Abstract
Background
Primary cytoreduction followed by platinum based chemotherapy is the primary treatment for advanced ovarian cancer. However neoadjuvant chemotherapy followed by interval debulking is an alternative option, particularly in those who may be poor surgical candidates.
Objective
The objective of this study was to determine factors associated with short term, significant perioperative morbidity and mortality for women undergoing surgery for ovarian cancer and to create a nomogram to predict the risk of adverse perioperative outcomes.
Study Design
We used the National Surgical Quality Improvement Program database to identify women with ovarian, fallopian tube, or primary peritoneal cancer who underwent surgery from 2011-2015. Demographic factors, clinical characteristics, comorbidity, functional status and the extent of surgery were used to predict the risk of severe perioperative complications or death using multivariable models. Multiple imputation methods were employed for missing data. A nomogram was developed based on the final model. The discrimination ability of the model was assessed with a calibration plot and discrimination C-index.
Results
We identified a total of 7,029 patients. Overall, 5.8% of patients experienced a Clavien-Dindo IV complication, 9.8% of patients were readmitted, 3.0% of patients required a reoperation, and 0.9% of patients died within 30 days. Among the baseline variables assessed, increasing age, emergent surgery, ascites, bleeding disorder, low albumin, higher ASA, and a higher extended procedure score were associated with serious perioperative morbidity or mortality. Of these factors, performance of ≥3 cytoreductive procedures (aOR 4.53, 95% CI 3.01-6.82), ASA ≥ class 4 (aOR 2.89, 95% CI 1.17-7.14), bleeding disorder (aOR 2.73, 95% CI 1.82-4.10), and age ≥80 years old (aOR 2.46, 95% CI 1.66-3.63) were most strongly associated with risk of an event. The final nomogram included the above variables and had an internal discrimination C-index of 0.71, with accurate predictions in an internal validation set, indicating a 71% correct identification of patients across all possible pairs.
Conclusion
Women undergoing surgery for ovarian cancer are at significant risk for the occurrence of adverse perioperative outcomes. Using readily identifiable characteristics, this nomogram can predict adverse outcomes.
Introduction
The primary treatment for advanced stage ovarian cancer is cytoreductive surgery followed by adjuvant platinum based chemotherapy. Optimal or complete resection of disease is associated with improved survival outcomes.1–4 In addition to hysterectomy, bilateral salpingoophorectomy, and omentectomy, complete resection of disease may also require radical surgery, including bowel resection, diaphragm stripping, splenectomy, liver resection, and other complex procedures.5–9
While aggressive surgery may be associated with increased overall survival, this benefit must be balanced against the significant risk of perioperative morbidity and mortality associated with radical cytoreductive surgery. 10,11 Patients diagnosed with ovarian cancer are often elderly, have multiple comorbidities and may experience less benefit from cytoreduction than is reported in clinical trials of highly selected patients. 11–15 Surgical complications in women with ovarian cancer are associated with significant pain and suffering, are costly to treat, and may lead to delay in the receipt of adjuvant chemotherapy.16
There has been an increasing interest in identifying patients who may be poor surgical candidates, given the risks associated with surgery. Neoadjuvant chemotherapy followed by interval debulking is an alternative to primary cytoreduction. In clinical trials, neoadjuvant chemotherapy has been associated with similar survival as primary cytoreduction, but is accompanied by significantly less perioperative morbidity and mortality. 17 A number of models have attempted to predict perioperative morbidity and mortality as a method of identifying patients who may benefit from neoadjuvant chemotherapy. Most models have used single institutional data to determine factors associated with short term morbidity and mortality and have included factors, such as age, ASA score, surgical complexity and tumor characteristics, such as stage, grade, and histology.18–24
The objective of our study was to use a large, national dataset to determine factors associated with short-term, significant perioperative morbidity and mortality and to create a nomogram to predict the risk of adverse perioperative outcomes. Using this nomogram, we hope to create a prediction tool for patients who are being considered for primary debulking or neoadjuvant chemotherapy.
