Abstract
We investigated the effect of baseline Aβ, sex, and APOE on longitudinal tau accumulation in cerebrospinal fluid (CSF) in clinically-normal older adults. 239 participants (aged 56–89 years, Clinical Dementia Rating=0) underwent serial CSF collection for Aβ1–42, total-tau (t-tau) and phospho-tau181P (p-tau). We used pre-processed data from fully-automated Roche Elecsys® immunoassays. A series of linear regressions were used to examine cross-sectional effects of Aβ1–42, sex, and APOEε4 on baseline CSF tau, and linear mixed models for longitudinal changes in CSF tau. Cross-sectionally, CSF t-tau and p-tau were associated with abnormal Aβ1–42 and APOEε4, but not with sex. Longitudinally, low baseline CSF Aβ1–42 levels, but not APOEε4 or sex, predicted faster p-tau accumulation. The relationship between baseline CSF Aβ1–42 and tau accumulation was strongest in APOEε4 carriers, and particularly female carriers, relative to other groups. The current findings support an association between baseline CSF Aβ1–42 and changes in CSF tau. Elevated risk in females, apparent only in carriers, reinforces findings of sex-related vulnerability in those with genetic predisposition for Alzheimer’s disease.
Keywords: Cerebrospinal fluid, Alzheimer’s disease, amyloid, tau, APOE, sex
Since the mid-1990s, amyloid-β (Aβ) and tau, the hallmark pathological proteins of Alzheimer’s Disease (AD), can be detected and quantified in the cerebrospinal fluid (CSF) (Blennow et al., 1995; Nitsch et al., 1995), including in normal older adults (Petrie et al., 2009). Given the close association between tau and cognition (Nelson et al., 2012), identifying biological factors associated with the accumulation of tau pathology is critical to our understanding of the disease. Lack of standardization across centers and poor test-retest reliability, however, have made longitudinal CSF studies difficult to conduct until recently. Using conventional assays, no relationships have been observed between baseline CSF Aβ and changes in tau (Donohue, M. C. et al., 2017) even though Aβ pathology is an important factor promoting tau pathology(Jack et al., 2013). With the advent of more sophisticated immunoassays for measuring changes in CSF Aβ and tau, such as the Roche Elecsys® in the ADNI cohort (Bittner et al., 2016; Schindler et al., 2018), investigating potential risk factors for tau accumulation is now possible in the preclinical, clinically-normal stage of the disease.
The female sex and carriage of apolipoprotein ε4 (APOEε4) have both been implicated in the early pathophysiology of AD. Sex-specific elevated risk for AD biomarkers in APOEε4 carriers is increasingly evidenced in cross-sectional studies of cerebrospinal fluid (CSF) markers across the diagnostic spectrum from the ADNI sample (Altmann et al., 2014; Damoiseaux et al., 2012), as well as in meta-analyses (Hohman et al., 2018). In patients with mild cognitive impairment (MCI) from ADNI, female APOEε4 carriers exhibit a more AD-like elevated pattern of CSF tau levels relative to males (Altmann et al., 2014). Clinically-normal female APOEε4 carriers may also exhibit elevated cross-sectional CSF t-tau relative to males (Damoiseaux et al., 2012; Hohman et al., 2018), however, this finding has not been proven as robust in more recent studies (Altmann et al., 2014; Hohman et al., 2018). By contrast, human studies do not report consistent evidence of sex by APOE effects on Aβ burden across a range of cohorts (Altmann et al., 2014; Buckley et al., 2018; Hohman et al., 2018; Morris et al., 2010), suggesting that an emergence of sex-specific biological risk may appear downstream of Aβ (Fisher et al., 2018). Further, although mounting evidence exists of sex-APOE effects at the cross-section, studies have yet to examine the modifying association of sex and APOE on longitudinal CSF changes.
The aim of the current study was to examine the effect of baseline Aβ, sex, and APOE on longitudinal changes in CSF tau in clinically-normal older adults. The primary hypothesis was abnormal CSF Aβ would lead to greater CSF tau accumulation. We hypothesized that this relationship would be exacerbated in APOEε4 carriers, and that clinically-normal female APOEε4 carriers would show greater longitudinal changes in CSF tau in comparison with males.
Methods
Participants
Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). From this publicly available dataset, 239 who were diagnosed as clinically-normal participants at baseline (52% Female, Age = 74 (5.9) years [56–89 years]) were selected based on their availability of serial CSF collection. In this study, participants had a median of 2 visits of CSF collection and a range of 2 to 7 visits. To be classified as clinically-normal, participants were required to score 0 on the clinical dementia rating (CDR) scale global score, greater than 24 on the Mini-Mental State Examination, less than 6 on the Geriatric Depression Scale (short form) and perform within validated education-adjusted norms on Logical Memory II delayed recall. To be included in this study, participants were required to possess at least two annual CSF collections: 139 only completed two visits in all, 62 completed 3 visits, 12 completed 4 visits, 12 completed 5 visits, and 4 completed 6 visits, and 10 completed 7 visits. Baseline demographics can be found in Table 1. A blood sample for assessment of APOE genotype was also obtained for the purposes of grouping individuals as APOEε4 carriers and non-carriers (five individuals were APOEε4 homozygotes). Written informed consent was obtained from all individuals participating in the ADNI study. We conducted the procedures for this study under the ethical guidelines stipulated by the Partners Human Research Committee, which is the Institutional Review Board for the Massachusetts General Hospital and Brigham and Women’s Hospital.
Table 1.
