Abstract
The molecular mechanisms of postmenopausal heart diseases in women may involve the loss of estrogen-deactivation of its membrane receptor, G-protein coupled estrogen receptor (GPER), and subsequent activation of the cardiac NLRP3 inflammasome, a component of the innate immune system. To study the potential effects of cardiac GPER on NLRP3-mediated inflammatory pathways, we characterized changes in innate immunity gene transcripts in hearts from 6-month-old cardiomyocyte-specific GPER knockout (KO) mice and their GPER-intact wild type (WT) littermates using RT2 ProfilerTM real-time PCR array. GPER deletion in cardiomyocytes decreased %fractional shortening (%FS) and myocardial relaxation (e΄), and increased the early mitral inflow filling velocity-to-early mitral annular descent velocity ratio (E/e΄), determined by echocardiography, and increased the mRNA levels of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP), determined by real-time PCR. Of the 84 inflammasome-related genes tested, 9 genes were upregulated, including NLRP3 and IL-18, while 1 gene, IL-12a, was downregulated in GPER KO when compared to WT. The importance of NLRP3 upregulation in GPER KO-induced heart failure was further confirmed by an in vivo study showing that, compared to vehicle-treated KO mice, 8 weeks of treatment with a NLRP3 inhibitor, MCC950 (10 mg/kg, i.p., 3 times per week), significantly limited hypertrophic remodeling, defined by reductions in heart weight/body weight, and improved systolic and diastolic functional indices, including increases in %FS and e΄, and decreases E/e΄ (P<0.05). Both ANF and BNP mRNA levels were also significantly reduced by chronic MCC950 treatment. The findings from this study point toward a new understanding for the increased occurrence of heart diseases in women following loss or absence of estrogenic protection and GPER activation that involves cardiac NLRP3 inflammatory pathways.
Keywords: GPER, NLRP3, Inflammation, Heart failure
1. Introduction
Estrogen is widely accepted as a protector of the female heart against various hemodynamic stresses and sterile, low grade inflammatory processes, including hypertension, cardiac hypertrophy failure, atherosclerosis, ischemia–reperfusion injury and heart failure, while its loss contributes to an acceleration of cardiovascular diseases in women. Numerous preclinical studies demonstrate that estrogen protects the heart through both direct effects on cardiomyocytes, and indirectly via systemic effects [1]. However, hormone therapy over the past few decades has not shown clear cardiac benefits and might be associated with health risks including cancer, coronary heart disease, and stroke [2]. Furthering research on the mechanisms of estrogen in the attenuation of cardiovascular diseases, with a focus on roles of specific estrogen receptors, is critical in the development of more specific strategies for the treatment of cardiovascular diseases in women, with fewer or no side effects. A new potential target for drug therapy might be the G protein-coupled estrogen receptor (GPER), also known as G protein-coupled receptor 30 (GPR30). Activation of GPER by its agonist G1 protects the heart against various stresses including pressure-overload [3] , ischemia/reperfusion [4], high salt diet [5], estrogen loss and aging [6,7], while cardiomyocyte-specific GPER deletion induces cardiac remodeling and heart failure [8]. These findings strongly suggest that GPER mediates the cardioprotective effects of estrogen.
The contribution of inflammatory processes in the development and progression of cardiovascular disease is a topic of continuous research. One exciting area in this field is the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome, in which NLRP3, caspase-1, interleukin-1β (IL-1β) and IL-18 are involved [9]. The NLRP3 inflammasome, which is formed and activated by various stimuli such as PAMPs (pathogen-associated and danger) and DAMPs (damage-associated molecular patterns), participates in the pathogenesis of hypertension, diabetes, atherosclerosis, myocardial infarction, heart failure and other cardiovascular diseases [9]. Several preclinical models show that inhibition of NLRP3 protects the heart [10–12], while findings from recent clinical trials indicate that blockade of IL-1 with a modified version of the human interleukin 1 receptor antagonist AnakinraTM has cardiac beneficial effects in patients with coronary artery disease, heart failure and pericarditis [13–15]. No studies to-date have linked the NLRP3 inflammasome to the loss of estrogenic cardioprotection or inactivation of any one of the estrogen receptors. In the present study, using the cardiomyocyte-specific GPER knockout (KO) mouse, RT2 ProfilerTM real-time PCR array, and in vivo NLRP3 inhibition by its specific inhibitor, MCC950, we show, for the first time, that the NLRP3-related inflammatory pathway is involved in cardiac dysfunction related to GPER deficiency in cardiomyocytes.