Materials and Methods
We examined patients who underwent surgery for primary ovarian, fallopian tube, or peritoneal cancer in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database between 2011-2015. NSQIP collects preoperative, intraoperative, and 30-day postoperative data of patients undergoing major surgical procedures from participating hospitals to measure surgical quality. Data are abstracted from medical charts under a systematic sampling process which requires each participating hospital to submit data from 42 of the 46 8-day cycles equally spaced throughout the year. Data quality is ensured by conducting Inter-Rater Reliability audits regularly. 25
All patients in our cohort underwent oophorectomy with or without hysterectomy. For patients who had additional procedures for cytoreduction, we developed a surgical complexity score. For the procedure score, we assigned one point each for lymph node dissection, small bowel, colon, rectosigmoid, liver, bladder or diaphragm resection, and debulking. Each patient was thereby classified with a score of 0, 1, 2 or ≥3.
Demographic characteristics included age (<50, 50-59, 60-69, 70-79, ≥80 years), race/ethnicity (white, black, other), and whether the surgery was elective (yes, no). For each woman, the following preoperative conditions were recorded: body mass index (BMI, normal <25 kg/m2, overweight 25 to <30 kg/m2, obese ≥30 kg/m2), diabetes mellitus (insulin dependent, or non-insulin dependent), tobacco use within one year, history of severe chronic obstructive pulmonary disease (COPD), ascites, congestive heart failure (CHF) within 30 days before surgery, hypertension requiring medication, bleeding disorder, American Society of Anesthesiology (ASA) classification score (≤1, 2, 3, ≥4), serum albumin (>4, 3.5-4, <3.5 g/dL) and hematocrit (<36%, ≥36%). Year of operation, length of stay (0, 1, 2, ≥3) and discharge status (home, dead, facility) were reported descriptively. Missing data were reported as the “unknown” category. The primary outcome was Clavien-Dindo IV complications (including postoperative sepsis, shock, cardiac arrest, myocardial infarction, pulmonary embolism, ventilation >48 hours, or unplanned intubation) or death within 30 days after surgery. 26
Fifteen predictors were initially evaluated for statistically significant associations (P-value <0.05) with the outcome using bivariate logistic regression models. Missing data were noticed in race/ethnicity, elective surgery, BMI, albumin and hematocrit and were accounted for using multiple imputation with chained equations with M=100 imputations. The discriminant function method was used to impute the categorical variables of race/ethnicity and elective surgery. Height, weight, albumin, and hematocrit were imputed using linear regression models assuming normality, and then categorized as BMI, albumin and hematocrit groups. To avoid bias, all the variables in the analysis model, including height, weight, each cytoreductive procedure (yes/no) from the procedure score and the outcome variable were included in the imputation model, along with year of operation.27,28 Race, BMI, preoperative diabetes mellitus, tobacco use, COPD and CHF were excluded because of P-values ≥0.05. All two-way interaction terms were evaluated between the remaining predictors. The interactions between ascites and hematocrit, and between hypertension and bleeding disorder had a P-value <0.1, but neither showed clinically differentiable ORs; therefore, only the main predictors were included in the multivariable model. Hypertension and hematocrit were no longer significant (P-value <0.05) after adjusting for the other covariates and were excluded. The final model included procedure score, age, elective surgery, preoperative ascites, bleeding disorder, albumin, and the ASA classification score.
A nomogram was developed based on the final model. The discrimination ability of the model was reported as the calibration plot with the 95% confidence interval. The concordance index (C-index) was reported as a measure of internal validation using both 10-fold cross-validation repeated for 20 times, and bootstrap validation of 200 resamples the same size as the original cohort with replacement. We performed sensitivity analysis with complete cases excluding patients with missing data, or classifying them as the unknown group. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
We identified a total of 7,029 patients for whom surgeries were performed between 2011-2015. Age was well represented across all groups with 19.4% of patients age <50 years, 26.5% 50-59 years old, 29.3% 60-69 years, and 24.8% ≥70 years (Table 1). Most patients were white, overweight or obese, non-smokers, and non-diabetic. Preoperatively, most patients had no ascites, a normal albumin, and were not anemic. Most patients underwent one extended procedure (49.7%), with the most common being debulking (49.8%) and lymph node dissection (43.4%), followed by rectosigmoid resection (6.8%), other large bowel resection (3.9%), and small bowel resection (3.1%). Postoperatively, most patients had a hospital length of stay of 3 days or longer and 92.4% of patients were discharged home (Table 1).
