Abstract
Aging is associated with increased difficulty in facial emotion identification, possibly due to age-related network change. The neuropeptide oxytocin (OT) facilitates emotion identification but this is understudied in aging. To determine the effects of OT on dynamic facial emotion identification across adulthood, 46 young and 48 older participants self-administered intranasal OT or a placebo (P) in a randomized, double-blind procedure. Older participants were slower and less accurate in identifying emotions. Although there was no behavioral treatment effect, partial least squares (PLS) analysis supported treatment effects on brain patterns during emotion identification that varied by age and emotion. For young participants, OT altered the processing of sadness and happiness, while for older participants OT only affected the processing of sadness (15.3% covariance, p = 0.004). Further, seed PLS analysis showed that older participants in the OT group recruited a large-scale amygdalar network that was positively correlated for anger, fear, and happiness whereas older participants in the P group recruited a smaller, negatively correlated network (7% covariance, p = 0.002). Advancing the literature, these findings show that OT alters brain activity and amygdalar connectivity by age and emotion.
Keywords: oxytocin, aging, emotion, amygdala, functional connectivity
1. Introduction
Accurately interpreting facial expressions is a crucial skill for successfully navigating the social world (Calder et al., 2003). Aging is robustly associated with increased difficulty in this ability (Ruffman et al., 2008), with negative impacts on social functioning (Noh & Isaacowitz, 2013). The mechanisms underlying age-related deficits in facial emotion identification, however, are still unknown (Ebner & Fischer, 2014) but age-related change in brain connectivity may be at work. Age-related changes in brain connectivity can be adaptive (e.g., increased prefrontal and reduced amygdala recruitment during emotion processing; St Jacques et al., 2010; Ziaei et al., 2017), but they can also be associated with cognitive challenges (e.g., decreases in salience network connectivity; Onoda et al., 2012, reduced suppression of default network; Turner & Spreng, 2015). Understanding the neural underpinnings of age-related changes in emotion processing can help to characterize healthy aging and identify opportunities for intervention.
Intranasal administration of the neuropeptide oxytocin (OT) may be a promising tool for optimizing emotion processes in aging, based on evidence that OT improves the perception of social signals (Kéri & Benedek, 2009), enhances facial emotion identification (Leppanen et al., 2017), and is associated with large-scale network change in young adults (Brodmann et al., 2017). In fact, to date, OT’s effects have mostly been examined in young men (Ebner et al., 2013) and only very few studies have investigated OT’s effects on task-related functional connectivity with mixed findings (Bethlehem et al., 2013). Recent evidence, however, points to possible age-by-sex variations in OT’s effects on affective processes (Campbell et al., 2014; Ebner et al., 2015) and functional coupling at rest (Ebner et al., 2016).
The amygdala, in particular, has been identified as a primary region of interest (ROI) in OT-related connectivity research (Bethlehem et al., 2013) and in research on age-related network change (Sakaki et al., 2013). The amygdala is a hub that is well-connected to cortical regions relevant to emotion processing and social cognition (Bickart et al., 2014; Sergerie et al., 2008). Importantly, amygdalar connectivity is impacted by aging and may be a major underlying factor for increased difficulty in emotion identification with age (Huffmeijer et al., 2013; St Jacques et al., 2010). Although the amygdala has been strongly implicated as a mediator of OT’s effects, it is still unclear how aging would impact OT’s functions in emotion processing (Huffmeijer et al., 2013). Therefore, examining amygdalar connectivity is a promising avenue for synthesizing two previously parallel lines of research on age- and OT-related brain network change.
To address this gap in the literature, the present study examined age-related differences in behavior, whole-brain patterns, and amygdalar functional connectivity during a dynamic facial emotion identification task, and in this context considered the role of intranasal OT administration on altering behavior and brain patterns. We predicted:
better identification of, particularly negative, facial emotions in young than older participants (Ruffman et al., 2008);
better facial emotion identification in the OT than the placebo (P) group, particularly among older individuals (Campbell et al., 2014; Leppanen et al., 2017);
greater recruitment of anterior and midline regions, consistent with observed default network activity during facial emotion identification (Martins & Mather, 2016), in older than young participants;
greater recruitment of regions implicated in dynamic facial emotion identification (Kessler et al., 2011; LaBar et al., 2003) and the salience network (e.g., anterior insula (AI), anterior cingulate cortex (ACC)) in the OT than the P group across age groups (Uddin, 2015);
age-specific patterns of amygdalar connectivity during dynamic facial emotion identification (i.e., an amygdalar network connected to more frontal regions in older participants vs. an amygdalar network connected to more posterior regions in young participants) (Huffmeijer et al., 2013; St Jacques et al., 2010); and
OT-related change in functional connectivity, compared to placebo (P), in these amygdalar networks across age groups and emotion (sadness, anger, fear, happiness).
2. Material and Methods
2.1. Participants.
The behavioral sample comprised 48 young (M = 22.4 years, SD = 3.0, 18–31 years, 48% female) and 54 older (M = 71.2 years, SD = 4.9, 63–81 years, 56% female) generally healthy adults, of which 26 young (46% female) and 27 older (56% female) were randomly assigned to the OT group and 22 young (50% female) and 27 older (56% female) to the P group. Eight participants were excluded from the fMRI analysis for either corrupted images, excessive head motion, outlying brain scores, or missing data. Thus, the fMRI sample comprised 46 young (M = 22.5 years, SD = 3.1, 18–31 years, 47.8% female) and 48 older (M = 71.1 years, SD = 5.2, 63–81 years, 56.3% female) adults, of which 26 young (46% female) and 24 older (50% female) were assigned to the OT group and 20 young (50% female) and 24 older (63% female) to the P group.
All participants were white, English-speaking with no history of neurological or psychiatric disorder and perfect or corrected vision. All older participants scored ≥ 30 on the Telephone Interview for Cognitive Status (Brandt et al., 1988). Age and treatment group differences in sample-descriptive data were assessed using separate one-way between-subjects ANOVAs. See Table 1 in the Supplementary Material for details on sample-descriptive data. In particular, age groups were comparable in education level and subjective ratings of physical and mental health but differed in sensorimotor processing speed (Digit Symbol Substitution Test (DSST); Wechsler, 1981) (F(1,100) = 110.0, p < 0.05, ƞp2 = 0.5) and short-term verbal learning memory (Rey Auditory Verbal Learning Test (RAVLT); Rey, 1964) (F(1,100) = 14.9, p < 0.05, ƞp2 = 0.1) in line with the literature (Hoyer et al., 2004; Vakil & Blachstein, 1997). Both age groups reported comparable pre-treatment negative mood, but older (M = 3.5, SD = 0.6) compared to young (M = 2.8, SD = 0.7) participants reported higher positive mood (Positive Affect Negative Affect Schedule (PANAS); Watson et al., 1988) (F(1,100) = 29.0, p < 0.05, ƞp2 = 0.2). The treatment groups did not differ in any of these measures.