2. Materials and methods
2.1. Animals and treatments
Cardiomyocyte-specific GPER KO mice were generated in our laboratory, as described previously [8]. GPERf/f/Cre (GPER KO) and GPERf/f littermates (GPER WT) were studied at 6 months of age in 2 separate cohorts. In the first cohort, heart structure and functions were assessed with echocardiography, followed by euthanasia in both GPER KO and WT mice (n = 6 per group). In the second cohort, an equal number of male and female GPER KO mice received chronic treatment with a NLRP3 inhibitor, MCC950 (MedChemExpress LLC, Monmouth Junction, NJ; Catalog Number: HY-12815; 10 mg/kg, i.p., 3 times per week; n=6 per group), or its vehicle control (1% DMSO in PBS; n=6) for eight weeks, followed by echocardiography, and euthanasia. MCC950 is a potent, highly specific small molecule inhibitor of the NLRP3 inflammasome [16]. The dose of MCC950 was selected based on a mouse model of atherosclerosis [11]. The treatment groups were sex-mixed given our previous report of differentially expressed inflammatory-related gene family within cardiomyocytes from both male and female KO versus their respective wild type (WT) littermates [8].
All mice were housed in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care, with a 12-hour light-dark cycle and constant temperature and humidity. Mice had ad-libitum access to standard chow (Nestle Purina, St. Louis, Mo) and tap water. Body weight and water intake were monitored regularly throughout the study. At the time of sacrifice, the mice were administered with pentobarbital sodium (Akorn Inc, Lake Forest, Ill; 100 mg/kg body weight) by intraperitoneal injection. Upon verification of deep anesthesia by the absence of response to tail and toe pinches, the chest was opened, and the heart was quickly excised and trimmed. Whole heart and left ventricle (LV) weights were measured and normalized to body weight. The LV was sectioned and snap frozen in liquid nitrogen and stored at −80°C in cryogenic tubes for ribonucleic acid (RNA) extraction. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, published by National Institutes of Health (NIH Publication #8023, revised 1978), and study approval was obtained from the Animal Care and Use Committee of Wake Forest University Health Sciences (protocol # A15–191).
2.2. Blood pressure (BP) measurement
Systolic blood pressure (SBP) was measured noninvasively using a volume-pressure recording tail-cuff method on conscious, restrained mice using the CODA 6 system (Kent Scientific Corp, Torrington, CT). Briefly, mice were gently placed in the restrainer and allowed to rest quietly for 10 min at 30°C prior to obtaining 5 acclimation cycles followed by 10 measurement cycles. SBP measurements were collected and averaged. All measurements were performed between 9:00–11:00 a.m. for all groups, to account for any diurnal variations.
2.3. Exercise capacity test
The maximal exercise capacity test (time to exhaustion during a standardized exercise protocol) was performed using a motorized treadmill (Scientific Instruments, Stoelting, Wood Dale, IL). Mice were familiarized with the motor-driven, one-lane rodent treadmill by walking at a speed of 20 cm/s, 10 min/d, for 1 week. Each exercise test was performed after at least 1 day of rest. The protocol for exercise capacity evaluation consisted of 3 min at 12 meters/min, with 1.2-meter/min increases in speed every 3 min until the mouse reached exhaustion. Time to exhaustion (in min) was determined when the mouse was unable to continue running and sat at the lower end of the treadmill for more than 5 s, despite gentle nudging.
