Abstract
During a quit attempt, high negative affect predicts relapse to smoking. In this study, we evaluated bidirectional longitudinal associations between smoking and negative affect among cancer patients treated with varenicline. Participants (N=119, 50% female, Mage=59 years) were smokers (≥5 cigarettes/week) who were diagnosed with cancer and were recruited for a 24-week trial of extended duration varenicline plus behavioral counseling; data for this secondary analyses were drawn from the 12-week open-label phase of the trial. Smoking was assessed via self-reported number of cigarettes in the past 24 hours. Negative affect was assessed using the Positive and Negative Affect Scale (PANAS). Data were collected at pre-quit (week 0), target quit day (week 1), week 4, and week 12. We evaluated cross-lagged panel models for negative affect and smoking using PROC CALIS in SAS. Models were run separately for participants who were adherent (≥80% of medication taken) or nonadherent to varenicline. Among adherent participants (n=96), smoking accounted for up to 22% of variance in subsequent negative affect throughout treatment. Cross-lagged associations were not observed between smoking and negative affect among non-adherent participants (n=23). Negative affect did not predict subsequent smoking among either adherent or nonadherent participants. These results suggest that varenicline may attenuate abstinence-induced negative affect among cancer patients treated for nicotine dependence.
Keywords: smoking cessation, affect, adherence, cancer, randomized controlled trial
1. Introduction
Cigarette smoking continues to be a great public health concern, contributing to an estimated three in ten cancer-related deaths in the U.S. (Jacobs et al., 2015). Continued smoking amongst cancer patients perpetuates adverse treatment outcomes, including a second primary cancer, worse side effects, and poorer survival (USDHHS, 2014), and yet nearly half of smokers continue to smoke after a cancer diagnosis (Cox et al., 2003). To provide effective smoking cessation treatments for this high-risk population, a better understanding of the reasons why cancer patients continue to smoke is critical.
In the general population, one proposed mechanism of relapse is that abstinence-related hedonic changes, including increased negative affect (NA) and decreased positive affect (PA), drive smokers to resume smoking (Cook et al., 2010; Copeland et al., 2009; Doran et al., 2006). While this mechanism has not been tested among smokers with cancer, cancer patients report high levels of psychological distress (Brown et al., 2013), and smokers with cancer and comorbid psychiatric conditions are less likely to quit (Blalock et al., 2011). However, the associations between successful smoking cessation and affect among cancer patients have not been evaluated.
First-line pharmacotherapies can improve the odds of successful cessation two- or threefold (Fiore et al., 2008). The seven FDA-approved smoking cessation pharmacotherapies, including five nicotine replacement therapies and two medications, are designed to ameliorate nicotine withdrawal symptoms, including affective changes (Fiore et al., 2008). Varenicline, a first-line smoking cessation pharmacotherapy that is safe and effective among cancer patients (Schnoll et al., 2019), may reduce psychological distress among those who achieve abstinence by improving NA and enhancing PA (Doran et al., 2018; Patterson et al., 2009). A better understanding of the impact of affective changes on quitting behavior amongst these smokers, while using first-line treatments, is crucial for supporting this high-risk population to quit smoking and thus improve their physical and psychological health.
The aim of this study was to better understand the relationship between smoking and affect among cancer patients using varenicline to quit smoking. Compared to previous studies that only evaluated NA and PA as baseline predictors or as outcome measures following abstinence, we assessed a bidirectional model to elucidate directionality between smoking and affect by assessing smoking, NA, and PA contemporaneously among cancer patients who were and were not adherent to varenicline during the 12-week, open-label phase of a clinical trial. This design allowed us to evaluate smoking level and affective changes within the context of an effective treatment that has demonstrated affective benefits during abstinence. Based on studies in the general population (Cook et al., 2010; Doran et al., 2018), we hypothesized that higher levels of NA and lower levels of PA would be associated with a greater likelihood of subsequent smoking, particularly among those who were adherent to varenicline.
2. Materials and Methods
2.1. Participants
Eligible participants were men and women ≥18 years old who reported smoking ≥5 cigarettes per week and diagnosed with cancer within 5 years. Participants were excluded for: daily use of non-cigarette tobacco products; current smoking cessation treatment; medical contraindications for varenicline; or bipolar disorder, psychotic disorder, or current suicidality. Further inclusion/exclusion information can be found elsewhere (Crawford et al., 2018).