Baseline demographics and CSF biomarkers
| Overall (n=239) | Females (n=125) | Males (n=114) | ||||
|---|---|---|---|---|---|---|
| APOEε4- (n = 98) | APOEε4+ (n = 27) | APOEε4- (n = 83) | APOEε4+ (n = 31) | Uncorrected group comparison p value | ||
| Mean (SD) | ||||||
| Age (years) | 74.3 (5.9) | 74.3 (5.6) | 72.9 (6.1) | 74.8 (5.5) | 73.9 (7.4) | 0.54 |
| Education (years) | 16.4 | 15.8 (2.5) | 16.2 (2.9) | 17.1 (2.5) | 16.5 (2.8) | 0.18 |
| Race (% white) | 90 | 89 | 93 | 93 | 87 | 0.71 |
| CSF Aβ1–42 | 1331.8 (651.4) | 1434.7 (652.6) | 999.2 (606.2)a | 1418.4 (587.5) | 1064.2 (706.7)a | <.001 |
| CSF t-tau | 239.8 (90.9) | 234.2 (96.5) | 283.4 (99.8)a | 221.9 (73.3) | 267.4 (93.1)a | <.001 |
| CSF p-tau | 22.0 (9.4) | 21.0 (9.2) | 27.8 (11.6)a | 20.1 (7.8) | 25.3 (9.8)a | <.001 |
| Aβ (% positive*) | 41 | 32 | 67 | 34 | 65 | <.001 |
CSF = cerebrospinal fluid, t-tau = total-tau, p-tau = phospho-tau.
Based on published cutoff: positive = <1100 pg/ml (Hansson et al., 2018).
significant difference between APOEε4+ and APOEε4−
Cerebrospinal fluid
Data for cerebrospinal fluid analyses were accessed from previously processed samples that were available through the ADNI website (http://loni.adni.usc.edu/). Lumbar punctures were performed as previously described in the ADNI procedures manual (http://www.adni-info.org/). CSF samples were frozen on dry-ice soon after collection (~1 hour) and shipped to the UPenn Medical Center ADNI Biomarker Core laboratory. 0.5mL aliquots were prepared from these and stored in polypropylene tubes at −80°C.
For the current study, pre-processed LONI data using the fully automated Roche Elecsys® immunoassays (Bittner et al., 2016; Shaw et al., 2016) for Aβ1–42, total-tau (t-tau) and phospho-tau181P (p-tau) were used for analyses (http://loni.adni.usc.edu/). Unthawed aliquots of ADNIGO/2 CSF samples were analyzed by the electrochemiluminescence immunoassays (ECLIA) for the three analytes on a fully automated Elecsys cobas e 601 instrument (software v05.02) and a single lot of reagents for each biomarker. These measures were gathered via a Roche Study Protocol at the UPenn/ADNI Biomarker Laboratory. Quantification of these measures was performed using 36 runs, with each sample running a single time for each of the 3 CSF analytes. For each run, quality control results were required to adhere to within stated limits to meet acceptance criteria for validation. Although each of the three CSF analytes were treated as continuous measures in analyses (in picograms per milliliter [pg/mL]), a cut-off for CSF Aβ1–42 of 1100pg/ml was also used that best demarcated PET-positive and PET-negative groups from a previous publication (Bittner et al., 2016; Hansson et al., 2018).
Analyses
We used R (version 3.3.3) software to conduct a series of linear regressions and linear mixed models ascertaining the relationship between sex, APOEε4 and CSF Aβ1–42 on CSF t-tau and p-tau. Linear regression models were constructed to ascertain the effects of sex, APOEε4 and baseline CSF Aβ1–42 on CSF t-tau and p-tau at baseline after adjusting for the effect of age. Linear mixed effects models examined the influence of sex, APOEε4 and baseline CSF Aβ1–42 over time on longitudinal CSF t-tau and p-tau. Fixed effects of time were considered as both a main effect and in interaction with other predictors. In these models, random effects of intercept and slope were modeled using maximum likelihood estimation, while co-varying for age at baseline. The following fully-factorial linear mixed-effects models were examined:
Model 1: CSF taua ~ Baseline CSF Aβ1–42 OR sex OR APOEε4 * time + age * time
Model 2: CSF taua ~ Baseline CSF Aβ1–42 * sex * time + age * time
Model 3: CSF taua ~ Baseline CSF Aβ1–42 * APOEε4 * time + age * time
Model 4: CSF taua ~ Baseline CSF Aβ1–42 *sex * APOEε4 * time + age * time
atau = CSF t-tau or CSF p-tau
We report all longitudinal analyses, but also adjust for 12 multiple comparisons in our longitudinal analyses, using a Sidak-corrected α = 0.004). As baseline analyses have been previously reported in the literature, and as such were not of direct interest, we did not include these comparisons in our significance adjustment. We ran post-hoc analyses constraining CSF Aβ1–42 to the technical limits of 200–1700 (i.e. 58 data points sat above the 1700 range, with none below, and so were constrained to 1700 but not removed from analyses) to confirm findings were not driven by outliers.
Results
Demographics
Subject demographics can be found in Table 1. There was no difference in the frequency of APOEε4 status between males and females (χ2 = 0.73, p = 0.39). APOEε4 carriers exhibited abnormal baseline CSF Aβ1–42 and CSF tau. There was no significant difference by sex and APOE status with regard to length of time in the study (F = 0.24, p = 0.65). There were no differences in progression rates to MCI or dementia by sex (Hazard Ratio [HR] = 1.10 [95% CI: 0.53–2.29], p = 0.79), APOEε4 status (HR = 1.93 [95% CI: 0.82–4.54], p = 0.79), or the interaction between these factors (HR = 1.92 [95% CI: 0.38–9.81], p = 0.43), after adjusting for age or CSF Aβ1–42, t-tau or p-tau slopes. In all, 32 individuals progressed to MCI or dementia over the course of the current study, with 15% female APOEε4 carriers, 10% female noncarriers, 13% male carriers and 17% male non-carriers progressing over approximately 4.43 years (SD = 3.1). The R2 between p-tau and t-tau was 0.95, and as such, observed identical results with both outcomes for the cross-sectional analyses below. By contrast, the R2 between t-tau and p-tau slopes was 0.74, and as such, their trajectories were highly, but not perfectly, correlated.