2.4. Echocardiography
Heart structure and function were determined using a commercially available echocardiograph equipped with both PureWave 12–4 MHz sector and 15–7 MHz linear transducers (CX50 Compact Xtreme System; Philips Medical Systems), as described previously [8]. In brief, mice were anesthetized with an isoflurane (1.5%) oxygen mixture by nose cone and secured in the supine position to a warm (37.5°C) imaging platform. A 15 MHz linear probe was used to obtain 2D-guided, M-mode images using parasternal long and short axis views for measures of end diastolic and end systolic dimensions (LVEDD and LVESD) and posterior and anterior wall thicknesses (PWTed and AWTed) at end diastole. A 12 MHz phased array probe was used to obtain the apical 4-chamber view for transmitral inflow Doppler (early transmitral filling or maximum E-wave velocity) and septal tissue Doppler measurements (early mitral annular descent or e′) of diastolic function. Heart rate was determined from five consecutive RR intervals from pulsed-Doppler inflow tracings. The fractional shortening (FS) was expressedas %FS = (LVEDD-LVESD)/LVEDD × 100. The relative wall thickness (RWT) was calculated as (PWTed + AWTed)/LVEDD. The early mitral inflow filling velocity-to-early mitral annular descent velocity ratio, or E/e′, was used to estimate LV filling pressure. E/e′ is a useful measure to assess the severity of LV stiffness or diastolic dysfunction.
2.5. Mouse inflammasomes assay using RT2 Profiler PCR Array
Total RNA isolation and cDNA synthesis were performed as described before [8]. Realtime PCR array was performed using mouse inflammasomes RT2 ProfilerTM PCR Array kit (Cat. no. PAMM-097Z; SuperArray Bioscience, Frederick, MD), which profiles the expression of 84 key genes involved in the function of inflammasomes, protein complexes involved in innate immunity, as well as general NOD-like receptor (NLR) signaling. The array was performed according to the manufacturer’s instruction using RT2 SYBR Green/Rox PCR master mix (Cat. no. PA-012, SuperArray Bioscience) on a QuantStudio 3 Real-Time PCR system (Applied Biosystems). The integrated web-based software package for the PCR array system automatically performed all comparative threshold cycle (ΔΔCt)-based fold-change calculations from the uploaded data. Three mice per group were used for this analysis. A two-fold or greater change in expression with P ≤ 0.05 was considered significant.
2.6. Regular real-time qPCR analysis
Relative quantification of mRNA levels by real-time qPCR was performed using a SYBR Green PCR kit (Qiagen Inc), as previously described [8]. Amplification and detection were performed with the ABI7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Sequence-specific oligonucleotide primers were designed according to published GenBank sequences and confirmed with OligoAnalyzer 3.0. The relative target mRNA levels in each sample were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Expression levels are reported relative to the geometric mean of the control group.
2.7. Statistical analyses
All results are expressed as mean ± standard error of the mean (SEM). Data were analyzed using a 2-tailed unpaired Student t test with the software GraphPad Prism Version 6 (GraphPad Software, Inc, La Jolla, Calif). P <0.05 was considered statistically significant.
