Abstract
A fixed dose combination of bupropion (BPP) and naltrexone (NTX), Contrave®, is an FDA approved pharmacotherapy for the treatment of obesity. A recent study found that combining BPP with low-dose NTX reduced alcohol drinking in alcohol-preferring male rats. To explore potential pharmacological effects of the BPP+NTX combination on alcohol drinking, both male and female C57Bl/6J mice were tested on one-week drinking-in-the dark (DID) and three-week intermittent access (IA) models. Neuronal proopiomelanocortin (POMC) enhancer knockout (nPE−/−) mice with hypothalamic-specific deficiency of POMC, and its bioactive peptides melanocyte stimulating hormone and beta-endorphin, were used as a genetic control for the effects of the BPP+NTX. A single administration of BPP+NTX (10 mg/kg+1 mg/kg) decreased alcohol intake after DID in C57Bl/6J males, but not females. Also in C57Bl/6J males, BPP+NTX reduced intake of the caloric reinforcer sucrose, but not the non-caloric reinforcer saccharin. In contrast, BPP+NTX had no effect on alcohol DID in nPE−/− males. Pretreatment with the selective melanocortin 4 receptor (MC4R) antagonist HS014 reversed the anti-dipsogenic effect of BPP+NTX on alcohol DID in C57Bl/6J males. In the 3-week chronic IA model, single or repeated administrations for four days of BPP+NTX reduced alcohol intake and preference in C57Bl/6J males only. The behavioral measures observed in C57Bl/6J mice provide clear evidence that BPP+NTX profoundly reduced alcohol drinking in males, but the doses tested were not effective in females. Furthermore, our results suggest a hypothalamic POMC/MC4R-dependent mechanism for the observed BPP+NTX effects on alcohol drinking in male mice.
Keywords: Bupropion, naltrexone, alcohol, MC4R, nPE knockout, sex
1. INTRODUCTION
A fixed dose, extended-release oral formulation containing bupropion (BPP, 90mg) and naltrexone (NTX, 8mg) has been developed to reduce excessive eating. This combination of BPP and NTX, marketed with the trade name of Contrave®, has been shown to reduce body weight [Toll et al., 2008; Greenway et al., 2009; Sherman et al., 2016]. It is known that obese individuals treated with this BPP+NTX combination have less cravings for palatable foods, and more control over their drive to consume food [Greenway et al., 2010]. Similarly, in rats, the reduction of food consumption by BPP+NTX was observed when the BPP+NTX was administered systemically or infused directly into the ventral tegmental area of male rats [Billes et al., 2014; Levy et al., 2018]. BPP (dopamine and norepinephrine reuptake inhibitor and nicotine receptor antagonist) is an antidepressant and smoking cessation agent, while NTX (a non-selective mu-opioid receptor [MOP-r] antagonist) is prescribed for both obesity and alcohol addiction [O’Malley et al., 1992, 2002; Greig and Keating, 2015; Karoly et al., 2015]. Of interest is the possibility that the combination of low doses of BPP with NTX may have greater effects than either drug alone, with less adverse consequences [e.g., Chandler and Herxheimer 2011; Reeves and Ladner 2013].
In pre-clinical models of alcohol addiction, there are several precedents to test NTX in combination with other compounds, including acamprosate, prazosin, varenicline or V1b antagonists [Heyser et al., 2003; Froehlich et al., 2013, 2016; Zhou et al., 2018]. The focus of the current study was to explore potential pharmacological actions of the BPP+NTX on alcohol drinking behaviors in mice. Specifically, it was explored whether BPP+NTX could alter alcohol drinking in both drinking-in-the dark (DID) and intermittent access (IA) models. Hence, we determined the effect of BPP+NTX in a DID paradigm with limited access (4 h/day) and relatively low alcohol intake (<5–6 g/kg/day) which models “binge” drinking to the point of intoxication in mice [Rhodes et al., 2005; Zhou et al., 2017a, 2018]. Because BPP+NTX increase proopiomelanocortin (POMC) expression and neuronal activity in the hypothalamus of rats [Greenway et al., 2009; Levy et al., 2018], we then investigated whether BPP+NTX could alter DID in neuronal POMC enhancer (nPE−/−) knockout mice (with resultant loss of hypothalamic POMC) [Bumaschny et al., 2012; Lam et al., 2015], to explore potential neuronal mechanisms for the BPP+NTX effects. Two main neuropeptides beta-endorphin and melanocortin derived from POMC activate MOP-r and melanocortin receptors to increase and decrease alcohol consumption, respectively [Olney et al., 2014]. As NTX in BPP+NTX blocks MOP-r, it was further explored whether the combination could reduce DID through a melanocortin-mediated mechanism via MC4 receptors (MC4R).