Baseline CSF t-tau and p-tau
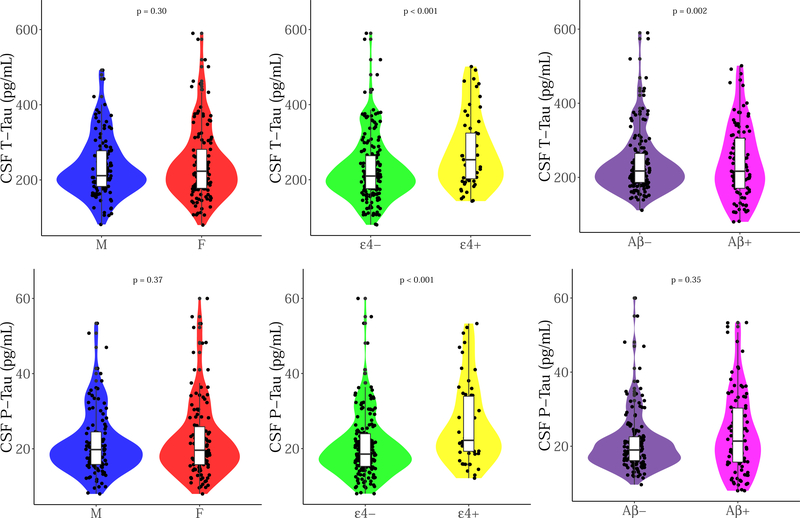
Figure 1 represents violin plots of sex, APOE and CSF Aβ1–42 on CSF t-tau and p-tau. After adjusting for age, both APOE and CSF Aβ1–42 were significantly associated with greater CSF t-tau (βAPOE = 0.23, p < 0.001; βAβ = 0.20, p = 0.002), while APOE alone was associated with greater p-tau (βAPOE = 0.28, p < 0.001; βAβ = 0.06, p = 0.35). Sex had no association with baseline CSF t-tau (β = 0.07, p = 0.30) or CSF p-tau (β = 0.06, p = 0.37) after adjusting for covariates. There was no sex-APOE interaction on baseline CSF tau (βt-tau = 0.03, p = 0.83; βp-tau = 0.10, p = 0.50), and no sex-CSF Aβ1–42 interaction on baseline CSF tau (βt-tau = < −0.001, p = 0.49; βp-tau < −0.001, p = 0.39), however, CSF Aβ1–42-APOE was associated with CSF tau (βt-tau < −0.001, p = 0.03; βp-tau < −0.001, p = 0.03). A borderline three-way interaction between sex, APOE and CSF Aβ1–42 was found with CSF t-tau (β < −0.001, p = 0.05), but was sub-threshold for p-tau (β < −0.001, p = 0.07).
Figure 1. Baseline CSF t-tau and p-tau by (A) sex (B) APOEε4 status and (C) CSF Aβ1–42 status.
The x-axis represents CSF t-tau or p-tau pg/mL at baseline. Each violin plot represents the density of the data at each level of pg/mL, with a boxplot overlaid to indicate the median level for each group.
Longitudinal CSF t-tau and p-tau
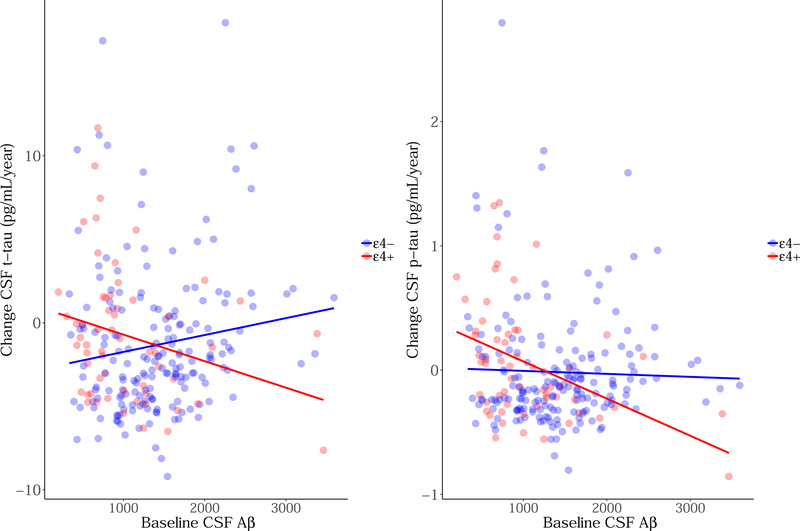
Model estimates can be found in Table 2 (with full models in Appendix A). After adjusting for age, baseline CSF Aβ1–42 was trend-associated with increasing CSF p-tau levels (tt-tau = −0.93, p = 0.35; tp-tau = −2.50, p = 0.01), however, this did not survive multiple comparison. This effect appeared after the first year of follow-up (p<1 year = 0.94; p>1 year ~ 0.01–0.002); in order to determine this significance, we subset data in the analyses according to follow-up year. Neither sex nor APOE genotype was associated with increasing CSF tau over time. No interactions between sex and CSF Aβ1–42 were found on longitudinal CSF tau (tt-tau = 0.53; p = 0.59; tp-tau = 1.15; p = 0.25). An interaction between APOE and CSF Aβ1–42 exhibited a trend-level association with changing CSF t-tau and p-tau (tt-tau = −2.12; p = 0.03; tp-tau = −2.50; p = 0.01; see Figure 2) that did not survive multiple comparison. A post-hoc analysis revealed that lower baseline CSF Aβ1–42 was associated with increasing CSF t-tau and p-tau in APOEε4 carriers (tt-tau = −1.93, p = 0.05; tp-tau = −2.36, p = 0.02), but not in non-carriers (tt-tau = −0.03, p = 0.97; tp-tau = −0.92, p = 0.36). When constraining CSF Aβ1–42 to the technical limits of 200–1700 pg/mL (not removing these data points from the model), the interaction was not significant (tt-tau = −1.04, p = 0.30; tp-tau = −1.77, p = 0.08).