Table 1.
Descriptive statistics of patient characteristics
| N | % | ||
|---|---|---|---|
| All | 7,029 | (100.0) | |
| Year of operation | |||
| 2011 | 878 | (12.5) | |
| 2012 | 1,082 | (15.4) | |
| 2013 | 1,510 | (21.5) | |
| 2014 | 1,606 | (22.9) | |
| 2015 | 1,953 | (27.8) | |
| Age (in years) | |||
| <50 | 1,366 | (19.4) | |
| 50-59 | 1,864 | (26.5) | |
| 60-69 | 2,057 | (29.3) | |
| 70-79 | 1,291 | (18.4) | |
| ≥80 | 451 | (6.4) | |
| Race/ethnicity | |||
| White | 5,380 | (76.5) | |
| Black | 445 | (6.3) | |
| Other | 384 | (5.5) | |
| Unknown | 820 | (11.7) | |
| Elective surgery | |||
| Yes | 6,370 | (90.6) | |
| No | 632 | (9.0) | |
| Unknown | 27 | (0.4) | |
| BMI | |||
| Normal | 2,435 | (34.6) | |
| Overweight | 2,056 | (29.3) | |
| Obese | 2,495 | (35.5) | |
| Unknown | 43 | (0.6) | |
| Diabetes | |||
| Insulin | 221 | (3.1) | |
| Non-insulin | 555 | (7.9) | |
| No | 6,253 | (89.0) | |
| Tobacco use | 928 | (13.2) | |
| COPD | 190 | (2.7) | |
| Ascites | 1,323 | (18.8) | |
| CHF | 19 | (0.3) | |
| Hypertension | 2,852 | (40.6) | |
| Bleeding disorder | 183 | (2.6) | |
| Albumin (g/dL) | |||
| <3.5 | 1,033 | (14.7) | |
| 3.5-4 | 1,902 | (27.1) | |
| >4 | 1,974 | (28.1) | |
| Unknown | 2,120 | (30.2) | |
| Hematocrit | |||
| <36% | 2,780 | (39.6) | |
| ≥36% | 4,073 | (58.0) | |
| Unknown | 176 | (2.5) | |
| ASA classification score | |||
| ≤1 | 204 | (2.9) | |
| 2 | 2,959 | (42.1) | |
| 3 | 3,595 | (51.2) | |
| ≥4 | 271 | (3.9) | |
| Procedure score | |||
| 0 | 1,586 | (22.6) | |
| 1 | 3,493 | (49.7) | |
| 2 | 1,618 | (23.0) | |
| ≥3 | 332 | (4.7) | |
| Extended procedures | |||
| LND | 3,047 | (43.4) | |
| Small bowel resection | 217 | (3.1) | |
| Colon resection | 272 | (3.9) | |
| Rectosigmoid resection | 475 | (6.8) | |
| Liver resection | 123 | (1.8) | |
| Bladder resection | 21 | (0.3) | |
| Diaphragm resection | 154 | (2.2) | |
| Debulking | 3,503 | (49.8) | |
| Length of stay | |||
| 0 | 110 | (1.6) | |
| 1 | 498 | (7.1) | |
| 2 | 619 | (8.8) | |
| ≥3 | 5,798 | (82.5) | |
| Unknown | 4 | (0.06) | |
| Discharge status | |||
| Home | 6,494 | (92.4) | |
| Dead | 40 | (0.6) | |
| Facility | 480 | (6.8) | |
| Unknown | 15 | (0.2) | |
Overall, 5.8% of patients experienced a Clavien-Dindo IV complication, 9.8% of patients were re-admitted, and 3.0% of patients required a reoperation. Of the Clavien-Dindo IV complications, the most common were sepsis (2.4%) and pulmonary embolism (1.7%) (Table 2). The perioperative mortality rate within 30 days of surgery was 0.9%.
Table 2.