3. Results
3.1. Cardiomyocyte-specific GPER deletion impaired heart structure and functions
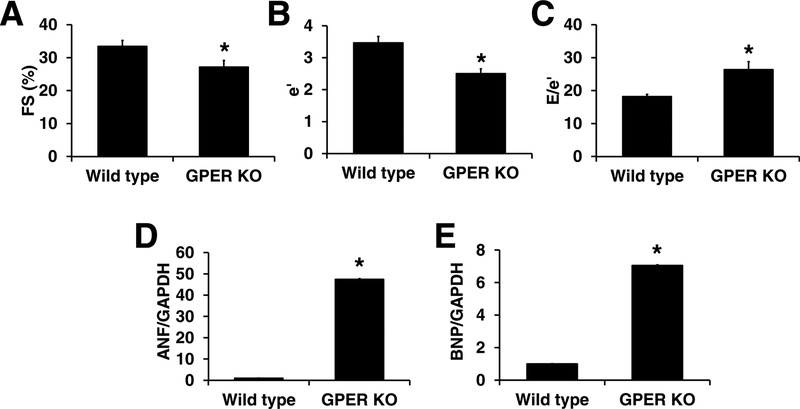
Consistent with our previous report, findings from echocardiographic examinations revealed decreaed fractional shortening (FS) (27.1±2.0 vs. 33.5±1.8, P<0.05. Figure 1A), decreased e΄ (2.5±01 vs. 3.5±0.2, P<0.05. Figure 1B), and increased E/e΄ (26.4±2.4 vs. 18.2±0.7, P <0.05. Figure 1C) in hearts with cardiomyocyte-specific GPER deletion. The mRNA levels of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP), two important biomarkers of heart failure, were significantly increased in GPER KO vs. WT mice by 46- and 6-fold, respectively, as determined by real-time PCR (Figure 1D,1E).
Fig. 1. Cardiomyocyte-specific GPER deletion induced heart failure.
Cardiomyocyte-specific GPER deletion decreased %fractional shortening (%FS) (A), decreased e΄ (B), increased E/e΄ (C), determined by echocardiography, and increased cardiac ANF (D) and BNP (E) mRNA determined by real-time PCR. n=6/group. * P <0.05 vs wild type.
3.2. GPER deletion changed NLRP3 inflammasome-related gene expression
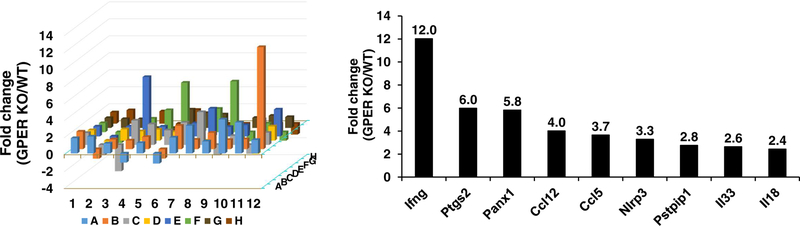
Of the 84 inflammasome-related genes tested, 10 were differentially expressed in GPER KO mice compared with WT littermates, including 9 upregulated genes and 1 downregulated gene (Figure 2). Genes that were significantly upregulated in GPER KO include interferon gamma (Ifng), prostaglandin endoperoxide synthase 2 (Ptgs2), pannexin 1 (Pnax1), chemokine (C-C motif) ligand 12 (Ccl12), chemokine (C-C motif) 5 (Ccl15), NLR family pyrin 3 (NLRP3), proline-serine-threonine phosphatase-interacting protein 1 (Pstpipi1), interleukin 33 (IL-33), interleukin 18 (IL-18). Interleukin 12a (IL-12a) was decreased by 68% in hearts of GPER KO vs. WT mice (P<0.05) (Figure 2).
Fig. 2. PCR microarray analysis of inflammatory genes in cardiomyocytes from GPER knockout (KO) vs. wild type (WT) mice.
(A) 3-D plot representing real-time RT PCR microarray analysis of 84 inflammatory genes. Columns pointing up (with z-axis values > 1) indicate an upregulation of gene expression, in the test sample (GPER KO) vs. control (WT). (B) Data represent those inflammatory genes significantly increased by 2-fold or more and P<0.05 in KO vs. WT hearts; n=3/group. Ifng, interferon gamma; Ptgs2, prostaglandin endoperoxide synthase 2; Pnax1, Pannexin 1; Ccl12, chemokine (C-C motif) ligand 12; Ccl15, Chemokine (CC motif) 5; Nlrp3, NLR family, pyrin 3; Pstpipi1, Proline-serine-threonine phosphatase-interacting protein 1; Il33, interleukin 33; Il18, interleukin 18.