2.2. Study design and procedures
The parent trial was designed to evaluate the efficacy of extended (24-week) varenicline compared to standard (12-week) varenicline treatment, plus 24 weeks of behavioral counseling provided to all participants (NCT01756885). The present study is a secondary analysis from the 12-week open-label phase of the trial. Primary recruitment efforts focused on oncology clinics in Chicago, IL and Philadelphia, PA between May, 2013 and June, 2017. Potential participants were screened for initial eligibility via telephone, and final eligibility was confirmed at an in-person session where participants provided written informed consent and completed assessments. Eligible participants were then scheduled for a Pre-Quit (PQ) session, where they initiated varenicline following standard dosing: 0.5 mg once per day for days 1–3, 0.5 mg twice per day for days 4–7, and 1.0 mg twice per day for the remainder (through day 84 for the 12-week course). Participants were instructed to set their target quit date (TQD) for the day they started the full dose of varenicline. Smoking cessation counseling (based on PHS Guidelines; Fiore et al., 2008) was provided in-person at PQ (Week 0), TQD (Week 1), Week 4, and Week 12 and via telephone at Week 8. At each in-person session, participants completed assessments including medication adherence and side effects, smoking, and psychological symptoms. All procedures were approved by the institutional review boards at each site.
2.3. Measures
2.3.1. Sociodemographics and smoking history
At baseline, participants self-reported gender, race/ethnicity, and age; how long they had been smoking and how many cigarettes per day (CPD) they smoked on average; level of dependence as assessed by the Fagerström Test for Cigarette Dependence (FTCD; Fagerstrom, 2012; α=0.61).
2.3.2. Current smoking
At each in-person visit, participants were asked, “Have you smoked a cigarette, even a puff, in the past 24 hours?” and, if they answered yes, “How many cigarettes have you smoked in the past 24 hours?” Past 24-hour cigarette use (CPD) was used for the analyses, given that this value can be dynamic throughout a quit attempt.
2.3.3. Affect
Participants completed the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) at each in-person visit. The PANAS is a self-report measure on which participants rated their current state on a scale from 1 to 5 of 10 negative feelings (e.g., irritable, jittery; α=0.80, 0.86, 0.85, and 0.87 at PQ, TQD, W4, and W12 respectively) and 10 positive feelings (e.g., interested, enthusiastic; α=0.93, 0.90, 0.93, and 0.92 at PQ, TQD, W4, and W12 respectively). Total scores on each scale range from 10 to 50, with higher scores indicating higher levels of NA or PA.
2.3.4. Treatment adherence
We chose to stratify the analyses by varenicline adherence because participants who were nonadherent would be unlikely to experience the NA or PA benefits it offers. At each visit, participants self-reported varenicline use by pill count via timeline follow-back and confirmed by medication blister pack contents. Participants were categorized as adherent (took ≥80% of pills) consistent with previous studies (Carroll et al., 2018; Catz et al., 2011).
2.4. Data analysis
All analyses were conducted using SAS version 9.4. The primary analysis was a cross-lagged panel model, which allowed us to test bidirectional pathways while accounting for past and contemporaneous factors. We completed four models using PROC CALIS to evaluate associations between smoking (CPD) and NA or PA (PANAS scores) separately by adherence status (adherent or nonadherent). The models included data from: PQ, TQD, Week 4, and Week 12. Participants who attended all sessions were included because complete data are required for this analysis. We assessed autocorrelations (within CPD or within NA/PA; e.g., PQ CPD to TQD CPD) and cross-lagged associations (between CPD and affect over the course of treatment; e.g., PQ CPD to TQD NA). Error covariances within each session (e.g. PQ CPD and PQ PA) were included in each model. Model fit was described using χ2, standardized root mean squared residual (SRMR), and comparative fit index (CFI) (Hu and Bentler, 1999).
3. Results
3.1. Sample characteristics
Demographic and cancer-related characteristics of the sample are presented in Table 1. A total of 119 participants attended all 4 in-person sessions during the open-label treatment phase. The 88 participants in the primary study who were excluded from this analysis did not differ from included participants on any of the baseline characteristics (demographics, cancer-related factors, smoking variables, or PANAS scores). Ninety-six participants (81%) were classified as adherent. No significant differences on baseline characteristics were observed between adherent and nonadherent participants.
Table 1.