2.2. Procedure.
This study utilized a randomized, double-blind, between-group design that comprised an initial phone call to determine study eligibility (~30 min), an in-person screening session (~45 min), and an in-person full session (~3 h). Only measures relevant to the present analyses are reported (Ebner et al., 2015, 2016, 2018).
All participants provided informed consent before enrollment. Participants were instructed to stay hydrated and to abstain from substance use and caffeine for 24 hours and to abstain from food, exercise, and sexual activity at least two hours before the sessions. All in-person sessions took place at ~8:00 AM. This study was approved by the University Institutional Review Board (IRB#39–2013) and pre-registered with ClinicalTrials.gov (NCT01823146).
During the in-person screening session, participants completed an intake interview on behavior from the past 24 hours, short questionnaires, the DSST, and the RAVLT. Saliva and blood sampling was conducted along with a health review by a clinician. During the in-person full session, participants underwent MRI safety determination. Participants completed another intake interview, the PANAS, and underwent saliva sampling. Following recommendations for the standardized administration of intranasal OT (Guastella et al., 2013), participants self-administered 24 international units (IU; one puff per nostril) of OT or P, which contained the same ingredients as the OT spray except for the OT. Compounding, dispensing, and randomization was overseen by the dispensing pharmacy.
Prior to scanning, participants received instructions on scanning procedure and the tasks, including practice runs, and were settled into the 3T MRI scanner ~45 minutes after self-administration. Participants underwent anatomical image acquisition followed by functional image acquisition across four tasks (see also Ebner et al., 2016). Participants engaged in the dynamic facial emotion identification task ~90 minutes after self-administration and completed, outside the scanner, social and affective questionnaires plus a post-event questionnaire about their experience with the spray and scanner, before being debriefed and compensated. Approximately one week later, participants received a follow-up call to assess any side effects of the spray. No consistent or adverse side effects were reported.
2.3. Dynamic Facial Emotion Identification Task.
This task was modeled after Lischke et al. (2012) for fMRI use in Experiment Builder (http://www.sr-research.com/eb.html). Twelve high-quality images of young adults (6 male and 6 female) were chosen from the FACES database (Ebner et al., 2010) with neutral expressions and four corresponding emotional expressions: sadness, anger, fear, and happiness. Following a jittered fixation cross ranging between 5, 7, and 9 seconds (M = 7 s), a neutral face was presented that morphed into an emotive face at 5% increments of emotion intensity per image. Each image in a set for a specific facial identity and emotion was presented for 1 second (21 images per set). Participants were asked to identify, as quickly and as accurately as possible, the emotion presented via a button press. A four-button MagConcepts Inc. response box was used for emotion identification that rested on the participants’ abdomen during the task in the scanner. The assignment of fingers to emotions was kept consistent across participants (sadness, index; anger, middle; fear, ring; happiness, pinky). The emotion continued to develop after a response was made. No feedback was provided.
Stimuli were counterbalanced by facial emotion, sex, and identity across four lists. Facial identities were presented in the same order for each list with no more than two presentations of the same sex in a row. Each identity was used once before showing the same identity again with a different emotion. No more than two faces with the same emotion were repeated in a row and every emotion was presented three times per run (16 faces per run, 7.7 min per run, 3 runs per session).
2.4. Image Acquisition and Preprocessing.
A 3T Philips Achieva MR Scanner (Philips Medical Systems, Best, The Netherlands) acquired images using a 32-channel head coil. Whole-brain high-resolution three-dimensional T1-weighted anatomical reference images were acquired using an MP-RAGE sequence (sagittal plane, 170 slices, FOV 240×240 mm2; 1 mm³ isotropic voxels). Functional images were obtained using whole-head echo-planar imaging and a single-shot gradient echo (38 interleaved slices, TR 2 s, TE 30 ms, FOV 252×252 mm2, flip angle 90°, in-plane resolution of 3.15×3.15 mm2, slice thickness 3.5 mm, no skip). A total of 234 TRs per participant were acquired for each of the three runs.
Data preprocessing was conducted using the CONN default preprocessing pipeline for volume-based analysis to MNI space (Whitfield-Gabrieli & Nieto-Castanon, 2012). This included functional realignment and unwarping, slice-timing correction, structural segmentation and normalization, functional normalization, outlier detection, and smoothing. Functional images were normalized into standard stereotaxic MNI space with a target voxel size of 2 mm and smoothed with an 8 mm Gaussian kernel. Regression of white matter and cerebrospinal fluid signal was conducted using aCompCor (Behzadi et al., 2007). Motion artifacts greater than 2 mm were coded as a separate variable by ArtRepair (Mazaika et al., 2009) and removed for individual time series.
2.5. Behavioral Analysis.
To determine age and treatment effects on behavioral performance in dynamic facial emotion identification, two separate repeated measures ANOVAs on reaction time (ms for correct trials) and accuracy (percent correct trials), respectively, were conducted with age (young vs. older) and treatment (OT vs. P) as between-subject variables and facial emotion as the within-subject variable. The significance threshold for the behavioral results was set to p < 0.05.
2.6. Task Partial Least Squares (PLS) Analysis.
To determine OT-related brain patterns associated with dynamic facial emotion identification by age and treatment, event-related mean-centered task PLS (Type 2, which removes the grand mean for all participants and conditions) was implemented. PLS is a model-free, multivariate, relational measure of patterns of activation and connectivity, which allowed us to examine OT-related networks over time (McIntosh & Lobaugh, 2004). This data-driven approach determines orthogonal whole-brain patterns of activity as latent variables (LVs), which optimally relate blood oxygen-level dependent (BOLD) signal with the experimental design. However, unlike principal component analysis, the number of LVs is constrained by experimental conditions. Unlike univariate analysis, PLS detects brain-wide systems that covary with the experimental design in a single step (i.e., decomposition and resampling are concurrently applied to all voxels). Therefore, multiple comparisons correction is not required (McIntosh et al., 1996; McIntosh & Lobaugh, 2004).
For each trial, activity for each voxel was averaged across runs for each condition and normalized to activity at trial onset. The data matrix was expressed as a voxel-by-voxel deviation from the grand mean across the entire experiment. This matrix was analyzed with singular value decomposition to derive optimal effects in the data. The results provide a set of regions wherein activity was reliably related to task conditions and groups for each LV.