3.3. MCC950 treatment improved heart function in GPER knockout mice
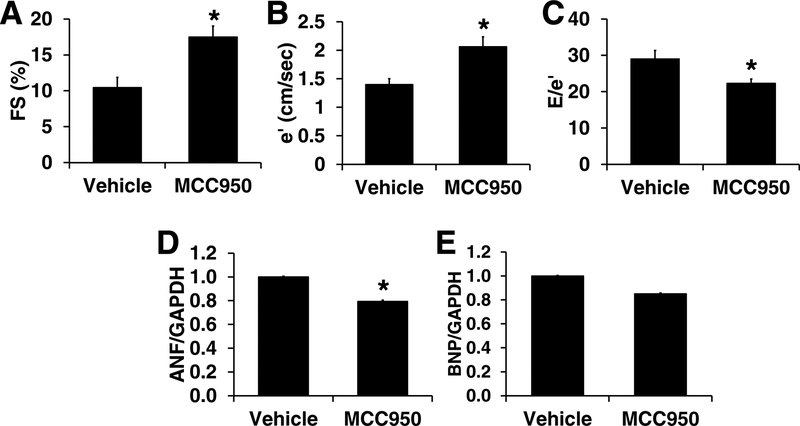
The 8-week treatment with MCC950, a NLRP3 inhibitor, in GPER KO mice did not change body weight or systolic blood pressure (Table 1). However, compared to vehicle-treated KO mice, MCC950 decreased heart weight normalized to body weight (HW/BW) by 26% (P<0.05; Table 1) and improved LV function as depicted by increases in %FS and e΄, and decreases in Doppler-derived filling pressure, or E/e΄, in GPER KO mice (P<0.05. Figure 3). Results from real-time PCR demonstrate that ANF mRNA level decreased by 21% in MCC950 vs. vehicle-treated animals (P<0.05), while BNP mRNA was not significantly affected (0.85±0.01 vs. 1.0±0.01, P >0.05) (Figure 3).
Table 1.
Body weight, blood pressure, heart weight, and treadmill time in GPER knockout mice treated with vehicle and MCC950
| Vehicle (n=6) | MCC950 (n=6) | P-value | |
|---|---|---|---|
| Body weight (g) | 30.2±2.1 | 29.5±1.4 | 0.782 |
| Heart weight (mg) | 176±31 | 126±8 | 0.095 |
| HW/BW (mg/g) | 5.73±0.72 | 4.24±0.01 | 0.038 |
| Systolic BP (mmHg) | 105±2.5 | 106±3 | 0.894 |
| Treadmill time (min) | 20.1±5.0 | 29.8±1.9 | 0.068 |
HW, heart weight; BW, body weight; BP, blood pressure
Fig. 3. Eight weeks of treatment with NLRP3 inhibitor, MCC950, improved heart function in GPER knockout mice.
MCC950 treatment increased %fractional shortening (FS) (A), increased e΄ (B), decreased E/e΄ (C), determined by echocardiography, and decreased cardiac ANF (D) and BNP (E) mRNA determined by real-time PCR. n=6/group. * P <0.05 vs vehicle treated GPER knockout mice.
Consistent with these cardiac changes, MCC950 treatment tended to increase exercise capacity, as evidenced by a 49% increase in time to exhaustion (P=0.068, Table 1).