Morbidity and mortality outcomes of patients
| N | % | |
|---|---|---|
| Readmission | 688 | (9.8) |
| Reoperation | 214 | (3.0) |
| Death | 64 | (0.9) |
| Clavien-Dindo IV complications | 409 | (5.8) |
| Sepsis | 166 | (2.4) |
| Shock | 63 | (0.9) |
| Cardiac arrest | 15 | (0.2) |
| Myocardial infarction | 22 | (0.3) |
| Pulmonary embolism | 116 | (1.7) |
| Ventilation > 48 hours | 69 | (1.0) |
| Unplanned intubation | 65 | (0.9) |
| Death or Clavien-Dindo IV complications | 434 | (6.2) |
Among the baseline variables assessed in multivariable models, increasing age, emergent surgery, ascites, bleeding disorder, low albumin, higher ASA, and a higher extended procedure score were significantly associated with serious perioperative morbidity or mortality. Of these factors, performance of ≥3 cytoreductive procedures (aOR 4.53, 95% CI 3.01-6.82), ASA ≥ class 4 (aOR 2.89, 95% CI 1.17-7.14), bleeding disorder (aOR 2.73, 95% CI 1.82-4.10), and age ≥80 years old (aOR 2.46, 95% CI 1.66-3.63) a were most strongly associated with risk of an event (Table 3).
Table 3.
Multivariable model for predictors of death or Clavien-Dindo IV complication
| aOR | ||
|---|---|---|
| Procedure score | ||
| 0 | Referent | |
| 1 | 1.41 (1.04-1.92)* | |
| 2 | 2.26 (1.63-3.11)* | |
| ≥3 | 4.53 (3.01-6.82)* | |
| Age (in years) | ||
| <50 | 1.32 (0.94-1.85) | |
| 50-59 | Referent | |
| 60-69 | 1.39 (1.04-1.87)* | |
| 70-79 | 1.81 (1.32-2.46)* | |
| ≥80 | 2.46 (1.66-3.63)* | |
| Elective surgery | ||
| Yes | Referent | |
| No | 1.72 (1.29-2.29)* | |
| Ascites | 1.58 (1.26-1.99)* | |
| Bleeding disorder | 2.73 (1.82-4.10)* | |
| Albumin | ||
| >4 | Referent | |
| 3.5-4 | 1.42 (1.06-1.90)* | |
| <3.5 | 1.93 (1.39-2.70)* | |
| ASA classification score | ||
| ≤1 | Referent | |
| 2 | 1.28 (0.55-2.98) | |
| 3 | 1.61 (0.70-3.72) | |
| ≥4 | 2.89 (1.17-7.14)* | |
The final multivariable logistic regression model included age, elective surgery, preoperative ascites, bleeding disorder, albumin level, ASA classification score and procedure score. Multiple imputation using chained equations (MICE) with m=100 imputations were performed for patients with missing data in demographic characteristics.
p-value <0.05.
The final nomogram included the above variables and had an initial discrimination C-index of 0.71 indicating a 71% correct identification of patients across all possible pairs. A 10-fold cross validation with 20 replications resulted in a C index of 0.70 and the bootstrap validation with 200 resamples resulted in a C index of 0.71 indicating acceptable discriminatory ability. The bias corrected C-index with these validation sets closely matched the initial C index. The final model showed good internal calibration with predicted outcomes matching closely with observed outcomes (Figure 1). The nomogram seen in Figure 2 uses individual patient characteristics to predict risk of a Clavien-Dindo IV event or 30-day mortality postoperatively. Complete case analyses showed similar results.
Figure 1.