Table 2.
Unstandardized model estimates in association with longitudinal CSF t-tau and p-tau
| CSF t-tau | CSF p-tau | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std.Error | DF | t value | p value | Estimate | Std.Error | DF | t value | p value | ||
| Model 1A: Baseline CSF Aβ*Time | Model 1A: Baseline CSF Aβ*Time | ||||||||||
| CSF Aβ | 0.02 | 0.01 | 236 | 2.97 | 0.003 | CSF Aβ | 0.001 | 0.001 | 236 | 0.84 | 0.40 |
| CSF Aβ:Time | −0.001 | 0.11 | 423 | −0.93 | 0.35 | CSF Aβ:Time | −0.0002 | 0.0001 | 423 | −2.50 | 0.01 |
| Model 1B: Sex*Time | Model 1B: Sex*Time | ||||||||||
| Sex (F) | 12.27 | 11.63 | 236 | 1.06 | 0.29 | Sex (F) | 1.06 | 1.21 | 236 | 0.88 | 0.38 |
| Sex (F):Time | 0.19 | 1.29 | 423 | 0.15 | 0.88 | Sex (F):Time | 0.02 | 0.14 | 423 | 0.17 | 0.87 |
| Model 1C: APOE*Time | Model 1C: APOE*Time | ||||||||||
| APOEε4+ | 50.73 | 13.22 | 236 | 3.84 | <0.001 | APOEε4+ | 6.22 | 1.36 | 236 | 4.58 | <0.001 |
| APOEε4+:Time | −1.74 | 1.54 | 423 | −1.13 | 0.26 | APOEε4+:Time | 0.07 | 0.17 | 423 | 0.40 | 0.67 |
| Model 2: Baseline CSF Aβ*Sex*Time | Model 2: Baseline CSF Aβ*Sex*Time | ||||||||||
| CSF Aβ:Sex (F):Time | 0.001 | 0.002 | 421 | 0.53 | 0.59 | CSF Aβ:Sex (F):Time | 0.0002 | 0.0002 | 421 | 1.15 | 0.25 |
| Model 3: Baseline CSF Aβ*APOE*Time | Model 3: Baseline CSF Aβ*APOEε4+*Time | ||||||||||
| CSFAβ:APOEε4+ :Time | −0.005 | 0.003 | 421 | −2.12 | 0.03 | CSFAβ:APOEε4+ :Time | 0.001 | 0.0002 | 421 | −2.50 | 0.01 |
| Model 4: Baseline CSF Aβ*Sex*APOE*Time | Model 4: Baseline CSF Aβ*Sex*APOE*Time | ||||||||||
| CSFAβ:Sex(F):A POEε4+:Time | −0.01 | 0.005 | 417 | −1.99 | 0.04 | CSFAβ:Sex(F):A POEε4+:Time | −0.001 | 0.001 | 417 | −1.77 | 0.08 |
| Post-hoc Model in FEMALES: Baseline CSF Aβ*APOE*Time | |||||||||||
| CSFAβ:APOEε4+ :Time | −0.01 | 0.004 | 213 | −2.52 | 0.01 | ||||||
| Post-hoc Model in MALES: Baseline CSF Aβ*APOE*Time | |||||||||||
| CSFAβ:APOEε4+ :Time | −0.003 | 0.003 | 203 | −1.06 | 0.29 | ||||||
Figure 2. Longitudinal CSF t-tau and p-tau slopes by baseline CSF Aβ1–42 as stratified by APOEε4 status.
The y-axis represents slopes of CSF tau change in pg/mL per year (extracted from linear mixed models), while the x-axis represents baseline CSF Aβ1–42. The colors represent: red = APOEε4 carriers and blue = APOEε4 non-carriers.