3.4. MCC950 regulated inflammasome-related gene expression in the heart
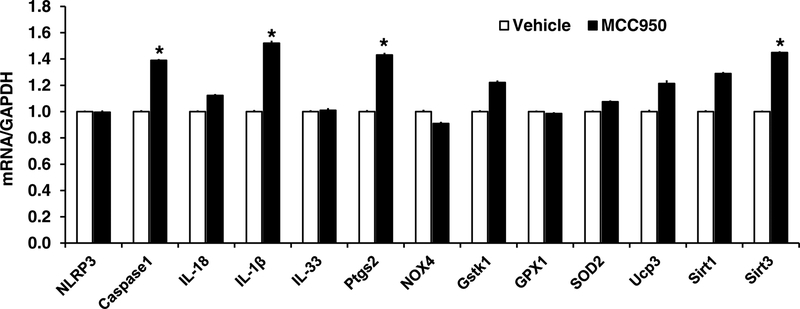
As shown in Figure 4, MCC950 treatment did not change NLRP3 mRNA, but significantly increased the mRNA levels of caspase 1, IL-1β, Ptgs2, and Sirtuin 3 (Sirt 3) (P<0.05). While mRNA levels of IL-18, glutathione S-transferase kappa 1 (GSTK1), uncoupling protein 3 (UCP3), and Sirt 1 also increased, these changes did not reach statistical significance (Figure 4).
Fig. 4. Inflammatory and related gene changes in GPER knockout mice treated with MCC950 vs. vehicle.
n=6/group. * P <0.05 vs vehicle treated GPER knockout mice.
4. Discussion
The main findings of this study are: (1) cardiomyocyte-specific GPER deficiency-induced heart dysfunction occurs with increases in NLRP3 inflammasome-related gene expression; and (2) chronic NLRP3 inhibition with MCC950 treatment improves heart function in GPER KO mice. These results suggest that the NLRP3-mediated inflammatory response pathway may contribute to heart failure progression. Further studies should clarify whether this inflammatory mechanism plays a role in the development of heart disease in women after the loss of ovarian estrogen and subsequent GPER inactivation.
As reviewed elsewhere [9], the NLRP3 inflammasome has been shown to be involved in various cardiovascular diseases, such as vascular damage spanning from atherosclerosis, aneurysmal disease, and arteritis; ischemic heart disease; and nonischemic heart diseases including diabetic cardiomyopathy, chronic heart failure, and pressure overload-induced hypertrophy, and virus-induced cardiac dysfunction. Once the cytosolic innate immune signaling receptor NLRP3 is activated by cardiac stimuli, it nucleates the assembly of an inflammasome, leading to caspase 1-mediated proteolytic activation of the cytokines including IL-1β and IL-18, and induces an inflammatory, proptotic cell death. The cardioprotective effects of NLRP3 inhibition has been demonstrated in animal models of hypertension, atherosclerosis, and myocardial infarction using its selective inhibitor, MCC950 [10–12]. MCC950 is a potent highly specific small molecule inhibitor of both canonical and noncanonical activation of the NLRP3 inflammasome [16]. However, to our knowledge, there are no studies on the roles of NLRP3-mediated inflammatory responses in heart diseases that implicate loss of estrogen or deactivation of estrogen receptors. A recent study demonstrated that estrogen deficiency increases NLRP3 inflammasome activation and pro-inflammatory cytokine production in mouse models of allergic airway inflammation [17], hippocampal inflammation [18], and human fibroblast-like synoviocytes [19]. In these models of increased NLRP3 activation, 17β-estradiol (E2) treatment inhibited the inflammatory response. Consistent with these findings, the present study shows for the first time that activation of the NLRP3-inflammatory pathway might be a key mediator in the development of heart diseases associated with estrogen loss and the subsequent deactivation of the membrane estrogen receptor, GPER.