In an IA model, mice develop high alcohol consumption (15–25 g/kg/day) after 3 weeks of access to alcohol every other day (two-bottle choice for 24 hours) [Hwa et al., 2011; Zhou et al., 2017a, 2018]. Therefore, we investigated whether single or repeated administrations of BPP+NTX altered excessive alcohol intake after chronic alcohol drinking, to evaluate BPP+NTX’s potential as a therapeutic agent for alcoholism. Since BPP is a psychomotor stimulant [Wright and Rogers 2013], we tested locomotor activity to examine potential psychomotor side effects of BPP+NTX.
A recent study found that combining BPP with NTX was effective in reducing alcohol drinking in alcohol-preferring male rats [Nicholson et al., 2018]. It has been shown that there are sex differences in alcohol drinking behavior in animals and humans [Becker and Koob, 2016; Erol et al., 2019]. Therefore, the present study was designed to examine the effects of BPP+NTX in both male and female mice.
2. MATERIAL AND METHODS
2.1. Animals.
Adult C57BL/6J (B6) mice of both sexes were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in a temperature-controlled (21°C) room on a 12-hour reverse light-dark cycle (lights on at 7:00 pm). Mice (9–10 weeks of age) were individually housed in ventilated cages fitted with steel lids and filter tops, and given ad libitum access to a standard chow and water for at least 7 days prior to the beginning of the experiment. The present study also used singly-housed male mice with a targeted deletion of the POMC neuronal enhancers nPE1 and nPE2 and insertion of a transcriptional blocking neo cassette in the enhancer locus (nPE−/−) [Bumaschny et al., 2012; Lam et al., 2015]. Male nPE−/− mice and nPE+/+ littermates derived from heterozygous nPE+/− parents used in the recent [Zhou et al., 2017b, 2018] and current studies were bred at the Rockefeller University animal facility and had been backcrossed for 14–16 serial generations onto the B6 background strain. nPE−/− mice exhibit nearly undetectable Pomc expression in the hypothalamic arcuate nucleus, without altered Pomc expression in pituitary, thereby maintaining function of the hypothalamic-pituitary-adrenal axis. During the experiments (age 9–10 weeks), nPE−/− mice had greater body weight (~ 40g in males and 35g in females) than their nPE+/+ siblings (~ 27g in males and 23g in females). The housing conditions were identical to those for B6 mice. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996) and were approved by the Animal Care and Use Committee of the Rockefeller University. Unless noted, 6–9 mice of each sex and condition were used in all experiments.
2.2. Materials.
Bupropion hydrochloride and naltrexone-hydrochloride (Sigma Aldrich, USA) or HS014 (Tocris, USA) were dissolved in 0.9% saline and administered within a range of doses known to affect alcohol drinking or food consumption in rodents [Greenway et al., 2009; Zhou et al., 2017a; Levy et al., 2018]. The BPP+NTX combination was studied at a ratio of 10 BPP/1 NTX in most experiments to model the ratio employed in Contrave® [Greenway et al., 2009]. The alcohol solution from 190 proof absolute ethyl alcohol (Pharmco-AAPER, Brookfield, CT), sucrose or saccharin [Sigma-Aldrich Inc., St. Louis, MO, USA) was diluted in tap water.
2.3. Experiments.
2.3.1. Effects of single BPP+NTX, BPP or NTX administration on alcohol drinking in drinking-in-the-dark (DID) in B6 and nPE knockout mice.
Based on the original publication [Rhodes et al., 2005] with our recent modifications [Zhou et al., 2016], the basic protocol used in the present study was as follows: after 3 hours of lights off, a water bottle was replaced with a 10-ml pipette tube fitted with a stainless steel straight sipper tubing, filled with alcohol at a concentration of 15% for 4 hours. Alcohol solution was prepared fresh every 24 hours by mixing alcohol with tap water to reach the appropriate (v ⁄ v) alcohol concentration. On the drinking days, alcohol intake value (ml) was recorded after 4 hours of alcohol access (to the nearest 0.1 ml). These data were used for calculating alcohol intake (g ⁄ kg).