Each voxel is given a singular value weight (i.e., a salience) which is proportional to the covariance of activity with the task contrast on each LV. Multiplying the salience by the BOLD signal value in that voxel and summing the product across all voxels gives a composite brain activity score for each participant on a given LV. These scores are used to examine similarities and differences in activity across conditions, as greater activity in regions with positive (or negative) weights on an LV will yield positive (or negative) mean scores for a given condition. The significance of each LV (p < 0.01) and their corresponding patterns was assessed through permutation testing (500 permutations) by randomly reassigning the order of conditions for each participant. PLS is recalculated for each permutation sample and the frequency with which the permuted singular value exceeds the observed singular values is determined and expressed as a probability. In an independent step, the reliability of the saliences for voxels across participants, characterizing each pattern identified by a LV, was determined by iterative bootstrap resampling with replacement (100 iterations) to estimate the standard errors for each voxel. Confidence intervals (CIs) (95%) for the mean composite brain activity score in each condition and group were calculated from the bootstrap, and differences in activity between conditions were determined via a lack of overlap in these CIs.
We limited the time window for our brain analyses to the first eight lags (1 lag or 1 TR), which corresponded to 16000 ms after trial onset, to capture the temporal variation in emotion processing across task conditions and groups. Our interpretations of brain activity were drawn from the lags that maximally dissociated conditions and groups, which varied per LV.
Clusters larger than 10 voxels with a ratio of the salience to the bootstrap standard error values (i.e., bootstrap ratio; BSR) greater than ±2.58 (p < 0.01) were considered reliable (Krishnan et al., 2011) and presented in the brain maps (Figures 1–4) using Caret v5.65 (Van Essen, 2005). We defined the local maximum for each cluster as the voxel with a BSR higher than any other voxel in a 2 cm cube centered on that voxel. Regions were defined from peak MNI coordinates using Neurosynth (Yarkoni et al., 2011) and MRIcron (Rorden & Brett, 2000).
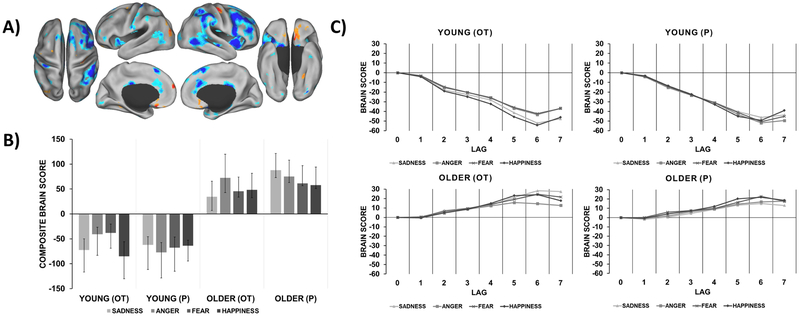
Figure 1.
Age-specific brain patterns relating to dynamic emotion identification. A) Functional activation map with warm colors that correspond to the brain pattern recruited by older participants and cool colors that correspond to the brain pattern recruited by young participants. This brain pattern was mapped at the time point of greatest temporal dissociation between age groups (lag 6). B) Composite brain scores plot depicting the contrast in pattern recruitment for each condition and group (95% CI). C) Temporal brain scores plot that shows brain scores corresponding to each lag in the designated time window after trial onset.
Figure 4.
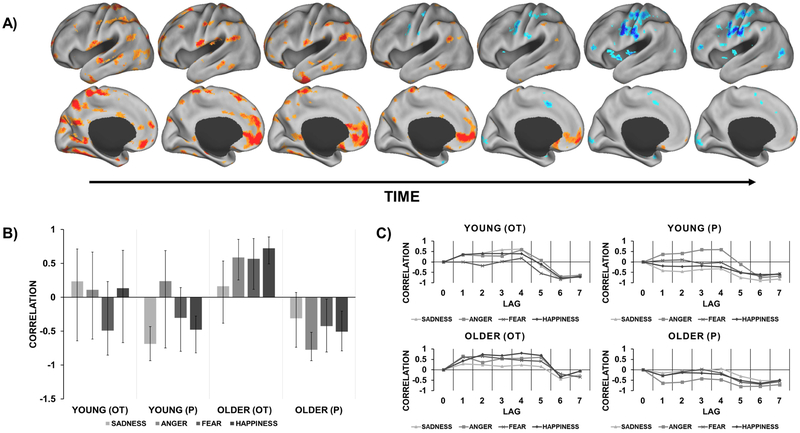
Emotion-specific amygdalar networks for older participants that varied by OT vs. P. A) Functional connectivity maps (1 map per lag in medial and lateral view) that represents the temporal unfolding of two distinct age- and emotion-specific amygdala networks further distinguished by treatment (OT vs. P). Warm colors correspond to an amygdala network recruited by older participants in the OT group for anger, fear, and happiness that emerged early in the task. Cool colors correspond to an amygdala network recruited by older participants in the P group for these same emotions that emerged later in the task. B) Composite correlation plot depicting amygdalar connectivity for each condition and group (95% CI). Note that these networks were not reliable for older participants in response to sadness due to CIs crossing zero. C) Temporal correlation plot that shows correlations corresponding to each lag in the designated time window after trial onset.
2.7. Seed PLS Analysis.
Seed PLS was conducted to determine if there were age and treatment differences in amygdalar connectivity during dynamic facial emotion identification and if connectivity varied by emotion. Seed PLS investigates the relationship between a seed ROI and the whole-brain voxel response (Krishnan et al., 2011). The a priori functional loci were theoretically backed (Bethlehem et al., 2013; Huffmeijer et al., 2013) and were determined via the task PLS analysis from a peak voxel corresponding to the left amygdala (MNI = −28, −6, −10; lag 6; BSR = −5.73; neighborhood size = 1) (Tzourio-Mazoyer et al., 2002). Amygdala seed values were correlated with activity in all voxels across participants. This correlation matrix was then analyzed with singular value decomposition, assessed for statistical significance by permutation testing, and for reliability by bootstrap resampling, as described above.
3. Behavioral Results
3.1. Reaction Time.
The average reaction time across emotion and treatment was 8764 ms (SD = 1651) in young participants and 10202 ms (SD = 2537) in older participants. There were significant main effects for age (F(1,98) = 14.6, p < 0.001, ƞp2 = 0.1) and facial emotion (F(2.8,270.0) = 190.5, p < 0.001, ƞp2 = 0.7) and a significant age-by-emotion interaction (F(2.8,270.0) = 5.1, p = 0.02, ƞp2 = 0.1). Older participants (sadness: M = 12311 ms, SD = 3020, anger: M = 10783 ms, SD = 3297, fear: M = 10825 ms, SD = 2841) were slower than young participants (sadness: M = 10243 ms, SD = 2098, anger: M = 8935 ms, SD = 2002, fear: M = 9006 ms, SD = 1717) in correctly identifying dynamic facial emotions for all emotions except for happiness, for which the reaction time difference was only marginally significant between young and older participants (p = 0.06). No other effects were significant.