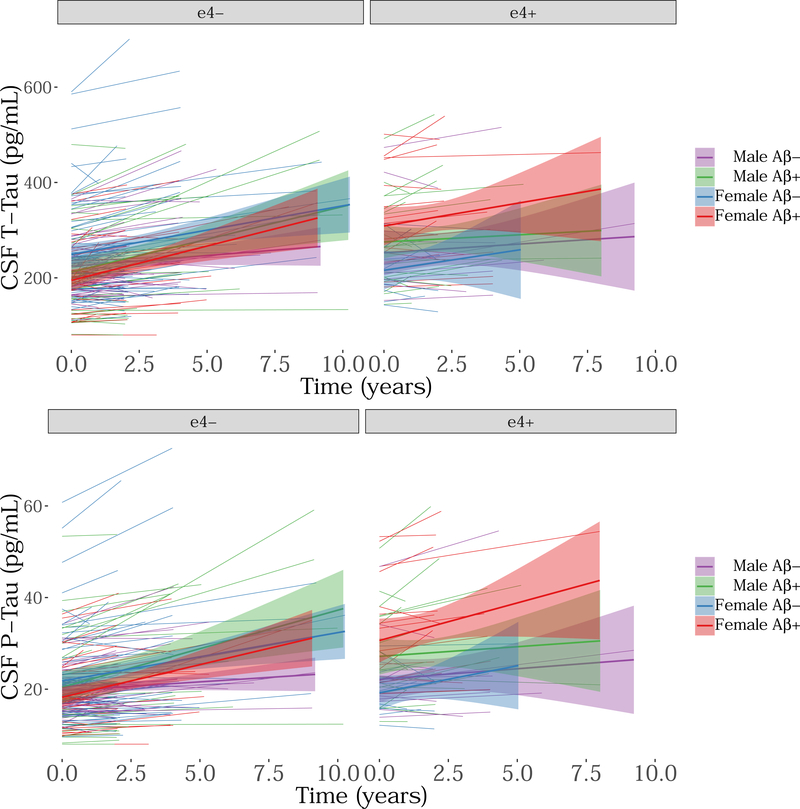
A three-way interaction between sex, APOEε4 status, and CSF Aβ1–42 was found only in association with rates of accumulation of CSF t-tau (t = −1.95, pt-tau = 0.04; t = −1.73, pp-tau = 0.08; see Figure 3). After adjusting for multiple comparisons, however, this relationship was not considered significant. In addition, we found that one female APOEε4 carrier outlier exhibited a strong influence on this relationship (pt-tau = 0.40), and as such, this finding needs to be interpreted with caution. A post-hoc analysis revealed a significant interaction between baseline CSF Aβ1–42 and APOEε4 status on CSF t-tau change in females (t = −2.52, p = 0.01), but not in males (t = −1.11, p = 0.29). That is, in the female group, APOEε4 carriers showed greater CSF t-tau change in those with abnormal CSF Aβ1–42 in comparison with non-carriers. When constraining CSF Aβ1–42 to the technical limits of 200–1700 pg/mL, the above three-way interaction was not significant (t = −1.81, pt-tau = 0.07; t = −1.84, pp-tau = 0.07).
Figure 3. Longitudinal CSF t-tau and p-tau accumulation by sex, APOE and baseline CSF Aβ1–42 (depicted here as a dichotomous variable).
The y-axis represents CSF tau pg/mL while the x-axis represents time in the study (in years). The colors represent: purple = male Aβ−, green = male Aβ+, blue = female Aβ−, red = female Aβ+.
Discussion
We present preliminary findings suggesting a trend towards greater CSF tau accumulation in clinically-normal APOEε4 carriers with abnormal CSF Aβ1–42 than non-carriers. In addition, these data provide preliminary evidence of a trend this greater tau accumulation occurring in female carriers. Due to the trend-level associations reported in our results, however, replication is necessary in other longitudinal cohorts. Accumulating evidence supports an important role of the interaction between Aβ and tau in the earliest stages of AD pathophysiology. This Aβ-tau interaction has been shown to have greater impact than either pathology alone on glucose metabolism (Hanseeuw et al., 2017), resting-state functional connectivity (Schultz et al., 2017), retrospective (Schöll et al., 2016) or prospective cognitive decline (Sperling et al., 2018), and clinical progression (Desikan et al., 2012; Hansson et al., 2006). In this longitudinal CSF dataset, we observed a significant association between baseline CSF Aβ1–42 and the rate of CSF p-tau accumulation using the Roche Elecsys immunoassay, indicating that both pathologies interact in clinically-normal older adults. Baseline CSF Aβ1–42 did not predict t-tau change as some APOEε4 non-carriers with normal Aβ had an increase in t-tau but not p-tau (see Figure 2) supporting the notion that t-tau changes may be less specific to AD physiopathology. Both analytes are proximal to clinical progression of the disease (Mattsson et al., 2009) and tau-PET topographies (Brier et al., 2016), however, and are traditionally highly correlated together (Mattsson et al., 2009), although their slopes do not correlate as well as their baseline values. Among APOEε4 carriers, we found baseline CSF Aβ1–42 was associated with both t-tau and p-tau changes. By contrast, previous reports using overlapping data with the xMAP immunoassay have not reliably revealed an association between baseline CSF Aβ and longitudinal CSF p-tau (Donohue, Michael C et al., 2017) underscoring the use of the more advanced assay to interrogate CSF tau accumulation.
This study is the first to describe the interactive effect of APOE genotype and CSF Aβ1–42 on longitudinal measures of CSF t-tau and p-tau in a CN cohort. Previous cross-sectional findings of clinically-normal older adults did not find a relationship between APOE and CSF tau (Morris et al., 2010). Indeed, APOE has been more closely associated with Aβ than tau at the cross-section (Morris et al., 2010), and has also been associated with faster Aβ accumulation in CN older adults with sub-threshold levels of baseline Aβ (Lim et al., 2017). Similar to our findings, an earlier study in ADNI using the xMAP immunoassay did not report a main effect of APOE on longitudinal changes in CSF tau (Toledo et al., 2013). Our findings suggest that APOEε4 carriers do exhibit greater tau accumulation, but only in those with abnormal Aβ, and this effect was subtle. Mouse models support Aβ-associated neuritic degeneration exacerbated by the presence of the apolipoprotein E protein (that is, in apoE+ mice)(Holtzman et al., 2000). Although interactive effects of APOE and baseline Aβ on changes in tau have not yet been reported, effects on downstream cognitive decline have been repeatedly shown (Lim et al., 2015; Mormino et al., 2014), highlighting the deleterious effect of APOE on pathological processes in AD. Effects of APOE and baseline Aβ on neurodegeneration are less robust (Jack et al., 2015; Villemagne et al., 2013), suggesting that APOE genotype may express only subtle effects on downstream pathology. Due to issues of power, we did not explore dose-response effects based on heterozygotic or homozygotic genotype; it is possible that stronger effects exist in homozygotes, which may be masked by the rarity of this variant.