Body weights were matched within each group of each sex before assignment to treatment. After 4 days of DID, mice within each sex that had similar alcohol intake 24 hours before the test day were assigned to vehicle- and drug-treated groups. The compounds were dissolved in vehicle and administered by an experimenter blind to the treatments assigned to the experimental groups. All drugs were administered by intraperitoneal injections. The control and experimental groups received the same volume of injections and the same amount of vehicle solution.
We first tested the dose-response of each drug alone: [A] BPP at 5, 10 or 20 mg/kg; and [B] NTX at 1 and 2 mg/kg in both male and female B6 mice. On the test day, alcohol was presented 30 min after a single injection of BPP or saline, or 10 min after a single injection of NTX or saline and then alcohol intake values were recorded after 4 hours.
Based on the single-dose results, the BPP+NTX combination dose-response was then tested BPP+NTX at 5+0.5, 10+1, 20+1 or 20+2 mg/kg in both sexes of B6 mice. In these BPP+NTX experiments, the mice received BPP followed by NTX 20 min later. Then alcohol was presented 10 min later and then alcohol intake values were recorded. The doses of the BPP+NTX combinations, BPP and NTX doses were suggested by the publications on rodent studies [Rhodes et al., 2005; Greenway et al., 2009; Wright and Rodgers, 2013; Zhou et al., 2017a, 2017b; Levy et al., 2018]. Based on our preliminary results, the best combination of BPP (10 mg/kg) with sub-effective doses of NTX (1 mg/kg) was further studied in all the following experiments.
To test whether the effect of BPP+NTX was mediated by MC4R, mice were pre-treated with the selective MC4R antagonist HS014. Male mice received HS014 (0.5 μmol/kg) in vehicle (saline) 60 min before the 4-h drinking test, followed by one BPP (10 mg/kg) 30 min later and then NTX (1 mg/kg) 20 min after BPP or vehicle (saline) injections before the 4-h drinking test. Only males were tested because it was found that BPP+NTX had no effect on alcohol drinking in females in the current experiments.
Lastly, a single administration of BPP+NTX (10 +1 mg/kg) was tested in the DID in male nPE mice. The effects of BPP+NTX were measured on alcohol intake in two genotypes (nPE+/+ and nPE−/−), and the dose of BPP+NTX was identical to one in B6 mice in the above DID experiments. Male mice were assigned to one of 4 treatment groups: [1] nPE+/+ with vehicles as control; [2] nPE+/+ with BPP+NTX; [3] nPE−/− with vehicles as control; and [4] nPE−/− with BPP+NTX.
2.3.2. Effects on single BPP+NTX administration on 4% sucrose or 0.1% saccharin drinking in DID in B6 mice.
Since alcohol is a caloric reinforcer, the specificity of BPP+NTX (10 +1 mg/kg) effects on alcohol intake was verified on sucrose (caloric reinforcer) and saccharin (non-caloric reinforcer) in the DID protocol. In these control experiments, sucrose or saccharin was provided, using the procedures described above. For each sucrose or saccharin experiment, separate groups of mice of both sexes were used: [1] Sucrose drinking (4% [w/v]) with single BPP+NTX (10+1 mg/kg); [2] Saccharin drinking (0.1% [w/v]) with single BPP+NTX (10+1 mg/kg); and [3] Sucrose or saccharin drinking with vehicles (saline) as controls. Of note, NTX at 1 mg/kg had no effect on sucrose or saccharin drinking in either male or female mice [Rhodes et al., 2005; Zhou et al., 2017a, 2017b].
2.3.3. Effects of single or repeated administrations of BPP+NTX (10 +1 mg/kg) on alcohol drinking in chronic (3-week) intermittent access (IA) in B6 mice.
The IA protocol used was identical to our recent reports [Zhou et al., 2017a, 2017b], that were modified from the early report [Hwa et al., 2011]: mice had 24 hours of access to 15% alcohol every other day on a two-bottle (water vs. alcohol) choice paradigm for 3 weeks with food available. For IA alcohol drinking, the main procedure was identical to that of the above DID model with some exceptions: on setting at 3 hours after lights off, the two10-ml pipette sipper tubes (water and 15% alcohol) were placed in the home cages for 24 hours. The left ⁄right position of the two tubes was randomized (the bottle side switched within a cage across days). On the drinking days, both alcohol and water intake values (ml) were recorded after 4, 8 and 24 hours of alcohol access. These data were used for calculating alcohol intake (g ⁄ kg) and preference ratio for alcohol (alcohol intake ⁄ total fluid intake). Body weights were matched within each group of each sex before assignment to treatment. After 3 weeks of IA, mice within each sex that had similar alcohol intake 24 hours before the test day were assigned to vehicle- and drug-treated groups.