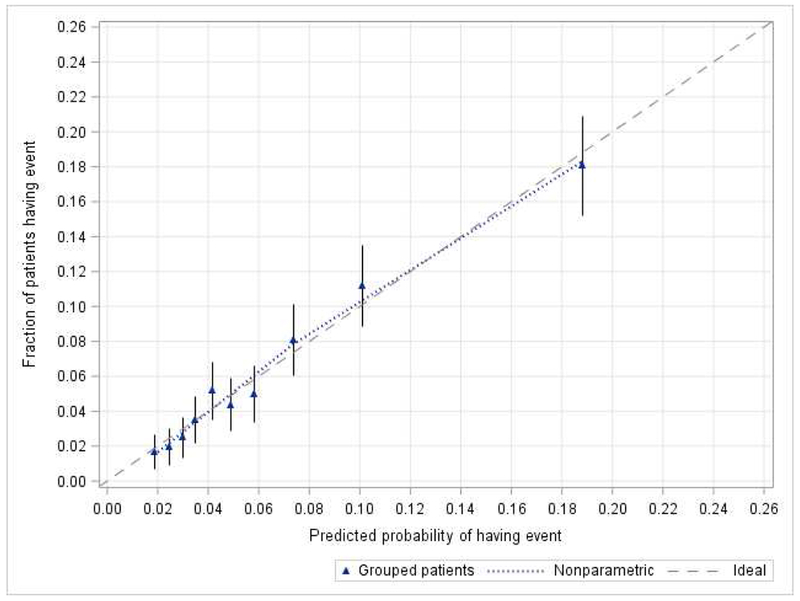
Calibration of the nomogram for Clavien-Dindo IV complication or mortality. Dashed line (the 45-degree line) indicated the ideal reference line where the predicted probabilities of having an event would match the observed factions. Blue triangles represented nomogram-predicted probabilities versus the actual probability grouped for each of the ten decile groups, along with the 95% confidence intervals (error bars). The distance between the pair of nomogram-predicted versus observed and the ideal line showed the absolute error of the nomogram’s prediction.
Figure 2.
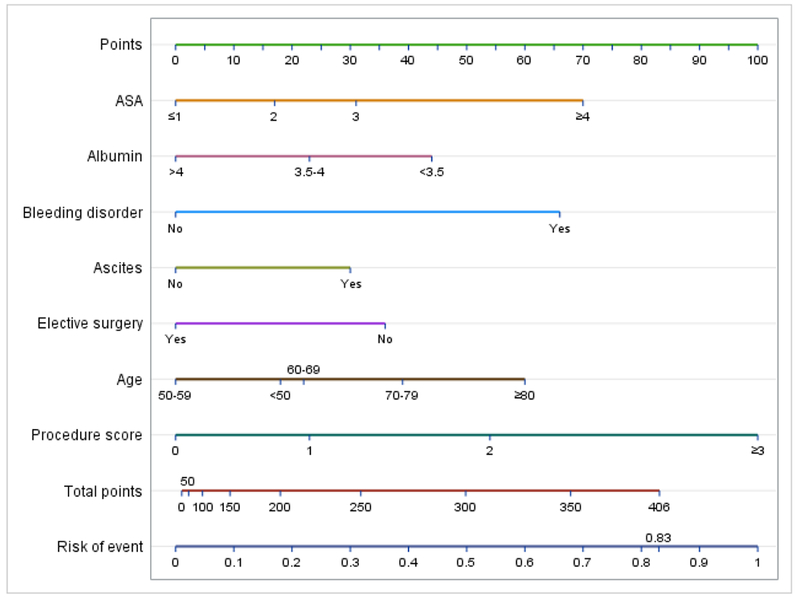
Nomogram for Clavien-Dindo IV complication or mortality.
Risk points for each variable are obtained by vertically mapping a patient’s category to the scale labeled “Points”. The predicted risk of Clavien-Dindo IV complication or mortality is obtained by vertically mapping the totaled points on the scale labeled “Total points” to the scale labeled “Risk of event”.
In one example, an 82 year old undergoing elective surgery for an ovarian mass with an ASA score of 2, normal albumin >4, no bleeding disorder, no ascites, and a procedure score of 1 (standard surgery with debulking) would be assigned 60 points for age ≥80, 23 points for a procedure score of 1, 17 points for an ASA of 2, and 0 points for an elective procedure, normal albumin, no ascites, and no bleeding disorder. Her total points would be 100 and her risk of a Clavien-Dindo IV complication or mortality would be 4.6%.