3.2. Accuracy.
There were significant main effects for age (F(1,98) = 22.8, p < 0.001, ƞp2 = 0.2) and facial emotion (F(3,294) = 30.4, p < 0.001, ƞp2 = 0.2) and a significant age-by-emotion interaction (F(3,294) = 6.1, p < 0.001, ƞp2 = 0.1). Older participants (sadness: M = 75.5%, SD = 23.1, anger: M = 77.6%, SD = 21.1, fear: M = 79.6%, SD = 20.1, happiness: M = 91.4%, SD = 15.5) were less accurate than young participants (sadness: M = 88.9%, SD = 9.6, anger: M = 93.8%, SD = 9.5, fear: M = 94.8%, SD = 8.4, happiness: M = 97.4%, SD = 5.5) across all emotions. Difference scores between young and older participants were smaller for happiness (6.0%) than for each negative emotion (sadness: 13.4%; anger: 16.1%; fear: 15.2%). Both young (M = 97.4%, SD = 5.5) and older (M = 91.4%, SD = 15.5) participants had the least difficulty identifying happiness and both young (M = 88.9%, SD = 9.6) and older (M = 75.5%, SD = 23.1) participants had the most difficulty accurately identifying sadness. No other effects were significant. Table 2 in the Supplementary Material presents details on the behavioral results.
4. Task PLS Results
Task PLS analysis of the four age-by-treatment groups (Young OT, Young P, Older OT, Older P) and the four task conditions (sadness, anger, fear, happiness) resulted in two significant LVs representing multivariate dissociations of brain patterns relating to dynamic facial emotion identification. The first significant LV accounted for 31.4% covariance (p = 0.002) and showed an age-driven dissociation. As depicted in Figure 1, the age groups recruited two distinct patterns involved in the processing of all facial emotions. In particular, young participants recruited a pattern including the AI, cerebellum, inferior frontal gyrus (IFG), postcentral gyrus, precentral gyrus, superior parietal lobule, temporoparietal junction (TPJ), ACC, and thalamus (cool regions). In contrast, older participants recruited the precentral gyrus, ventromedial prefrontal cortex, medial temporal lobe, medial prefrontal cortex (mPFC), middle temporal cortex, and lateral cortex (warm regions). See Table 3 in the Supplementary Material for peak activations from these regions. While young participants in the OT and P groups recruited cool regions similarly across all emotions (CIs overlapping), older participants in the OT compared to the P group showed lower recruitment in the warm regions for the processing of sadness.
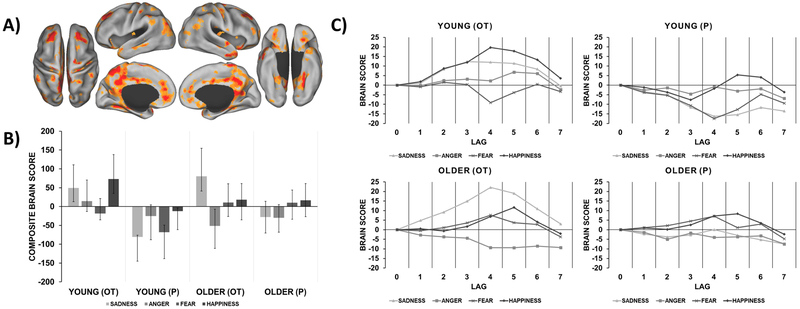
The second significant LV accounted for 15.3% covariance (p = 0.004) and showed an OT-related brain pattern recruited by both age groups for specific emotions. As presented in Figure 2, this pattern was recruited by young participants in the OT group (compared to P) for both happiness and sadness and by older participants in the OT group (compared to P) for sadness only. This treatment-by-emotion specific pattern included the superior frontal cortex, postcentral gyrus, fusiform gyrus (FG), temporal pole, inferior temporal gyrus, superior temporal sulcus (STS), middle temporal gyrus, posterior insula, parahippocampal gyrus, cerebellum, superior occipital cortex, and TPJ. See Table 4 in the Supplementary Material for peak activations from these regions.
Figure 2.
Treatment- and emotion-specific brain pattern relating to dynamic facial emotion identification. A) Functional activation map with warm colors that correspond to the brain pattern recruited by young participants in the OT group for sadness and happiness and older participants in the OT group for sadness only. This brain pattern was mapped at the time point of greatest temporal dissociation between treatment groups (lag 4). B) Composite brain scores plot depicting the contrast in pattern recruitment for each condition and group (95% CI). C) Temporal brain scores plot that shows brain scores corresponding to each lag in the designated time window after trial onset.
5. Seed PLS Results
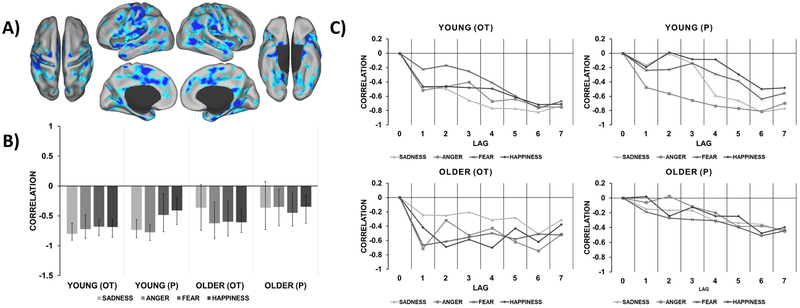
Seed PLS analysis determined two significant LVs associated with amygdalar connectivity relating to dynamic facial emotion identification. The first significant seed LV explained 56.0% covariance (p = 0.002). As depicted in Figure 3, much of the covariance in the data was explained by a robust amygdalar network for both age groups. This amygdalar network included other amygdalar clusters, cerebellum, inferior temporal gyrus, primary visual cortex, inferior occipital cortex, and the ACC. See Table 5 in the Supplementary Material for peak activations from these regions. This pattern applied to all emotions in young participants but was only specific to anger, fear, and happiness in older participants (CIs crossing zero for sadness).
Figure 3.
Amygdalar network recruited by both age groups for all dynamic facial emotions. A) Functional connectivity map with cool colors that correspond to a large-scale amygdalar network was recruited by both young and older participants for all emotions. This network was mapped at the time point of greatest temporal dissociation between age groups (lag 2). B) Composite correlation plot depicting amygdalar connectivity for each condition and group (95% CI). Note that this network was not reliable for older participants in response to sadness due to CIs crossing zero. C) Temporal correlation plot that shows correlations corresponding to each lag in the designated time window after trial onset. There were several timepoints of greatest temporal dissociation between age groups, which varied by emotion (lag 2: happiness and fear, lag 4: anger), however, this network was mapped at lag 2 only as this widespread pattern persisted for the entire time window.