The NLRP3 inflammasome exerts an inflammatory effect by activating caspase-1 and proinflammatory cytokines [9]. In the current study, GPER deletion significantly increased cardiac mRNA levels of NLRP3, IL-18, IL-33, and other inflammatory response-related genes including Ifng, Ptgs2, Pnax1, Ccl12, Ccl15, and Pstpipi1. However, while NLRP3 inhibition by MCC950 improved heart function in GPER KO mice, MCC950 increased cardiac mRNA levels of caspase-1, IL-1β, and Ptgs2, and did not change NLRP3, IL-18, and IL-33 gene expressions. The increased caspase-1, IL-1β, and Ptgs2 mRNA levels might reflect a compensatory mechanism while the activity of the cytokines is inhibited. The activities of NLRP3 and other related cytokines will be measured in future studies. Interestingly, NLRP3 inhibition significantly increased Sirt3 mRNA by 45% compared with vehicle treated PGER KO mice. Sirt3, the primary mitochondrial deacetylase, plays a significant role in enhancing the function of mitochondrial proteins. Recent studies have suggested an interaction between Sirt3 and NLRP3 inflammasome. Macrophages obtained from Sirt3 knockout mice showed significant increases in NLRP3 inflammasome activation, while the Sirt3 activator viniferin reduced NLRP3 activation and the production of proinflammatory cytokines in WT mice [20]. Similarly, in western diet-fed mice, Sirt3 deletion increased NLRP3 inflammasome activation and markers of neuroinflammation in the brain [21]. In human aortic endothelial cells (HAECs), Sirt3 overexpression attenuated NLRP3 inflammasome activation, while Sirt3 deletion accelerated its activation and also impaired endothelial dysfunction [22]. How NLRP3 inhibition upregulates Sirt3 gene expression, as found in the current study, will require future evaluation.
Our study provides new insight into the mechanism by which estrogen loss and deactivation of GPER might contribute to heart disorders and opens opportunities for additional research. Even so, we also realize the limitations of this study. First, we used a “loss of function” model, where GPER was knocked down in the cardiomyocyte. Indeed, regulation of NLRP3 by GPER needs to be tested in other models, as well using a “gain of function” paradigm. Interestingly, in the female mRen2.Lewis rat, an estrogen-sensitive hypertensive animal model, we found that chronic GPER activation by G1 decreased cardiac mRNA levels of NLRP3, caspase 1, IL-18, and IL-1β in of ovariectomized rats (unpublished data). Second, since NLRP3 mainly affects the activities [9], and not the transcriptional level of caspase-1, IL-18, IL-1β, the activities of NLRP3 and related cytokines need to be measured to further our understanding of the NLRP3 inflammasome in estrogen loss-associated heart disease. Finally, this study demonstrated that estrogen’s effects on NLRP3 inflammasome might be mediated by GPER, but how GPER regulates NLRP3 inflammasome at the gene, protein, and activity levels needs further study.
In summary, postmenopausal heart disease, mainly characterized of diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF) is a major health problem with significant morbidity and mortality in old women. There are no proven pharmacologic therapies that delay or reverse these age-related heart abnormalities in women. Further understanding of the mechanisms may lead to better personalized treatment of postmenopausal heart diseases. The findings from this study reveal that deactivation of GPER accelerates NLRP3-mediated inflammatory pathways, which might have important roles in the pathogenesis of heart disorders after estrogen loss.
Supplementary Material
Highlights.
GPER deletion in cardiomyocytes induce left ventricular dysfunction.
GPER deletion increases NLRP3 inflammasome-related gene expression.
NLRP3 inhibition by MCC950 improves heart function in GPER KO mice.
Funding
This work was funded by grants from the National Heart Lung and Blood Institute (CMF and LG) P01-HL051952 and the National Institute on Aging (LG) AG033727 of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflict of interest to report.