In contrast, a 65 year old woman with medical comorbidities including poorly controlled diabetes and hypertension and an ASA class of 3, undergoing elective surgery for widely disseminated disease with an anticipated procedure score of ≥3 (debulking, rectosigmoid, small bowel, and diaphragm resection), ascites, hypoalbuminemia, but without a bleeding disorder would be assigned 22 points for age, 31 points for ASA of 3, 100 points for her procedure score, 31 points for ascites, 44 points for hypoalbuminemia, 0 points for an elective procedure and no bleeding disorder. Her total points would be 228 and her risk of a Clavien-Dindo IV complication or mortality would be 25.1%.
Comment
We noted that women undergoing surgery for ovarian cancer are at significant risk for the occurrence of adverse perioperative outcomes. Cytoreduction with performance of multiple extended surgical procedures, higher ASA score, and advanced age were among the factors most strongly associated with adverse outcomes. Using readily identifiable clinical characteristics, we were able to develop a nomogram to predict adverse outcomes that was associated with strong internal calibration with a C index of 0.71, indicating that in 71% of cases the nomogram was able to correctly predict the actual outcome when tested across risk groups.
Cytoreductive surgery for ovarian cancer is associated with substantial morbidity. A systematic review of women who underwent surgery for ovarian cancer found that the overall risk of mortality was 3.7% in population-based studies and 2.5% in single center studies.10 One study using the Surveillance Epidemiology and End-Results (SEER) database indicated the 30 day mortality risk for stage II-IV epithelial ovarian cancer was 8.7%, with worse outcomes in the elderly, stage IV disease, and those with increasing comorbidity scores.14 In our prior published work using the NSQIP database, we found a perioperative complication rate of 9.5% with worsening outcomes in those with hypoalbuminemia or multiple cytoreductive procedures.29
NSQIP has a publicly available universal risk calculator that allows up to 20 variables to be input for a specified procedure but in prior studies has been poor performance for gynecologic oncology patients. 30,31 The strengths of our nomogram as compared to the universal risk calculator are that it includes only factors statistically and clinically associated with the primary outcomes, uses a surgical complexity score to account for multiple procedures during debulking surgery, and multiple imputation methods to complete data where missing.
Prior studies have attempted to create predictive models for both short and long-term postoperative outcomes in patients undergoing ovarian debulking surgery. One study of 620 patients with stage III/IV epithelial ovarian cancer reported a 22.3% rate of 30-day Clavien-Dindo III or higher complications and an 8.9% rate of 90-day mortality. Clavien-Dindo complications were significantly associated with age, BMI, ASA, albumin, stage, and surgical complexity (internal validation with C-index of 0.78). Stage and surgical complexity were no longer significant in 90-day mortality outcomes.19 Similarly, in another study of 219 patients, ASA score, surgical complexity score (based on difficulty and number of procedures performed), and age contributed to short term morbidity, while residual disease was the only factor contributing to 90-day mortality.18 One long-term survival nomogram examined 424 patients with bulky stage IIIC ovarian carcinoma and found age and residual disease were the greatest factors that contributed to 5-year survival probability (internal validation with C-index of 0.67).20 Our model using readily identifiable factors was associated with high internal and with a random holdout sample external validity.
An important goal of developing predictive nomograms for ovarian cancer is to help facilitate the triage of women at high-risk for adverse perioperative outcomes to neoadjuvant chemotherapy. Similar to prior work, our nomogram found the performance of extended cytoreductive procedures weighed more heavily than hypoalbuminemia, advanced age, ascites, or emergent surgery.11 An important goal of neoadjuvant chemotherapy is to reduce the need for extended cytoreductive procedures. In a randomized trial studying NACT, patients who underwent NACT had a lower perioperative mortality rate (0.7% vs 2.5%) and grade 3 and 4 hemorrhage (4% vs 7%).17 A second randomized trial also found a 10% increased rate of perioperative death or severe complications in those who underwent primary debulking surgery.32 A study of the National Cancer Database found that the increased regional use of NACT significantly reduced short and long term mortality within three years after diagnosis. 33 Given that both morbidity and mortality are lower with neoadjuvant chemotherapy compared to primary cytoreduction, there is a strong rationale to offer primary chemotherapy to the highest risk women. Using our nomogram, we were able to create a standardized objective algorithm to determine which patients may be at high risk who may be considered for neoadjuvant chemotherapy.