Demographics and cancer-related characteristics by study medication adherence status
| Nonadherent (n=23) | Adherent (n=96) | ||||
|---|---|---|---|---|---|
| Variable | N or M | (% or SD) | N or M | (% or SD) | p-value |
| Female, % | 12 | (52%) | 47 | (49%) | 0.782 |
| White, % | 13 | (57%) | 72 | (75%) | 0.078 |
| Age (years) | 58.6 | (9.0) | 59.4 | (8.4) | 0.712 |
| < College graduate, % | 14 | (61%) | 61 | (64%) | 0.812 |
| Employed, % | 11 | (48%) | 47 | (49%) | 0.922 |
| Years smoking | 40.9 | (8.9) | 40.9 | (10.4) | 0.981 |
| FTCD score | 4.7 | (2.5) | 4.4 | (2.1) | 0.673 |
| Tobacco-related cancer site,a % | 18 | (78%) | 77 | (80%) | 0.834 |
| Cancer stage | 0.223 | ||||
| Stage 0–2 | 6 | (26%) | 18 | (19%) | |
| Stage 3–4 | 6 | (26%) | 14 | (15%) | |
| Remission/not reported | 11 | (48%) | 64 | (67%) | |
| Current cancer treatment,b % | 7 | (30%) | 40 | (42%) | 0.375 |
| Chemotherapy | 3 | (13%) | 20 | (21%) | |
| Radiation treatment | 1 | (4%) | 7 | (7%) | |
| Surgery | 3 | (13%) | 12 | (13%) | |
| Hormone therapy | 2 | (9%) | 10 | (10%) | |
p-values indicate significance of differences between adherent vs. nonadherent sample by t-tests (continuous variables) or chi-square analyses (categorical variables).
Participants’ cancer types included: genitourinary (n=29), breast (n=20), skin (n=19), lung (n=16), hematological (n=15), head and neck (n=8), gastrointestinal (n=6), and kidney/pancreatic/liver (n=6).
Cancer treatments (chemotherapy, radiation treatment, surgery, and hormone therapy) are not mutually exclusive.
3.2. Panel analysis
The panel models of smoking and affect represented an adequate fit of the data among adherent participants (NA: χ2(df=12)=61.77, p<.001; SRMR=0.100; CFI=0.791; PA: χ2(df=12)=30.33, p=.003; SRMR=0.078; CFI=0.946) and nonadherent participants, though potentially overfitting the data for PA (NA: χ2(df=12)=14.52, p=0.269; SRMR=0.084; CFI=0.963; PA: χ2(df=12)=11.56, p=0.482; SRMR=0.051; CFI=1.000).
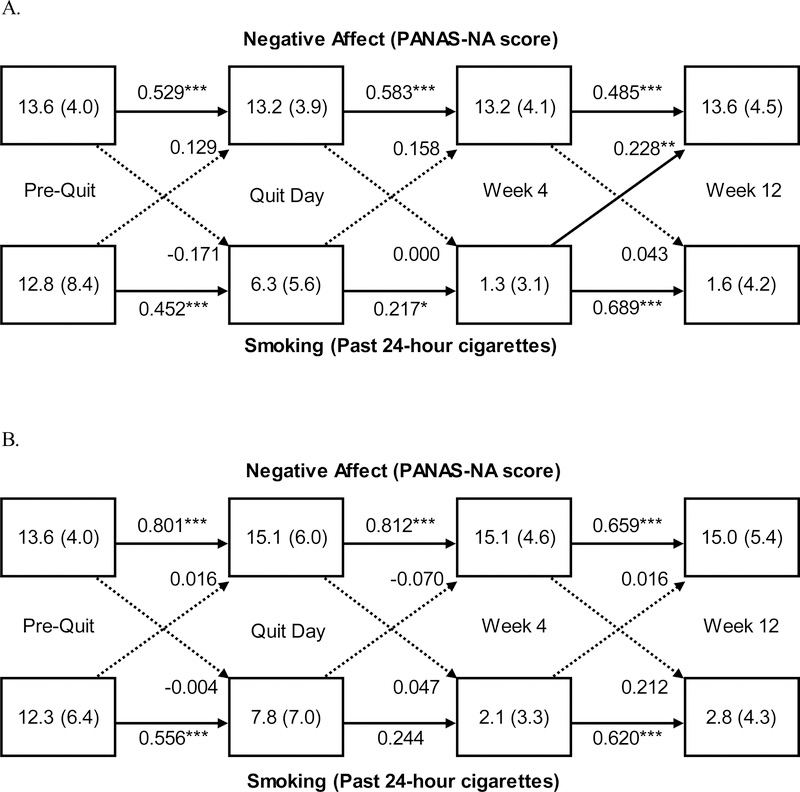
The panel models of smoking and NA are presented in Figure 1 by adherent (Panel A) and nonadherent participants (Panel B). Among adherent participants, cross-lagged associations from smoking to NA increased over time, from PQ to TQD (0.13, p=0.131), TQD to W4 (0.16, p=0.050), and W4 to W12 (0.23, p=0.007), indicating that higher levels of smoking predicted higher levels of NA at the subsequent visit. This pattern was not observed among nonadherent participants. Cross-lagged associations from NA to smoking were negligible throughout treatment (all ps>0.05). None of the cross-lagged associations between smoking and PA reached significance among either adherent or nonadherent participants (Supplemental Material).