Using the IA model, we determined whether there was any potential effect of BPP+NTX treatments on drinking behavior after IA with any sex differences. Separate groups of mice of both sexes were used in the following experiments: [1] IA with single BPP+NTX (10+1 mg/kg) (Table 1A); and [2] IA with four daily repeats of BPP+NTX (10+1 mg/kg) (Table 1B). Mice received the 1st injection BPP+NTX one day before the drinking session, followed by the 2nd administration immediately before the drinking session on the same day, and we then repeated the 3rd and 4th administrations and recorded alcohol drinking after the 2nd and 4th administration (Table 1B). In both single and repeated experiments, 15% alcohol was presented after the BPP+NTX injections or vehicles, and then alcohol and water intake values were recorded at 3 time points as described above.
Table 1.
Experimental timelines
| Week 1–3 | Day 21 |
| IA 24-h alcohol vs water | Single BPP+NTX with 4, 8 and 24 h recording |
| Week 1–3 | Day 22 | Day 23 | Day 24 | Day 25 |
| IA 24-h alcohol vs water | 1st BPP+NTX | 2nd BPP+NTX with 4, 8 and 24 h recording | 3rd BPP+NTX | 4th BPP+NTX with 4, 8 and 24 h recording |
This low-dose combination of BPP+NTX with short-period treatments of 4 days did not change the body weight in either male or female mice, consistent with several prior reports showing that BPP+NTX did not reduce body weight in rats after relatively chronic administrations [Wright and Rodgers, 2013; Levy et al., 2018; Nicholson et al., 2018].
2.3.4. Effects of BPP+NTX (10 +1 mg/kg) on locomotor activity in B6 mice.
In another set of mice of both sexes, locomotor activity was tested 3 hours after lights off (identical time for starting alcohol drinking sessions) in activity chambers for 30 min. In the first test of two tests, half the mice were placed in the black chamber 30 min after a single injection of BPP (10 mg/kg) followed by a single injection of NTX (1 mg/kg) 20 min later and another half in the white one. To avoid natural preference for the two different chambers, the chambers were reversed in the second test. The average value of the two tests was used as the locomotor activity score.
2.4. Data analysis.
In the DID experiments with single BPP, NTX or BPP+NTX alone in both B6 males and females or nPE males, alcohol (or sucrose or saccharin) intake (dependent variable) differences across the different groups were analyzed using 2-way ANOVA for treatment (vehicle vs BPP+NTX, BPP or NTX) and sex (male vs female) or genotype (male nPE+/+ vs male nPE−/−). In the DID experiments with single BPP+NTX after HS014 in males, alcohol intake (dependent variable) differences across the different groups were analyzed using 2-way ANOVA for pretreatment (vehicle vs HS014) and for treatment (vehicle vs BPP+NTX). In the IA experiments with BPP+NTX in males and females, alcohol intake, water intake, and preference ratio (dependent variable) differences across groups were analyzed using 2-way ANOVA for treatment and sex. In experiments examining group differences in locomotor activity, data were analyzed using 2-way ANOVA (treatment and sex). All the ANOVAs were followed by Newman-Keuls post-hoc tests. All the statistical analyses were performed using Statistica (version 5.5, StatSoft Inc, Tulsa, OK) and the accepted level of significance was p < 0.05.
3. RESULTS
3.1. Dose-responses of single BPP, NTX or BPP+NTX treatment on alcohol DID in B6 mice.
3.1.1. BPP treatment:
The response of single BPP at 5, 10 or 20 mg/kg on alcohol intake is presented in Table S2. Two-way ANOVA revealed no effect of BPP treatment or interaction between sex and BPP treatment, only with significant effects of sex at all doses [5 mg/kg, F(1,20)=9.6, p<0.01; 10 mg/kg, F(1,24)=14.2, p<0.001 and 20 mg/kg, F(1,24)=10.7, p<0.01]. Hence, females drank more alcohol than males after both vehicle and BPP [p<0.05 for all].
3.1.2. NTX treatment:
Table S3 presents the dose response of single NTX at 1 or 2 mg/kg on alcohol intake. At 1 mg/kg NTX, two-way ANOVA did not reveal any significant effects of NTX treatment alone [F(1,24)=1.7, p=0.25]. However, at 2 mg/kg, there was a significant effect of NTX treatment [F(1,20)=8.6, p<0.01] in both males and females [post-hoc tests p<0.05 for both], with no interaction between sex and NTX treatment. Females had more alcohol intake than males after either 1 mg/kg NTX [F(1,24)=8.7, p<0.05] or 2 mg/kg NTX [F(1,20)=7.4, p<0.05].