The second significant seed LV explained 7.0% covariance (p = 0.002) and represented a multivariate dissociation in amygdalar connectivity that was specific to age, treatment, and emotion. Figure 4 depicts two distinct amygdalar networks recruited by older participants that unfolded at different time points during the task. In particular, older participants in the OT group recruited a large-scale network early in the task that was positively correlated with the amygdala in response to anger, fear, and happiness (warm regions). This network included the mPFC, subgenual anterior cingulate gyrus, superior frontal gyrus, corpus callosum, STS, hippocampus, caudate, superior frontal gyrus, cerebellum, angular gyrus, parahippocampal gyrus, inferior temporal gyrus, and cuneus. In contrast, older participants in the P group recruited a smaller network later in the task that was negatively correlated with the amygdala in response to anger, fear, and happiness, which included the putamen and posterior parietal lobule (cool regions). Of note, both networks included overlapping regions such as the precentral and postcentral gyrus. See Table 6 in the Supplementary Material for peak activations from these regions. These networks were not reliable for older participants in response to sadness and among young participants (CIs crossing zero). Figure 4A and 4C show the change in network recruitment due to OT administration in lags 4 and 5. As evidenced by the composite brain scores plot (Figure 4B), this dissociation was reliable only when comparing older participants in the OT vs. the P group.
6. Discussion
Integrating parallel lines of research on age- and OT-related brain network change, the present study (i) identified brain activity and connectivity patterns underlying dynamic facial emotion identification across adulthood and (ii) determined the effects of intranasal OT administration on brain function and behavior related to emotion processing in aging. We found age-related differences persisted for dynamic (compared to static) facial emotion identification in terms of behavior and whole-brain pattern recruitment but not in amygdalar connectivity. Second, although we observed no behavioral treatment effect, OT altered brain activity and amygdalar connectivity by age and emotion. These novel findings will be discussed in detail next.
6.1. Older adults were slower and less accurate in dynamic facial emotion identification than young adults, but OT did not moderate this effect
As expected, older compared to young participants were slower and less accurate in dynamic facial emotion identification. These effects were pronounced for negative compared to positive emotions. These findings corroborate previous results with static emotions (Ebner & Johnson, 2009) and are among an emerging literature suggesting comparable age effects for dynamic and static facial emotions (Di Domenico et al., 2015).
However, we did not observe behavioral effects of OT administration on emotion identification. While some previous studies suggest that intranasal OT improves emotion identification (Leppanen et al., 2017; Shahrestani et al., 2013), other studies reported null effects of OT on emotion identification-related behavior in young (Chen et al., 2015; Lischke et al., 2012) and older (Grainger et al., 2018) adults, in line with the present study’s findings. For example, Lischke et al. (2012) found that OT increased accuracy for fear but not for happiness, anger, or sadness in young men (OT: n = 23, P: n = 24). Similarly, we did not find an OT-related behavioral increase in accuracy for these emotions, including fear, in young and older adults.
This lack of OT-related behavioral effects may be due to several factors. For example, the present study only assessed accuracy and reaction time, while other behavioral measures could be more sensitive to OT administration. Future studies may benefit from using behavioral measures such as eye- or mouse-tracking and by presenting finer-grained intensity levels of emotional stimuli and/or faster developing, more naturalistic facial emotion displays (Holland et al., 2018). Furthermore, tasks that incorporate naturalistic presentations of social emotions (e.g., pride, shame, jealousy) (Adolphs, 2002) or other visual and/or auditory emotional cues (Bänziger et al., 2012; Martinez et al., 2016) may generate greater inter-individual variability and may be more sensitive to OT’s effects.
It is also possible that high overall accuracy in the present study resulted in limited inter-individual variability reducing the likelihood to detect OT’s effects on dynamic facial emotion identification-related behavior. A crossover study in 203 men by Chen et al. (2015), using a similar task as in the present study, found that accuracy was near ceiling across participants (M = 88.0%, SD = 9.0%) and found no significant main effect of OT on reaction time. Given the lack of behavioral effects observed in multiple studies combined with evidence that OT facilitates early attention to social stimuli (Shamay-Tsoory & Abu-Akel, 2016), it is possible that OT impacts emotion identification at very early stages of facial processing. Thus, future studies incorporating methods that consider temporal variability (e.g., ERP, EEG) may be promising to elucidate the underlying processes of OT’s effects on face and emotion perception.
Moreover, while we considered age and treatment as between-group variables in the present study, we did not consider other, possibly relevant, inter-individual difference variables such as variations in endogenous OT levels and their interaction with exogenous OT (Chen et al., 2015). Attention to inter-individual differences is especially important for future research on OT’s effects in older adults given the diverse healthy or pathological aging trajectories individuals follow (Jesso et al., 2011) and the currently limited understanding of the OT system in human aging (Ebner et al., 2013).
Also, a growing literature suggests that sex constitutes an important moderator of OT’s effects, as gonadal hormones interact with OT (Ebner et al., 2015; Macdonald, 2013; Macdonald & Feifel, 2013). For example, in young men, intranasal OT reduced amygdala activity in response to unpleasant social (Gamer et al., 2010) and emotional stimuli (Domes et al., 2007; Kirsch et al., 2005), whereas intranasal OT increased activity in the amygdala in response to fear in young women (Domes et al., 2010) (see also Bethlehem et al., 2013; Domes et al., 2007; Lischke et al., 2012 for more on sexually dimorphic effects). To date, only very few studies have examined the effects of intranasal OT on social cognition with consideration of age and sex (Campbell et al., 2014; Ebner et al., 2015, 2016; Grainger et al., 2018) and future systematic work on this topic is needed. Campbell et al. found that older men who self-administered OT (compared to P) showed improved facial emotion identification. Similarly, in older men, OT (compared to P) was associated with enhanced awareness of their own emotions (Ebner et al., 2015). Intranasal OT also increased amygdala–mPFC coupling at rest for young women and trend-wise for older men but not in the other age-by-sex subgroups (Ebner et al., 2016). The present study was not sufficiently powered to consider sex as an additional design factor (over and above age, treatment, and facial emotion), which will have to be systematically addressed in future extensions of the current work.
Furthermore, the current study’s sample size may have resulted in limited statistical power to detect significant OT-related behavioral effects. A recent meta-analysis, published after the present study’s closure, recommends that behavioral studies on OT and emotion identification should have at least 64 participants in each group to reliably assess differences between two groups, such as OT vs. P (Effect Size ≥ 0.5) (Leppanen et al., 2017). Our treatment groups were slightly under this size (OT: n = 53, P: n = 49). Other studies using a similar task were also limited in terms of only focusing on OT’s effects in one sex and/or in small samples (Chen et al., 2015; Di Simplicio et al., 2009; Kim et al., 2015; Lischke et al., 2012). Thus, larger sample sizes in future extensions of this work are needed to not only drive neurophysiological responses to stimuli but also to distinguish OT-related behavioral responses.