References
- [1].Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L, Role of estrogen in diastolic dysfunction, Am J Physiol Heart Circ Physiol 306 (2014) H628–640. 10.1152/ajpheart.00859.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thaung Zaw JJ, Howe PRC, Wong RHX, Postmenopausal health interventions: Time to move on from the Women’s Health Initiative?, Ageing Res Rev 48 (2018) 79–86. 10.1016/j.arr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- [3].De Francesco EM, Angelone T, Pasqua T, Pupo M, Cerra MC, Maggiolini M, GPER mediates cardiotropic effects in spontaneously hypertensive rat hearts, PLoS One 8 (2013) e69322 10.1371/journal.pone.0069322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC, Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy, Br J Pharmacol 174 (2017) 4329–4344. 10.1111/bph.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L, Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats, PLoS One 5 (2010) e15433 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alencar AK, da Silva JS, Lin M, Silva AM, Sun X, Ferrario CM, Cheng C, Sudo RT, Zapata-Sudo G, Wang H, Groban L, Effect of Age, Estrogen Status, and Late-Life GPER Activation on Cardiac Structure and Function in the Fischer344xBrown Norway Female Rat, J Gerontol A Biol Sci Med Sci 72 (2017) 152–162. 10.1093/gerona/glw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L, Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats, Cardiovasc Res 94 (2012) 96–104. 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang H, Sun X, Chou J, Lin M, Ferrario CM, Zapata-Sudo G, Groban L, Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis, Biochim Biophys Acta Mol Basis Dis 1863 (2017) 1870–1882. 10.1016/j.bbadis.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou W, Chen C, Chen Z, Liu L, Jiang J, Wu Z, Zhao M, Chen Y, NLRP3: A Novel Mediator in Cardiovascular Disease, J Immunol Res 2018 (2018) 5702103 10.1155/2018/5702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, Robertson AAB, Cooper MA, Peter K, Latz E, Mansell AS, Sobey CG, Drummond GR, Vinh A, Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension, Cardiovasc Res 115 (2019) 776–787. 10.1093/cvr/cvy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I, Kuiper J, NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report, Arterioscler Thromb Vasc Biol 37 (2017) 1457–1461. 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- [12].van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE, The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction, Eur Heart J 38 (2017) 828–836. 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- [13].Buckley LF, Abbate A, Interleukin-1 Blockade in Cardiovascular Diseases: From Bench to Bedside, BioDrugs 32 (2018) 111–118. 10.1007/s40259-018-0274-5. [DOI] [PubMed] [Google Scholar]
- [14].Emmi G, Urban ML, Imazio M, Gattorno M, Maestroni S, Lopalco G, Cantarini L, Prisco D, Brucato A, Use of Interleukin-1 Blockers in Pericardial and Cardiovascular Diseases, Curr Cardiol Rep 20 (2018) 61 10.1007/s11886-018-1007-6. [DOI] [PubMed] [Google Scholar]
- [15].Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del Buono M, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E, Arena R, Abbate A , Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial), Circ Heart Fail 10 (2017). 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA, A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases, Nat Med 21 (2015) 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cheng C, Wu H, Wang M, Wang L, Zou H, Li S, Liu R, Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice, Biosci Rep 39 (2019). 10.1042/BSR20181117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X, NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice, Brain Behav Immun 56 (2016) 175–186. 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- [19].Shi J, Zhao W, Ying H, Zhang Y, Du J, Chen S, Li J, Shen B, Estradiol inhibits NLRP3 inflammasome in fibroblast-like synoviocytes activated by lipopolysaccharide and adenosine triphosphate, Int J Rheum Dis 21 (2018) 2002–2010. 10.1111/1756-185X.13198. [DOI] [PubMed] [Google Scholar]
- [20].Kurundkar D, Kurundkar AR, Bone NB, Becker EJ Jr., Liu W, Chacko B, Darley-Usmar V, Zmijewski JW, Thannickal VJ, SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury, JCI Insight 4 (2019). 10.1172/jci.insight.120722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tyagi A, Nguyen CU, Chong T, Michel CR, Fritz KS, Reisdorph N, Knaub L, Reusch JEB, Pugazhenthi S, SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain, Sci Rep 8 (2018) 17547 10.1038/s41598-018-35890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu P, Huang G, Wei T, Gao J, Huang C, Sun M, Zhu L, Shen W, Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation, Biochim Biophys Acta Mol Basis Dis 1864 (2018) 764–777. 10.1016/j.bbadis.2017.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.