3.1.3. BPP+NTX treatment:
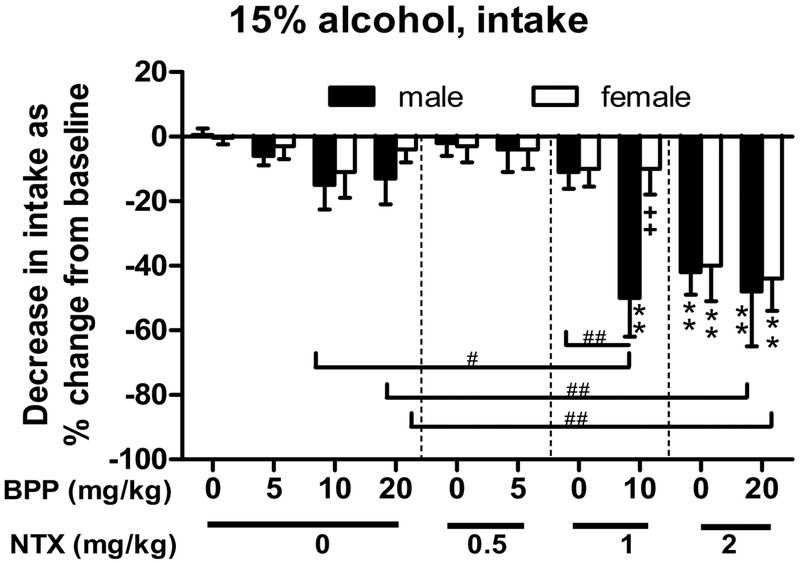
In Figure 1 for the full-dose response of BPP, NTX or BPP+NTX, data collected on the testing day (day 5) are expressed as a percentage of baseline alcohol intake (day 4) to account for the differences in baseline that contribute to variation between experiments. BPP (0, 5, 10 or 20 mg/kg) combined with NTX (0, 0.5, 1 or 2 mg/kg) reduced alcohol intake in a sex- and dose- dependent manner (data at the 4-hour time point are analyzed together). Two-way ANOVA showed significant effects of sex [F(1,114)=10.4, p<0.005], the BPP+NTX treatments [F(9,114)=31.5, p<0.0001], and their interaction [F(9,114)=4.1, p<0.001]. In comparison with the vehicle controls (0+0 dose), BPP+NTX at 10+1 mg/kg reduced alcohol intake in males [post-hoc test p<0.001], but not females [p=0.85]. Compared with the females, the males had more reduction in alcohol intake after the BPP+NTX (10+1) treatment [p<0.001]. Compared with either BPP alone at 10 mg/kg or NTX alone at 1 mg/kg, the combination at 10+1 mg/kg had more reduction in alcohol intake [p<0.001 for both]. BPP+NTX at 20+2 mg/kg, however, significantly reduced alcohol intake in both sexes [p<0.0005 for both]. Of note, NTX at 2 mg/kg alone showed significant reductions than vehicle control in both males and females [p<0.0005 for both], and the BPP+NTX treatment at 20+2 mg/kg dose did not differ from the NTX alone (Figure 1).
Figure 1.
Dose responses of single administration of bupropion (BPP, 0, 5, 10 or 20 mg/kg) alone or combined with naltrexone (NTX, 0, 0.5, 1 or 2 mg/kg) on reducing 15% alcohol intake in both male and female B6 mice (n=6–8) in a 5-day drinking-in-the-dark (DID) model. Data were collected at the 4-hour time point on the baseline and testing day (24 hours later) and are expressed as a percentage of baseline alcohol intake to account for the differences in baseline that contribute to variation between experiments and sexes. **p<0.01 vs. control (both BPP and NTX at 0 mg/kg); ++p<0.01 vs. male mice with the same treatment; and #p<0.05 or ##p<0.01 vs. the BPP or NTX alone groups. Data shown are mean + SEM.