6.2. OT altered brain patterns and amygdalar connectivity by age during dynamic facial emotion identification
Our findings regarding brain activity patterns and amygdalar connectivity were largely in line with our predictions. Young participants recruited the AI and ACC of the salience network during dynamic facial emotion identification. In contrast, older participants recruited more anterior and midline regions including the mPFC, a major node of the default network. These findings support age-related differences in brain activity patterns during dynamic emotion processing, which qualify previous work conducted in young and older adults using static facial emotions (Keightley et al., 2007; Ziaei et al., 2016). Also, while both age groups recruited frontal and temporoparietal regions during the task (Kessler et al., 2011; LaBar et al., 2003), amygdala activation only emerged as a significant node in the young adult brain pattern. This finding is in line with decreased amygdala and increased prefrontal recruitment during emotion processing in older adults (Keightley et al., 2007; but see Ziaei et al., 2017).
Regarding OT effects, we found that OT altered a brain pattern associated with the processing of sadness and happiness in young participants yet only for sadness in older participants. In contrast, there were no treatment-related effects in brain activity for anger and fear in either of the age groups. These results may reflect OT’s attention-orienting role in prompting emotion-specific prosocial responses to stimuli (e.g., sadness, empathic response; happiness, approach response) (Theodoridou et al., 2013; but see Tollenaar et al., 2013). Further, the finding that OT altered brain activity only for sadness in the older group vs. happiness and sadness in the young group could be linked to individual differences in the endogenous OT system. Previous research has shown that a specific polymorphism of the OT receptor gene (OXTR; AA compared to GA/GG carriers of OXTR rs237887) was associated with increased activity in the ACC and reduced reaction time for the identification of happiness in older adults. GA/GG carriers may leverage the ACC to accurately identify positive emotions (Ebner et al., 2013). A combined neural, behavioral, and genetic approach that examines interactions between intranasal OT and the OT system may elucidate individual differences in emotion processing in aging.
The present study’s seed PLS analysis showed a robust amygdalar network related to dynamic facial emotion identification in both age groups, suggesting that amygdalar connectivity for the identification of dynamic facial emotions may be preserved in aging. This finding contrasts with previous work on static emotion processing that showed age-related differences in amygdalar connectivity (Keightley et al., 2007; St Jacques et al., 2010). Follow-up studies will allow determination of age-related similarities and differences in connectivity relating to naturalistic (i.e., dynamic) emotion processing. Directly correlating behavioral performance or other relevant measures to task-related or resting-state networks will also further advance the characterization of individual socioemotional aging trajectories.
OT administration was associated with a more widespread amygdalar network in older (but not young) participants for anger, fear, and happiness, which are high-arousal emotions (Clark et al., 1984; Mauss & Robinson, 2009). This network was recruited early in the task. In contrast, older (but not young) participants in the P group recruited a smaller network which emerged later (lag 4). These findings contribute to an emerging literature suggesting that OT’s effects are temporally dependent (Piva & Chang, 2018) and that OT administration may shift attention early to facilitate the detection of socially salient stimuli (Domes et al., 2013; Shamay-Tsoory & Abu-Akel, 2016; but see Guastella et al., 2009).
This more widespread network recruited by older participants in the OT group included the mPFC and subgenual cingulate gyrus, regions implicated in emotional conflict regulation and appraisal (Etkin et al., 2011). In contrast, older participants in the P group recruited more parietal regions and the putamen. This pattern of findings suggests that OT may facilitate amygdalar communication with more anterior regions as a function of early orienting of attentional resources to socially relevant stimuli (Huffmeijer et al., 2013; Sander et al., 2003). OT’s effects may be particularly sensitive among older adults in specific emotional contexts. Intranasal OT in this respect may be beneficial for older adults’ processing of high-arousal emotions, given age-related declines in the experience of high-arousal emotions (Charles & Carstensen, 2010) and the impact of arousal on memory for high-priority information in aging (Mather & Sutherland, 2011). These hypotheses can be tested in the future by using experimental tasks that systematically incorporate emotion displays of varying valence (positive vs. negative) and arousal (low vs. high) levels.
Examination of functional connectivity can enhance our understanding of factors that impact brain processes, including age, health status, and sensitivity to pharmacological manipulation (Borsook et al., 2011; Sala-Llonch et al., 2015). Functional changes relating to healthy and pathological brain aging, in particular, have not been clearly distinguished yet in the context of social cognition (Natelson Love et al., 2015). To our knowledge, our study is the first to investigate the effects of OT on functional connectivity related to the processing of different facial emotions in healthy older adults. More research on healthy older populations is needed to follow up these findings towards uncovering functional changes relating to social cognition that stem from age-related neuropathology (Huffmeijer et al., 2013), such as in frontotemporal dementia (Jesso et al., 2011). Although a single dose of 24 IU is widely used for investigations on intranasal OT’s effects on social cognition (Quintana et al., 2018), we propose that in clinical extensions of this work, use of randomized, placebo-controlled, multi-dose and repeated administration paradigms will be beneficial towards determination of OT’s therapeutic efficacy by systematically comparing OT’s effects across different dosages and across multiple administrations (Macdonald & Feifel, 2013).
7. Conclusion
In conclusion, our study provides evidence for distinct age- and OT-related, emotion-specific brain patterns, and amygdalar connectivity during dynamic facial emotion identification and represents a crucial first step in a broader research program that probes the efficacy of intranasal OT on the brain and behavior in aging. Intranasal OT effects on brain pattern recruitment varied between young and older adults and by emotion. Further, OT administration in older adults resulted in an amygdalar network shift for the processing of high-arousal emotions (anger, fear, happiness). OT’s alteration of brain patterns and connectivity by age and emotion supports a discrete approach to emotion processing in adulthood and aging (Kunzmann et al., 2017). Together these findings suggest that intranasal OT effects on the brain in healthy aging may be temporally and contextually dependent. Moving forward, fine-grained investigations on OT’s temporal effects may be particularly informative for advancing understanding of OT’s role in aging and emotion processing.
Supplementary Material
Highlights.