While cross-sectional differences in CSF tauopathy predominantly exist in cognitively-impaired female APOEε4 carriers, regardless of Aβ (Altmann et al., 2014; Hohman et al., 2018), our findings, suggest that in clinically-normal individuals, changes in CSF tau can be detected in clinically-normal female APOEε4 carriers when CSF Aβ1–42 levels at baseline are abnormally low. This finding must be interpreted with caution as it did not survive multiple comparison adjustment and was influenced, to some degree, by outliers. Nevertheless consistent with our finding, Hohman and colleagues recently observed that CN female APOEε4 carriers with low CSF Aβ1–42 had higher tau levels at the cross-section in a meta-analysis of several independent cohorts (Hohman et al., 2018). It is very possible that the early appearance of Aβ in preclinical stages of the disease instigate downstream tauopathological events (Sperling et al., 2014), which may represent the crucial epicenter for emerging sex differences in AD risk. Taken together, our findings and those of others support the notion of an interaction between sex and APOE to play a disease modifying role on the Aβ-tau relationship. The current study, however, extends beyond cross-sectional evidence, and provides preliminary longitudinal evidence of sex-APOE effects on CSF tau changes in preclinical AD.
In transgenic mouse models, deposition of both Aβ and tauopathy are greater in females. In a mouse model that expresses both mutant tau (P301L) and Aβ precursor protein (APP), females show greater, and earlier, neurofibrillary deposition than males (Lewis et al., 2001). This same study also reported this female-bias exists to a greater extent in the double-mutation than solely in models of mutant tau alone. The authors posited this arose as a function of sex differences in initial levels of Aβ accumulation (supported by findings of sex differences in the Tg2576 mouse model that exhibits only mutant APP (Callahan et al., 2001)). Human studies have not replicated sex differences in Aβ burden with either CSF (Altmann et al., 2014) or PET imaging (Mielke et al., 2012; Morris et al., 2010), however, a recent study suggests clinically-normal females with familial history of AD dementia may exhibit greater Aβ accumulation than males with familial history proximal to estimated parental year of onset (Villeneuve et al., 2018). Further, recent studies also suggest that clinically-normal females display faster cognitive decline (Buckley et al., 2018) and hippocampal atrophy (Koran et al., 2017) than males despite similarly abnormal levels of Aβ, implying that female susceptibility to tauopathy and neurodegeneration may occur after the onset of Aβ abnormality. Further investigations are needed, however, to fully elucidate the temporal pattern of sex related differences along the AD pathophysiologic trajectory.
The current study has several limitations. A major drawback involves the trend-level results that we report in this study; although these findings allude to a promising relationship between sex, APOE and Aβ to influence CSF tau, it is imperative to replicate these data in other out-of-sample cohorts. These data also involve a convenience sub-sample of participants from the ADNI study who opted into serial CSF collection, and as such, are not representative of the wider population. In addition, the ADNI population includes largely highly-educated, less racially diverse, and higher-socioeconomic individuals, which also limits the generalizability of these findings. It will be important for future studies to examine other covariates, beyond age, that might influence sex and APOE relationships on CSF Aβ1–42 and longitudinal tau. Our analyses also included CSF Aβ1–42 data that was extrapolated beyond the technical limits of the 200–1700 pg/mL measuring range of the Elecsys assay. In order to account for this, we also carried out post-hoc analyses within these ranges and found the same pattern of results, however, these findings require replication in an independent sample. Furthermore, the issue of multiple comparisons and outlier influences, along with a large majority of sex difference findings in this area arising from ADNI, underscores the need for validation in another sample.
Further, post-mortem research has found that for a given level of clinical impairment at death, females exhibit greater expression of both neuritic plaques and neurofibrillary tangles (Barnes et al., 2005). Here, the authors implicated APOEε4 as the mechanistic pathway for female vulnerability. The biological mechanism explaining the greater impact of APOEε4 on females remains unclear, however, animal models have implicated the role of sex hormones (Pfankuch et al., 2005). Indeed, the menopausal phase cannot be discounted as a watershed moment in the critical loss of protection for females along the AD pathophysiological pathway, which may be exacerbated by APOEε4 (Hasanpour et al., 2018).
Conclusions
We provide evidence that clinically-normal APOEε4 carriers with abnormal baseline CSF Aβ1–42 exhibit accelerated rates of longitudinal CSF t-tau and p-tau change in comparison with non-carriers with similar levels of CSF Aβ1–42. Specifically, preliminary findings suggest that female APOEε4 carriers demonstrated a stronger Aβ-tau relation than males APOEε4 carriers. Mounting evidence implicates female- APOEε4 vulnerability to tau across the diagnostic spectrum. While recent work supports the notion of sex-APOE effects on CSF tau at the cross-section across multiple independent cohorts (Hohman et al., 2018), our findings reveal the potential early emergence of sex-APOE differences in longitudinal tau in preclinical AD, mirroring findings in transgenic mouse models of AD.
Supplementary Material
Highlights.