We recognize a number of important limitations. First, NSQIP lacks data on clinical and tumor characteristics, such as CA-125 levels, histology, and the amount of residual tumor at the completion of surgery. However, The focus of the current study was immediate postoperative morbidity and mortality and not long term outcomes. Similarly, we lack data on other diagnostic modalities, such as imaging and laparoscopic assessment of disease which might be useful in further improving the performance of our nomogram if available.34–37 Lastly, we are unable to distinguish whether a patient underwent primary or interval cytoreduction or the stage at time of diagnosis. A priori, the goal of this analysis was only to examine factors associated with complications regardless of the timing of surgery. However, the overall complication rate would likely have been higher if our study were limited to women who underwent primary surgery or had strictly stage III/IV disease.
In summary, these data demonstrate that it is feasible to create a highly predictive nomogram for adverse outcomes among women undergoing surgery for ovarian cancer. Extended cytoreductive procedures, ASA score, bleeding disorder, and age were all predictive of poor outcomes. Our nomogram is among the first to use nationwide data and its strengths include a large patient sample size and a strong C index of 0.71. This nomogram may be a valuable tool for decision making in guiding providers when considering primary debulking or NACT.
AJOG at a Glance:
Prediction of patients who are at high risk of adverse perioperative outcomes may help stratify patients between primary debulking and neoadjuvant chemotherapy
5.8% of patients experienced a Clavien-Dindo IV complication and 0.9% of patients died within 30 days. Among the baseline variables assessed, increasing age, emergent surgery, ascites, bleeding disorder, low albumin, higher ASA, and a higher extended procedure score were associated with serious perioperative morbidity or mortality and were included in our nomogram. The final nomogram had an internal discrimination C-index of 0.71.
Using readily identifiable characteristics, this validated nomogram may help predict patients at high risk of adverse outcomes and assist in stratifying ovarian cancer patients to primary debulking or neoadjuvant chemotherapy
Acknowledgements:
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. Dr. Neugut has served as a consultant to Pfizer, Teva, Eisai, Otsuka, and United Biosource Corporation. He is on the medical advisory board of EHE, Intl. No other authors have any conflicts of interest or disclosures.
Dr. Wright (NCI R01CA169121-01A1) is a recipient of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation: A nomogram developed and validated from a nationwide database may predict perioperative adverse outcomes in ovarian cancer debulking
References
- 1.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 2006;103:559–64. [DOI] [PubMed] [Google Scholar]
- 2.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 2006;107:77–85. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621–7. [DOI] [PubMed] [Google Scholar]
- 4.Colombo PE, Mourregot A, Fabbro M, et al. Aggressive surgical strategies in advanced ovarian cancer: a monocentric study of 203 stage IIIC and IV patients. Eur J Surg Oncol 2009;35:135–43. [DOI] [PubMed] [Google Scholar]
- 5.Tsolakidis D, Amant F, Van Gorp T, Leunen K, Neven P, Vergote I. Diaphragmatic surgery during primary debulking in 89 patients with stage IIIB-IV epithelial ovarian cancer. Gynecol Oncol 2010;116:489–96. [DOI] [PubMed] [Google Scholar]
- 6.Mourton SM, Temple LK, Abu-Rustum NR, et al. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol 2005;99:608–14. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol 2006;103:1083–90. [DOI] [PubMed] [Google Scholar]
- 8.Magtibay PM, Adams PB, Silverman MB, Cha SS, Podratz KC. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol Oncol 2006;102:369–74. [DOI] [PubMed] [Google Scholar]
- 9.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol 2006;100:283–7. [DOI] [PubMed] [Google Scholar]
- 10.Gerestein CG, Damhuis RA, Burger CW, Kooi GS. Postoperative mortality after primary cytoreductive surgery for advanced stage epithelial ovarian cancer: a systematic review. Gynecol Oncol 2009;114:523–7. [DOI] [PubMed] [Google Scholar]
- 11.Wright JD, Lewin SN, Deutsch I, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecol Oncol 2011;123:467–73. [DOI] [PubMed] [Google Scholar]