Older adults had more difficulty identifying dynamic emotions than young adults
OT effects on whole-brain patterns for happy faces differed by age group
Both age groups recruited a large-scale amygdalar network for emotion identification
OT altered amygdalar connectivity for high-arousal emotions in older adults
OT’s effects on connectivity appears temporally and contextually dependent in aging
Acknowledgments
This work was supported by the University of Florida Clinical and Translational Science pilot award (NIH/NCATS, UL1 TR000064 to N.C.E.); the Scientific Research Network on Decision Neuroscience and Aging pilot award (NIH/NIA, R24 AG039350 to N.C.E.); the NIA Pre-Doctoral Fellowship on Physical, Cognitive and Mental Health in Social Context (T32 AG020499 to M.H.); the Scientific Research Network on Decision Neuroscience and Aging Mentorship/Collaboration Award (NIH/NIA, R24-AG054355 to M.H. and R.N.S.); the University of Florida Psychology Department; the Center for Cognitive Aging & Memory; and the Claude D. Pepper Older Americans Independence Center. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. This work was also supported in part by a grant from the Canadian Institute for Health Research (to R.N.S.). The authors would like to thank Désirée Lussier, Elizabeth DuPre, Mark Schuster, Adam Soliman, and Paige Hespe for their input on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have no actual or potential conflicts of interest.
References
- Adolphs R (2002). Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews, 1(1), 21–62. 10.1177/1534582302001001003 [DOI] [PubMed] [Google Scholar]
- Bänziger T, Mortillaro M, & Scherer KR (2012). Introducing the Geneva Multimodal expression corpus for experimental research on emotion perception. Emotion (Washington, D.C.), 12(5), 1161–79. 10.1037/a0025827 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RAI, van Honk J, Auyeung B, & Baron-Cohen S (2013). Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38, 962–974. 10.1016/j.psyneuen.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, & Barrett LF (2014). The amygdala as a hub in brain networks that support social life. Neuropsychologia, 63, 235–48. 10.1016/j.neuropsychologia.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, & Becerra L (2011). Can Functional Magnetic Resonance Imaging Improve Success Rates in CNS Drug Discovery? Expert Opinion on Drug Discovery, 6(6), 597–617. 10.1517/17460441.2011.584529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, & Folstein M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 1(2), 111–117. [Google Scholar]
- Brodmann K, Gruber O, & Goya-Maldonado R (2017). Intranasal Oxytocin Selectively Modulates Large-Scale Brain Networks in Humans. Brain Connectivity, 7(7), 454–463. 10.1089/brain.2017.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, & Young AW (2003). Facial expression recognition across the adult life span. Neuropsychologia, 41(2), 195–202. 10.1016/S0028-3932(02)00149-5 [DOI] [PubMed] [Google Scholar]
- Campbell A, Ruffman T, Murray JE, & Glue P (2014). Oxytocin improves emotion recognition for older males. Neurobiology of Aging, 35(10), 2246–2248. 10.1016/j.neurobiolaging.2014.04.021 [DOI] [PubMed] [Google Scholar]
- Charles S, & Carstensen LL (2010). Social and emotional aging. Annual Review of Physiology, 61, 383–409. 10.1146/annurev.psych.093008.100448.Social [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, Dvorak F, Domes G, Yim OS, Ebstein RP, & Heinrichs M (2015). Genetic modulation of oxytocin sensitivity: a pharmacogenetic approach. Translational Psychiatry, 5(10), e664 10.1038/tp.2015.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Milberg S, & Erber R (1984). Effects of arousal on judgments of others’ emotions. Journal of Personality and Social Psychology, 46(3), 551–560. 10.1037/0022-3514.46.3.551 [DOI] [Google Scholar]
- Di Domenico A, Palumbo R, Mammarella N, & Fairfield B (2015). Aging and emotional expressions: is there a positivity bias during dynamic emotion recognition? Frontiers in Psychology, 6, 1130 10.3389/fpsyg.2015.01130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, & Harmer CJ (2009). Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology, 23(3), 241–248. 10.1177/0269881108095705 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, & Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–1190. 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, & Herpertz SC (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology, 35(1), 83–93. 10.1016/j.psyneuen.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, & Heinrichs M (2013). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology, 38(7), 1198–1202. 10.1016/j.psyneuen.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, & Cohen RA (2016). Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology, 69, 50–59. 10.1016/j.psyneuen.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, & Fischer H (2014). Emotion and aging: Evidence from brain and behavior. Frontiers in Psychology, 5, 996 10.3389/fpsyg.2014.00996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Horta M, Lin T, Feifel D, Fischer H, & Cohen RA (2015). Oxytocin modulates meta-mood as a function of age and sex. Frontiers in Aging Neuroscience, 7(SEP), 175 10.3389/fnagi.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, & Johnson MK (2009). Young and older emotional faces: Are there age group differences in expression identification and memory? Emotion, 9(3), 329–339. 10.1037/a0015179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Lin T, Muradoglu M, Weir DH, Plasencia GM, Lillard TS, … Connelly JJ (2018). Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. International Journal of Psychophysiology. 10.1016/j.ijpsycho.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, Macdonald K, Westberg L, & Fischer H (2013). Oxytocin and socioemotional aging: Current knowledge and future trends. Frontiers in Human Neuroscience, 7, 487 10.3389/fnhum.2013.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, & Lindenberger U (2010). FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362. 10.3758/BRM.42.1.351 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, & Büchel C (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences, 107(20), 9400–9405. 10.1073/pnas.1000985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger SA, Henry JD, Steinvik HR, Vanman EJ, Rendell PG, & Labuschagne I (2018). Intranasal oxytocin does not reduce age-related difficulties in social cognition. Hormones and Behavior, 99, 25–34. 10.1016/J.YHBEH.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, & Cox RE (2009). Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology, 34(2), 220–225. 10.1016/J.PSYNEUEN.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38, 612–625. 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Stawski RS, Wasylyshyn C, & Verhaeghen P (2004). Adult age and digit symbol substitution performance: A meta-analysis. Psychology and Aging, 19(1), 211–214. 10.1037/0882-7974.19.1.211 [DOI] [PubMed] [Google Scholar]
- Huffmeijer R, van Ijzendoorn MH, & Bakermans-Kranenburg MJ (2013). Ageing and oxytocin: A call for extending human oxytocin research to ageing populations--a mini-review. Gerontology, 59, 32–39. [DOI] [PubMed] [Google Scholar]
- Jesso S, Morlog D, Ross S, Pell MD, Pasternak SH, Mitchell DGV, … Finger EC (2011). The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain, 134(9), 2493–2501. 10.1093/brain/awr171 [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Winocur G, & Grady CL (2007). Age-related differences in brain activity underlying identification of emotional expressions in faces. Social Cognitive and Affective Neuroscience, 2(4), 292–302. 10.1093/scan/nsm024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéri S, & Benedek G (2009). Oxytocin enhances the perception of biological motion in humans. Cognitive, Affective, & Behavioral Neuroscience, 9(3), 237–241. 10.3758/CABN.9.3.237 [DOI] [PubMed] [Google Scholar]