No sex, sex-APOE or sex-Aβ1–42 effects on baseline CSF tau in healthy older adults
Accelerated CSF t-tau and p-tau change in older adults with lower CSF Aβ1–42
Female APOEε4 carriers with low Aβ1–42 show trends of greater CSF tau change
Acknowledgements
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org. Dr Buckley is funded by the NHMRC Dementia Research Fellowship (APP1105576). Dr Hanseeuw is funded by the Belgian National Fund for Scientific Research (FNRS grant #SPD 28094292) and the Belgian Foundation for Alzheimer Research (SAO-FRA grant #P16.008). This work was supported with funding from the National Institutes of Health, including P01 AG036694 (Sperling and Johnson), P50 AG005134 (Sperling, Johnson), K23 AG049087 (Chhatwal), K24 AG035007 (Sperling). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers S10RR021110, S10RR023401, and S10RR023043. For ADNI, data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Declaration of interests:
Dr Buckley is funded by the NHMRC Dementia Research Fellowship (APP1105576). Dr Schultz has been a paid consultant for Janssen Pharmaceuticals and Biogen. Dr Chhatwal is funded by NIH (K23 AG049087). Dr Rentz served as a consultant for Eli Lilly, Biogen Idec, Lundbeck Pharmaceuticals, and serves as a member of the Scientific Advisory Board for Neurotrack. Dr Gomez-Isla has participated as speaker in a Lilly sponsored educational symposium and serves as member of a Lilly Data Monitoring Committee (DMC). Dr Johnson has served as paid consultant for Bayer, GE Healthcare, Janssen Alzheimer’s Immunotherapy, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, ISIS Pharma, AZTherapy, GEHC, Lundberg, and Abbvie. He is a site coinvestigator for Lilly/Avid, Pfizer, Janssen Immunotherapy, and Navidea. He has spoken at symposia sponsored by Janssen Alzheimer’s Immunotherapy and Pfizer. K. Johnson receives funding from NIH grants R01EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497 and the Alzheimer’s Association grant ZEN-10-174210. Dr Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen. R. Sperling receives research support from Janssen Pharmaceuticals, and Eli Lilly and Co. These relationships are not related to the content in the manuscript. She also receives research support from the following grants: P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, Fidelity Biosciences, Harvard NeuroDiscovery Center, and the Alzheimer’s Association. Dr Hanseeuw is funded by the Belgian National Fund for Scientific Research (FNRS grant #SPD 28094292) and the Belgian Foundation for Alzheimer Research (SAO-FRA grant #P16.008).
Abbreviations
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s disease Neuroimaging Initiative
- APOE
apolipoprotein
- CN
clinically-normal
- CSF
cerebrospinal fluid
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachel F. Buckley, Florey Institutes of Neuroscience and Mental Health, Melbourne / Melbourne School of Psychological Science, University of Melbourne / Department of Neurology, Massachusetts General Hospital / Harvard Medical School
Elizabeth C. Mormino, Department of Neurology and Neurological Sciences, Stanford University
Jasmeer Chhatwal, Department of Neurology, Massachusetts General Hospital/ Harvard Medical School/ Center for Alzheimer Research and Treatment, Department of Neurology, Brigham and Women’s Hospital.
Aaron P. Schultz, Department of Neurology, Massachusetts General Hospital, Harvard Medical School/ Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital/ Department of Psychiatry, Massachusetts General Hospital
Jennifer S. Rabin, Department of Psychiatry, Massachusetts General Hospital/ Harvard Medical School
Dorene M. Rentz, Department of Neurology, Massachusetts General Hospital/ Harvard Medical School/ Center for Alzheimer Research and Treatment, Department of Neurology, Brigham and Women’s Hospital
Diler Acar, Department of Neurology, Brigham and Women’s Hospital.
Michael J. Properzi, Department of Neurology, Massachusetts General Hospital
Julien Dumurgier, Department of Neurology, Massachusetts General Hospital.
Heidi Jacobs, Department of Neurology, Massachusetts General Hospital, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Massachusetts General Hospital/Harvard Medical School.
Teresa Gomez-Isla, Department of Neurology, Massachusetts General Hospital/Harvard Medical School/Massachusetts Alzheimer’s Disease Research Center.
Keith A. Johnson, Department of Neurology, Massachusetts General Hospital/ Harvard Medical School/ Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital/ Center for Alzheimer Research and Treatment, Department of Neurology, Brigham and Women’s Hospital/ Division of Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital
Reisa A. Sperling, Department of Neurology, Massachusetts General Hospital/ Harvard Medical School/ Center for Alzheimer Research and Treatment, Department of Neurology, Brigham and Women’s Hospital
Bernard J. Hanseeuw, Department of Neurology, Massachusetts General Hospital/ Harvard Medical School/ Gordon Center for Medical Imaging, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital/Department of Neurology, Cliniques Universitaires Saint-Luc, Brussels, Belgium
References
- Altmann A, Tian L, Henderson VW, Greicius MD, 2014. Sex modifies the APOE‐related risk of developing Alzheimer disease. Annals of neurology 75(4), 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of general psychiatry 62(6), 685–691. [DOI] [PubMed] [Google Scholar]
- Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, Hubeek I, Gibson D, Chu DC, Eichenlaub U, Heiss P, Kobold U, Leinenbach A, Madin K, Manuilova E, Rabe C, Blennow K, 2016. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimer’s & Dementia 12(5), 517–526. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E, 1995. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 26(3), 231–245. [DOI] [PubMed] [Google Scholar]