- 12.Langstraat C, Aletti GD, Cliby WA. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer: a delicate balance requiring individualization. Gynecol Oncol 2011;123:187–91. [DOI] [PubMed] [Google Scholar]
- 13.Mahdi H, Lockhart D, Maurer KA. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for endometrial cancer. Gynecol Oncol 2015;137:106–11. [DOI] [PubMed] [Google Scholar]
- 14.Thrall MM, Goff BA, Symons RG, Flum DR, Gray HJ. Thirty-day mortality after primary cytoreductive surgery for advanced ovarian cancer in the elderly. Obstet Gynecol 2011;118:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters CL, Schneider KE, Whitworth JM, et al. Perioperative morbidity and mortality in octogenarians with ovarian cancer. Int J Gynecol Cancer 2013;23:1006–9. [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol 2012;120:871–81. [DOI] [PubMed] [Google Scholar]
- 17.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- 18.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol 2007;197:676 e1–7. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Janco JM, Mariani A, et al. Risk-prediction model of severe postoperative complications after primary debulking surgery for advanced ovarian cancer. Gynecol Oncol 2016;140:15–21. [DOI] [PubMed] [Google Scholar]
- 20.Chi DS, Palayekar MJ, Sonoda Y, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol 2008;108:191–4. [DOI] [PubMed] [Google Scholar]
- 21.Stashwick C, Post MD, Arruda JS, et al. Surgical risk score predicts suboptimal debulking or a major perioperative complication in patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer 2011;21:1422–7. [DOI] [PubMed] [Google Scholar]
- 22.Suidan RS, Leitao MM Jr., Zivanovic O, et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol 2015;138:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orskov M, Iachina M, Guldberg R, Mogensen O, Mertz Norgard B. Predictors of mortality within 1 year after primary ovarian cancer surgery: a nationwide cohort study. BMJ Open 2016;6:e010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Donato V, Kontopantelis E, Aletti G, et al. Trends in Mortality After Primary Cytoreductive Surgery for Ovarian Cancer: A Systematic Review and Metaregression of Randomized Clinical Trials and Observational Studies. Ann Surg Oncol 2017;24:1688–97. [DOI] [PubMed] [Google Scholar]
- 25.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998;228:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 28.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–91. [DOI] [PubMed] [Google Scholar]
- 29.Patankar S, Burke WM, Hou JY, et al. Risk stratification and outcomes of women undergoing surgery for ovarian cancer. Gynecol Oncol 2015;138:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivard C, Nahum R, Slagle E, Duininck M, Isaksson Vogel R, Teoh D. Evaluation of the performance of the ACS NSQIP surgical risk calculator in gynecologic oncology patients undergoing laparotomy. Gynecol Oncol 2016;141:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szender JB, Frederick PJ, Eng KH, Akers SN, Lele SB, Odunsi K. Evaluation of the National Surgical Quality Improvement Program Universal Surgical Risk Calculator for a gynecologic oncology service. Int J Gynecol Cancer 2015;25:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England) 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- 33.Melamed A, Fink G, Wright AA, et al. Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: quasi-experimental study. BMJ 2018;360:j5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutten MJ, van Meurs HS, van de Vrie R, et al. Laparoscopy to Predict the Result of Primary Cytoreductive Surgery in Patients With Advanced Ovarian Cancer: A Randomized Controlled Trial. J Clin Oncol 2017;35:613–21. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez N, Rauh-Hain JA, Shoni M, et al. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol 2012;125:362–6. [DOI] [PubMed] [Google Scholar]
- 36.Pelissier A, Bonneau C, Chereau E, et al. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol 2014;135:542–6. [DOI] [PubMed] [Google Scholar]
- 37.Eoh KJ, Yoon JW, Lee JY, et al. A novel algorithm for the treatment strategy for advanced epithelial ovarian cancer: consecutive imaging, frailty assessment, and diagnostic laparoscopy. BMC Cancer 2017;17:481. [DOI] [PMC free article] [PubMed] [Google Scholar]