- Kessler H, Doyen-Waldecker C, Hofer C, Hoffmann H, Traue HC, & Abler B (2011). Neural correlates of the perception of dynamic versus static facial expressions of emotion. Psycho-Social Medicine, 8, Doc03 10.3205/psm000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-R, Eom J-S, Yang J-W, Kang J, & Treasure J (2015). The Impact of Oxytocin on Food Intake and Emotion Recognition in Patients with Eating Disorders: A Double Blind Single Dose Within-Subject Cross-Over Design. PLOS ONE, 10(9), e0137514 10.1371/journal.pone.0137514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, … Meyer-Lindenberg A (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–11493. 10.1523/JNEUROSCI.3984-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, & Abdi H (2011). Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage, 56(2), 455–475. 10.1016/j.neuroimage.2010.07.034 [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Rohr M, Wieck C, Kappes C, & Wrosch C (2017). Speaking about feelings: Further evidence for multidirectional age differences in anger and sadness. Psychology and Aging, 32(1), 93–103. 10.1037/pag0000142 [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, & McCarthy G (2003). Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex (New York, N.Y. : 1991), 13(10), 1023–33. 10.1093/cercor/13.10.1023 [DOI] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, & Treasure J (2017). Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neuroscience & Biobehavioral Reviews, 78, 125–144. 10.1016/j.neubiorev.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, & Domes G (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37(4), 475–481. 10.1016/j.psyneuen.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Macdonald K (2013). Sex, receptors, and attachment: A review of individual factors influencing response to oxytocin. Frontiers in Neuroscience, 6, 194 10.3389/fnins.2012.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K, & Feifel D (2013). Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Frontiers in Neuroscience, 7, 35 10.3389/fnins.2013.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L, Falvello VB, Aviezer H, & Todorov A (2016). Contributions of facial expressions and body language to the rapid perception of dynamic emotions. Cognition and Emotion, 30(5), 939–952. 10.1080/02699931.2015.1035229 [DOI] [PubMed] [Google Scholar]
- Martins B, & Mather M (2016). Default Mode Network and Later-Life Emotion Regulation: Linking Functional Connectivity Patterns and Emotional Outcomes In Ong AD & Löckenhoff CE (Eds.), Emotion, Aging, and Health (pp. 9–29). American Psychological Association; 10.1037/14857-002 [DOI] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-Biased Competition in Perception and Memory. Perspectives on Psychological Science : A Journal of the Association for Psychological Science, 6(2), 114–33. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, & Robinson MD (2009). Measures of emotion: A review. Cognition & Emotion, 23(2), 209–237. 10.1080/02699930802204677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Hoeft F, Glover GH, & Reiss AL (2009). Methods and Software for fMRI Analysis of Clinical Subjects. NeuroImage, 47, S58 10.1016/S1053-8119(09)70238-1 [DOI] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, & Grady CL (1996). Spatial Pattern Analysis of Functional Brain Images Using Partial Least Squares. NeuroImage, 3(3), 143–157. 10.1006/nimg.1996.0016 [DOI] [PubMed] [Google Scholar]
- McIntosh AR, & Lobaugh NJ (2004). Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage, 23, S250–S263. 10.1016/j.neuroimage.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Natelson Love MC, Ruff G, & Geldmacher DS (2015). Social Cognition in Older Adults: A Review of Neuropsychology, Neurobiology, and Functional Connectivity. Medical & Clinical Reviews, 1(6), 1–8. 10.21767/2471-299X.1000006 [DOI] [Google Scholar]
- Noh SR, & Isaacowitz DM (2013). Emotional faces in context: age differences in recognition accuracy and scanning patterns. Emotion (Washington, D.C.), 13(2), 238–249. 10.1037/a0030234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, & Yamaguchi S (2012). Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience, 24(11), 2186–98. 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Piva M, & Chang SWC (2018). An integrated framework for the role of oxytocin in multistage social decision-making. American Journal of Primatology, e22735 10.1002/ajp.22735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Smerud KT, Andreassen OA, & Djupesland PG (2018). Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Therapeutic Delivery, 9(7), 515–525. 10.4155/tde-2018-0002 [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie. Presses Universitaires de France. [Google Scholar]
- Röcke C, Li S-C, & Smith J (2009). Intraindividual variability in positive and negative affect over 45 days: Do older adults fluctuate less than young adults? Psychology and Aging, 24(4), 863–878. 10.1037/a0016276 [DOI] [PubMed] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, & Phillips LH (2008). A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews, 32(4), 863–881. 10.1016/j.neubiorev.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Sakaki M, Nga L, & Mather M (2013). Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults’ memory. Journal of Cognitive Neuroscience, 25(8), 1206–24. 10.1162/jocn_a_00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, & Junqué C (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in Psychology, 6, 663 10.3389/fpsyg.2015.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, & Zalla T (2003). The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences, 14(4), 303–16. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, & Armony JL (2008). The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32(4), 811–30. 10.1016/j.neubiorev.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology, 38(10), 1929–1936. 10.1038/npp.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, & Cabeza R (2010). Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging, 31(2), 315–327. 10.1016/j.neurobiolaging.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Penton-Voak IS, & Rowe AC (2013). A Direct Examination of the Effect of Intranasal Administration of Oxytocin on Approach-Avoidance Motor Responses to Emotional Stimuli. PLoS ONE, 8(2), e58113 10.1371/journal.pone.0058113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Chatzimanoli M, van der Wee NJA, & Putman P (2013). Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology, 38(9), 1797–1802. 10.1016/J.PSYNEUEN.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Turner GR, & Spreng RN (2015). Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: The default–executive coupling hypothesis of aging. Journal of Cognitive Neuroscience, 27(12), 2462–2476. 10.1162/jocn [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Vakil E, & Blachstein H (1997). The clinical neuropsychologist Rey AVLT: Developmental norms for adults and the sensitivity of different memory measures to age Rey AVLT: Developmental norms for adults and the sensitivity of different memory measures to age. The Clinical Neuropsychologist, 11(4), 356–369. 10.1080/13854049708400464 [DOI] [Google Scholar]
- Van Essen DC (2005). A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage, 28(3), 635–662. 10.1016/j.neuroimage.2005.06.058 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). WAIS-R: Manual : Wechsler adult intelligence scale--revised. New York: Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–70. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei M, Burianová H, von Hippel W, Ebner NC, Phillips LH, & Henry JD (2016). The impact of aging on the neural networks involved in gaze and emotional processing. Neurobiology of Aging, 48, 182–194. 10.1016/j.neurobiolaging.2016.08.026 [DOI] [PubMed] [Google Scholar]
- Ziaei M, Salami A, & Persson J (2017). Age-related alterations in functional connectivity patterns during working memory encoding of emotional items. Neuropsychologia, 94, 1–12. 10.1016/j.neuropsychologia.2016.11.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.