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM, 2016. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science Translational Medicine 8(338), 338ra366–338ra366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, Papp KV, Jacobs H, Burnham S, Hanseeuw BJ, Doré V, Dobson A, Masters C, Waller M, Rowe CC, Maruff P, Donohue MC, Rentz DM, Kirn D, Hedden T, Chhatwal J, Schultz AP, Johnson KA, Villemagne VL, Sperling RA, 2018. Sex, Amyloid, and APOEe4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimer’s & Dementia 14(9), 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC, 2001. Augmented Senile Plaque Load in Aged Female β-Amyloid Precursor Protein-Transgenic Mice. The American Journal of Pathology 158(3), 1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD, 2012. Gender Modulates the APOE ε4 Effect in Healthy Older Adults: Convergent Evidence from Functional Brain Connectivity and Spinal Fluid Tau Levels. The Journal of Neuroscience 32(24), 8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM, Initiative A s.D.N., 2012. Amyloid-β–associated clinical decline occurs only in the presence of elevated p-tau. Archives of neurology 69(6), 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS, 2017. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA 317(22), 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS, Alzheimer’s Disease Neuroimaging I, 2017. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA 317(22), 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DW, Bennett DA, Dong H, 2018. Sexual Dimorphism in Predisposition to Alzheimer’s Disease. Neurobiology of Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Schultz AN, Papp K, Mormino EC, Sepulcre J, Bark J, Cosio D, LaPoint M, Chhatwal J, Rentz D, Sperling R, Johnson K, 2017. FDG metabolism associated with tau-amyloid interaction predicts memory decline. Annals of Neurology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM, 2018. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s & Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L, 2006. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology 5(3), 228–234. [DOI] [PubMed] [Google Scholar]
- Hasanpour M, Nourazarian A, Geranmayeh MH, Nikanfar M, Khaki-Khatibi F, Rahbarghazi R, 2018. The Dynamics of Neurosteroids and Sex-Related Hormones in the Pathogenesis of Alzheimer’s Disease. NeuroMolecular Medicine 20(2), 215–224. [DOI] [PubMed] [Google Scholar]
- Hohman T, Dumitrescu L, Barnes L, Thambisetty M, Beecham G, Kunkle B, Gifford K, Bush W, Chibnik L, Mukherjee S, De Jager P, Kukull W, Crane P, Resnick S, Keene C, Montine T, Schellenberg G, Haines J, Zetterberg H, Blennow K, Larson E, Johnson S, Albert M, Bennett D, Schneider J, Jefferson A, 2018. Sexspecific effects of Apolipoprotein E on cerebrospinal fluid levels of tau. JAMA Neurology 75(8), 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM, 2000. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences 97(6), 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12(2), 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, Lowe V, Senjem ML, Gunter JL, Machulda MM, Gregg BE, Pankratz VS, Rocca WA, Petersen RC, 2015. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurology 72(5), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran MEI, Wagener M, Hohman TJ, 2017. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior 11(1), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin W-L, Chisholm L, Corral A, Jones G, Yen S-H, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E, 2001. Enhanced Neurofibrillary Degeneration in Transgenic Mice Expressing Mutant Tau and APP. Science 293(5534), 1487–1491. [DOI] [PubMed] [Google Scholar]
- Lim YY, Mormino EC, Initiative F t.A.s.D.N., 2017. APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology 89(10), 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, Snyder PJ, Martins RN, Masters CL, Rowe CC, Maruff P, 2015. APOE ε4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging 36(3), 1239–1244. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, 2009. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama 302(4), 385–393. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM, Boeve BF, Senjem ML, 2012. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 79(15), 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA, 2014. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 82(20), 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA, 2010. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology 67(1), 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG, 2012. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71(5), 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch RM, Rebeck GW, Deng M, Richardson UI, Tennis M, Schenk DB, Vigo-Pelfrey C, Lieberburg I, Wurtman RJ, Hyman BT, et al. , 1995. Cerebrospinal fluid levels of amyloid beta-protein in Alzheimer’s disease: inverse correlation with severity of dementia and effect of apolipoprotein E genotype. Ann Neurol 37(4), 512–518. [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S, 2009. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol 66(5), 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfankuch T, Rizk A, Olsen R, Poage C, Raber J, 2005. Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Research 1053(1), 88–96. [DOI] [PubMed] [Google Scholar]
- Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, Wahl S, Benzinger TLS, Holtzman DM, Morris JC, Fagan AM, 2018. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer’s & Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, 2016. PET imaging of tau deposition in the aging human brain. Neuron 89(5), 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, Huijbers W, LaPoint M, Buckley RF, Johnson KA, 2017. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. Journal of Neuroscience 37(16), 4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Fields L, Korecka M, Waligórska T, Trojanowski JQ, Allegranza D, Bittner T, He Y, Morgan K, Rabe C, 2016. METHOD COMPARISON OF AB(1–42) MEASURED IN HUMAN CEREBROSPINAL FLUID SAMPLES BY LIQUID CHROMATOGRAPHY-TANDEM MASS SPECTROMETRY, THE INNO-BIA ALZBIO3 ASSAY, AND THE ELECSYS® B-AMYLOID(1–42) ASSAY. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 12(7), P668. [Google Scholar]
- Sperling RA, Mormino E, Johnson K, 2014. The Evolution of Preclinical Alzheimer’s Disease: Implications for Prevention Trials. Neuron 84(3), 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, Hanseeuw B, Buckley RF, Chhatwal J, Hedden T, Marshall GA, Quiroz YT, Donovan N, Jackson JD, Gatchel JR, Rabin JS, Jacobs H, Yang H-S, Properzi MJ, Kirn D, Rentz DM, Johnson K, 2018. The impact of Aβ and tau on prospective cognitive decline in older individuals. Annals of Neurology Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Xie SX, Trojanowski JQ, Shaw LM, 2013. Longitudinal change in CSF Tau and Aβ biomarkers for up to 48 months in ADNI. Acta Neuropathologica 126(5), 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins RN, Maruff P, Ames D, Rowe CC, Masters CL, 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12(4), 357–367. [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Vogel JW, Gonneaud J, Binette AP, Rosa-Neto P, Gauthier S, Bateman RJ, Fagan AM, Morris JC, Benzinger TL, 2018. Proximity to Parental Symptom Onset and Amyloid-β Burden in Sporadic Alzheimer Disease. JAMA neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.