 216 (1), 131–144. [DOI] [PubMed] [Google Scholar]
- 16.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama 2002; 287 (9), 1123–1131. [DOI] [PubMed] [Google Scholar]
- 17.Morley JE. Anticholinergic medications and cognition. Journal of the American Medical Directors Association 2011; 12 (8), 543–543.e541. [DOI] [PubMed] [Google Scholar]
- 18.Dawson GR, Iversen SD. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behavioural brain research 1993; 57 (2), 143–153. [DOI] [PubMed] [Google Scholar]
- 19.Verrico CD, Jentsch JD, Dazzi L, Roth RH. Systemic, but not local, administration of cannabinoid CB1 receptor agonists modulate prefrontal cortical acetylcholine efflux in the rat. Synapse 2003; 48 (4), 178–183. [DOI] [PubMed] [Google Scholar]
- 20.Mishima K, Egashira N, Matsumoto Y, Iwasaki K, Fujiwara M. Involvement of reduced acetylcholine release in Δ9-tetrahydrocannabinol-induced impairment of spatial memory in the 8-arm radial maze. Life sciences 2002; 72 (4–5), 397–407. [DOI] [PubMed] [Google Scholar]
- 21.Sofuoglu M, Sugarman DE, Carroll KM. Cognitive function as an emerging treatment target for marijuana addiction. Experimental and clinical psychopharmacology 2010; 18 (2), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farlow M A clinical overview of cholinesterase inhibitors in Alzheimer’s disease. International Psychogeriatrics 2002; 14 (S1), 93–126. [DOI] [PubMed] [Google Scholar]
- 23.Freo U, Pizzolato G, Dam M, Ori C, Battistin L. A short review of cognitive and functional neuroimaging studies of cholinergic drugs: implications for therapeutic potentials. Journal of Neural Transmission 2002; 109 (5–6), 857–870. [DOI] [PubMed] [Google Scholar]
- 24.Marco-Contelles J, do Carmo Carreiras M, Rodríguez C, Villarroya M, Garcia AG. Synthesis and pharmacology of galantamine. Chemical reviews 2006; 106 (1), 116–133. [DOI] [PubMed] [Google Scholar]
- 25.Ochoa EL, Clark E. Galantamine may improve attention and speech in schizophrenia. 2006. [DOI] [PubMed]
- 26.Khateb A, Ammann J, Annoni J-M, Diserens K. Cognition-enhancing effects of donepezil in traumatic brain injury. European neurology 2005; 54 (1), 39–45. [DOI] [PubMed] [Google Scholar]
- 27.Sitaram N, Weingartner H, Caine ED, Gillin JC. Choline: selective enhancement of serial learning and encoding of low imagery words in man. Life sciences 1978; 22 (17), 1555–1560. [DOI] [PubMed] [Google Scholar]
- 28.Davis KL, Mohs RC, Pfefferbaum A, Hollister L, Kopell B. Physostigmine: improvement of long-term memory processes in normal humans. Science 1978; 201 (4352), 272–274. [DOI] [PubMed] [Google Scholar]
- 29.Goekoop R, Rombouts SA, Jonker C, Hibbel A, Knol DL, Truyen L, Barkhof F, Scheltens P. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage 2004; 23 (4), 1450–1459. [DOI] [PubMed] [Google Scholar]
- 30.Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Experimental and clinical psychopharmacology 2011; 19 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll K, DeVito E, Shi J, Nich C, Sofuoglu M. A randomized trial of galantamine and computerized cognitive behavioral therapy for methadone maintained individuals with cocaine use disorder. The Journal of Clinical Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLean RR, Waters AJ, Brede E, Sofuoglu M. Effects of galantamine on smoking behavior and cognitive performance in treatment-seeking smokers prior to a quit attempt. Human Psychopharmacology: Clinical and Experimental 2018; 33 (4), e2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen KP, DeVito EE, Yip S, Carroll KM, Sofuoglu M. The Cholinergic System as a Treatment Target for Opioid Use Disorder. CNS drugs 2018; 32 (11), 981–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theunissen E, Heckman P, Perna EdSF, Kuypers K, Sambeth A, Blokland A, Prickaerts J, Toennes S, Ramaekers J. Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans. Psychopharmacology 2015; 232 (2), 343–353. [DOI] [PubMed] [Google Scholar]