Figure 1.
Panel analysis of negative affect (NA) and smoking among adherent participants (n=96; Panel A) and among nonadherent participants (n=23; Panel B) during the 12-week, open-label phase of a smoking cessation clinical trial using varenicline plus behavior counseling among adults with a cancer diagnosis. Values in the boxes indicate the Mean (Standard Deviation) NA score or smoking level, respectively. Solid lines represent significant path coefficients. Dotted lines represent non-significant path coefficients. Values on the lines represent standardized path coefficients, which indicate the proportion of variance in Y (e.g., TQD NA) that is explained by X (e.g., PQ Smoking). Error covariances of endogenous variables (e.g., PQ NA and PQ Smoking) were included in the model. NA was assessed by the Positive and Negative Affect Scale (PANAS) negative affect score. Smoking was self-reported number of cigarettes smoked in the past 24 hours. *p<.05 **p<.01 ***p<.001
The autocorrelations observed within smoking and affect provide face validity for these results. For example, smoking autocorrelations were moderate from PQ to TQD (range: 0.45–0.59; ps<.001), smaller between TQD and W4 (range: 0.21–0.24; ps<.05 for adherent, ps>.05 for nonadherent), and largest between W4 and W12 (range: 0.58–0.69; ps<.001). Within NA, adherent participants had moderate, significant effects throughout treatment (0.49–0.58, ps<.001) while nonadherent participants had large effects (0.66–0.81, ps<.001), suggesting limited variability across sessions among nonadherent participants. PA autocorrelations among both adherent and nonadherent participants were high (>0.70, all ps<.001), suggesting a potential ceiling effect of the PANAS PA scores, particularly among nonadherent participants.
4. Discussion
This study evaluated the bidirectional associations between smoking and affect among smokers with cancer during 12 weeks of varenicline. To our knowledge, this was the first study to explicitly evaluate the bidirectional associations between smoking and affect among cancer patients. Using an innovative application of cross-lagged panel analysis in a clinical trial, self-reported smoking predicted later NA, but not PA, amongst adherent participants; NA did not predict smoking in either model. A clearer understanding of this relationship may allow for more targeted smoking cessation interventions for smokers with cancer and reduce adverse treatment outcomes among this vulnerable population.
Specifically, only among participants who were adherent to varenicline, we observed that higher levels of smoking predicted higher levels of NA, while reducing or quitting smoking predicted lower levels of NA at the next visit, especially as participants progressed through treatment. On the other hand, these findings may be due to higher NA levels among those who are not successful in quitting (McClave et al., 2009) or because those with greater NA at baseline have more difficulty quitting smoking (Copeland et al., 2009). Thus, among cancer patients, varenicline adherence may ameliorate cessation-induced NA and support continued smoking abstinence. This is consistent with larger-scale trials that showed varenicline did not increase adverse neuropsychiatric events among smokers with and without psychiatric comorbidities (Anthenelli et al., 2016) and ameliorates abstinence-induced changes in affect (Doran et al., 2018; Patterson et al., 2009).
We did not find predictive associations between PA and smoking. This finding is in contrast with studies demonstrating that decreases in PA were predictive of relapse to smoking (Leventhal et al., 2014). Pre-existing deficits in PA has also been shown to predict further declines in PA upon smoking cessation (Cook et al., 2004). The lack of association between PA and smoking in this study suggests that varenicline may modulate PA, independent of smoking status. Other factors may influence the complicated relationships between affect and smoking cessation, such as whether the smoker’s cancer was caused by smoking (e.g., lung cancer), which should be investigated in future studies.
Despite the novel application, there are limitations to this analysis. First, due to model constraints, only participants with complete data were included. Second, PANAS scores had limited variability: NA scores demonstrated a large positive skew (1.78), reaching a maximum of 33 of 50 points, while PA scores had a moderate negative skew (−0.86), potentially reaching a ceiling at 50 of 50 points. These distributions may have limited our ability to detect significant changes in scores. Finally, only 24 participants were included in the nonadherent group, and thus these results should be interpreted with caution.
Using a cross-lagged panel analysis we found that self-reported smoking predicted later NA, but not PA, through 12 weeks of smoking cessation treatment amongst cancer patients who were adherent to varenicline. These findings further our understanding of these interrelationships, which may support the development of targeted interventions for this vulnerable population of high-risk smokers.