- 35.Giacobini E Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacological research 2004; 50 (4), 433–440. [DOI] [PubMed] [Google Scholar]
- 36.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. Journal of abnormal psychology 2003; 112 (3), 393. [DOI] [PubMed] [Google Scholar]
- 37.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 1999; 94 (9), 1311–1322. [DOI] [PubMed] [Google Scholar]
- 38.Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. Addiction 2001; 96 (7), 1023–1034. [DOI] [PubMed] [Google Scholar]
- 39.McNair D, Lorr M, Droppleman L. Manual for the profile of mood states (POMS). San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 40.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist 1998; 12 (1), 43–55. [Google Scholar]
- 41.Fray P J, Robbins T W, Sahakian B J. Neuorpsychiatyric applications of CANTAB. International journal of geriatric psychiatry 1996; 11 (4), 329–336. [Google Scholar]
- 42.Sahakian BJ, Owen A. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. Journal of the Royal Society of Medicine 1992; 85 (7), 399. [PMC free article] [PubMed] [Google Scholar]
- 43.Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol Psychiatry 2016; 79 (7), 557–567. [DOI] [PubMed] [Google Scholar]
- 44.Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Current drug abuse reviews 2008; 1 (1), 81–98. [DOI] [PubMed] [Google Scholar]
- 45.Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK, Group H. Demographically corrected norms for African Americans and Caucasians on the hopkins verbal learning test–revised, brief visuospatial memory test–revised, stroop color and word test, and wisconsin card sorting test 64-card version. Journal of clinical and experimental neuropsychology 2011; 33 (7), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabin RA, Barr MS, Goodman MS, Herman Y, Zakzanis KK, Kish SJ, Kiang M, Remington G, George TP. Effects of extended cannabis abstinence on cognitive outcomes in cannabis dependent patients with schizophrenia vs non-psychiatric controls. Neuropsychopharmacology 2017; 42 (11), 2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MA, Curran HV, Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA psychiatry 2016; 73 (3), 292–297. [DOI] [PubMed] [Google Scholar]
- 48.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences 2012, 201206820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindamood C, Colasanti BK. Effects of delta 9-tetrahydrocannabinol and cannabidiol on sodium-dependent high affinity choline uptake in the rat hippocampus. Journal of Pharmacology and Experimental Therapeutics 1980; 213 (2), 216–221. [PubMed] [Google Scholar]
- 50.Braida D, Sala M. Cannabinoid-induced working memory impairment is reversed by a second generation cholinesterase inhibitor in rats. Neuroreport 2000; 11 (9), 2025–2029. [DOI] [PubMed] [Google Scholar]
- 51.Nava F, Carta G, Colombo G, Gessa G. Effects of chronic Δ9-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology 2001; 41 (3), 392–399. [DOI] [PubMed] [Google Scholar]
- 52.Lichtman AH, Martin BR. Δ 9-Tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology 1996; 126 (2), 125–131. [DOI] [PubMed] [Google Scholar]
- 53.De Aquino JP, Sherif M, Radhakrishnan R, Cahill JD, Ranganathan M, D’Souza DC. The Psychiatric Consequences of Cannabinoids. Clinical therapeutics 2018. [DOI] [PubMed] [Google Scholar]
- 54.Englund A, Freeman TP, Murray RM, McGuire P. Can we make cannabis safer? The Lancet Psychiatry 2017. [DOI] [PubMed] [Google Scholar]
- 55.McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003; 28 (7), 1356. [DOI] [PubMed] [Google Scholar]
- 56.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive Measures in long-term cannabis users. The Journal of Clinical Pharmacology 2002; 42 (S1), 41S–47S. [DOI] [PubMed] [Google Scholar]
- 57.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 2013; 64, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]