Supplementary Material
Highlights.
Negative affect during a quit attempt predicted subsequent smoking
Smoking did not predict later negative affect among cancer patients trying to quit
Varenicline attenuated abstinence-induced negative affect among cancer patients
Acknowledgments
Role of funding sources: This research was supported by grants from the National Cancer Institute (R01 CA165001; R01 CA184211) and the National Institute on Drug Abuse (K24 DA045244). NCI and NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of interest: Dr. Schnoll receives medication and placebo free from Pfizer. Dr. Schnoll has provided consultation to Pfizer and GlaxoSmithKline, and consults with Curaleaf. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, et al. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. The Lancet, 387:2507–20. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- Blalock JA, Lam C, Minnix JA, Karam-Hage M, Gritz ER, Robinson JD, Cinciripini PM (2011). The effect of mood, anxiety, and alcohol use disorders on smoking cessation in cancer patients. Journal of Cognitive Psychotherapy, 25:82–96. doi: 10.1891/0889-8391.25.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Rand KL, Bigatti SM, Stewart JC, Theobald DE, Wu J, Kroenke K (2013). Longitudinal relationships between fatigue and depression in cancer patients with depression and/or pain. Health Psychology, 32:1199–208. doi: 10.1037/a0029773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AJ, Veluz-Wilkins AK, Blazekovic S, Kalhan R, Leone FT, Wileyto EP, Schnoll RA, Hitsman B (2018). Cancer-related disease factors and smoking cessation treatment: Analysis of an ongoing clinical trial. Psychooncology, 27:471–76. doi: 10.1002/pon.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, McAfee T, Richards J, Swan GE (2011). Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research, 13:361–68. doi: 10.1093/ntr/ntr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N (2010). Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research, 12:978–82. doi: 10.1093/ntr/ntq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D (2004). Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research, 6:39–47. doi: 10.1080/14622200310001656849 [DOI] [PubMed] [Google Scholar]
- Copeland AL, Kulesza M, Hecht GS (2009). Pre-quit depression level and smoking expectancies for mood management predict the nature of smoking withdrawal symptoms in college women smokers. Addictive Behaviors, 34:481–83. doi: 10.1016/j.addbeh.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Cox LS, Africano NL, Tercyak KP, Taylor KL (2003). Nicotine dependence treatment for patients with cancer. Cancer, 98:632–44. doi: 10.1002/cncr.11538 [DOI] [PubMed] [Google Scholar]
- Crawford G, Weisbrot J, Bastian J, Flitter A, Jao NC, Carroll A, Kalhan R, Leone F, Hitsman B, et al. (2018). Predictors of Varenicline Adherence among Cancer Patients Treated for Tobacco Dependence and its Association with Smoking Cessation. Nicotine Tob Res. doi: 10.1093/ntr/nty133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Dubrava S, Anthenelli RM (2018). Effects of Varenicline, Depressive Symptoms, and Region of Enrollment on Smoking Cessation in Depressed Smokers. Nicotine & Tobacco Research:nty033–nty33. doi: 10.1093/ntr/nty033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D (2006). Elevated positive mood: a mixed blessing for abstinence. Psychology of Addictive Behaviors, 20:36–43. doi: 10.1037/0893-164x.20.1.36 [DOI] [PubMed] [Google Scholar]
- Fagerstrom K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research, 14:75–8. doi: 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, SDorfman S, Froelicher E, Goldstein M, et al. (2008). Treating Tobacco Use and Dependence: 2008 Update. U.S. Department of Health and Human Services, Rockville, MD. [Google Scholar]
- Hu L.t., Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6:1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jacobs EJ, Newton CC, Carter BD, Feskanich D, Freedman ND, Prentice RL, Flanders WD (2015). What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Annals of Epidemiology, 25:179–82 e1. doi: 10.1016/j.annepidem.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW (2014). Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology, 82:122–9. doi: 10.1037/a0035046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH (2009). Associations between smoking cessation and anxiety and depression among U.S. adults. Addictive Behaviors, 34:491–97. doi: 10.1016/j.addbeh.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C (2009). Varenicline Improves Mood and Cognition During Smoking Abstinence. Biological Psychiatry, 65:144–49. doi: 10.1016/j.biopsych.2008.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao NC, Paul Wileyto E, Carroll AJ, Kalhan R, et al. (2019). A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: Efficacy, safety, and adherence. Psychooncology, 28:561–69. doi: 10.1002/pon.4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.