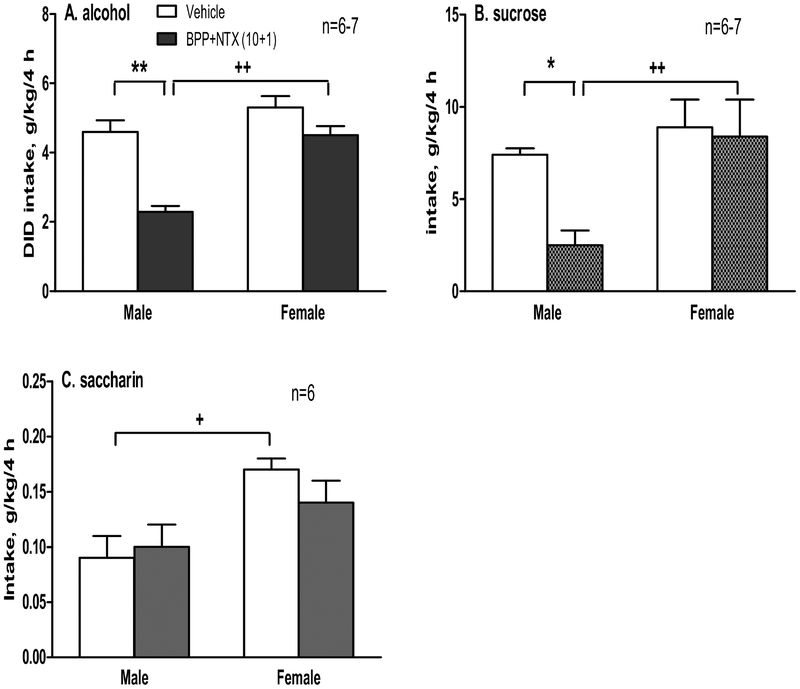
The combination of 5 mg/kg BPP and 0.5 mg/kg NTX had no effect on DID in either male or female mice (Table S4A), with only sex differences [F(1,20)=6.9, p<0.05]. However, 10 mg/kg BPP combined with 1 mg/kg NTX significantly reduced alcohol intake in males [F(1,22)=31.3, p<0.001; post-hoc test p<0.01], but not in females [p=0.09] (Figure 2A). Two-way ANOVA also showed a significant effect of sex [F(1,22)=27.4, p<0.001], and a significant interaction between the BPP+NTX treatment and sex [F(1,22)=6.8, p<0.05]. Female mice had more alcohol intake than male mice after BPP+NTX [post-hoc test p<0.01]. Similar to the 10+1 mg/kg combination, when the BPP at 20 mg/kg with 1 mg/kg NTX (Table S4B), there were significant effects of the 20+1 treatment in males [F(1,28)=15.5, post-hoc test p<0.05], but not in females [p=0.33]. There were also significant effects of sex [F(1,28)=11.7, p<0.05] and interaction between sex and treatment [F(1,28)=5.7, p<0.05]. When two highest doses of each drug combined (20 mg/kg BPP + 2 mg/kg NTX) were tested (Table S4C), there were significant effects of 20+2 treatment in both males and females [F(1,29)=10.8, p<0.01; post-hoc tests p<0.05 for both sexes], and sex [F(1,29)=7.4, p<0.05].
Figure 2.
Effect of single administration of bupropion (BPP, 10 mg/kg) combined with naltrexone (NTX, 1 mg/kg) on 15% alcohol intake (A), 4% sucrose intake (B) or 0.1% saccharin intake (C) in both male and female B6 mice (n=6–7) in a 5-day drinking-in-the-dark (DID) model in male and female mice. *p<0.05 or **p<0.01 vs. the vehicle-treated mice in the same sex; +p<0.05 or ++p<0.01 vs. male mice with the same treatment. Data shown are mean + SEM
In a new experiment, the BPP+NTX combinations at a ratio of 10 BPP/1 NTX were repeated with the following doses: 0+0, 5+0.5, 10+1 and 20+2 in both sexes (Table 2). Two-way ANOVA showed significant effects of sex [F(1,40)=27.6, p<0.001] and the BPP+NTX treatments [F(3,40)=13.8, p<0.001]. BPP+NTX at 20+2 mg/kg significantly reduced alcohol intake in both sexes [p<0.01 for both]. To test our a priori hypothesis that there were sex differences after the BPP+NTX treatments, we included the post-hoc result showing that BPP+NTX at 10+1 mg/kg reduced alcohol intake in males [p<0.05], but not females [p=0.68], with a significant sex difference (male vs female, p<0.01), even though 2-way ANOVA did not show a significant interaction between sex and treatment [F(3,40)=2.4, p=0.08].
Table 2.
BPP+NTX combinations’ dose responses (0+0, 5+0.5, 10+1 or 20+2) on reducing 15% alcohol intake in both male and female B6 mice (n=6) in a 5-day drinking-in-the-dark (DID) model. *p<0.05 or **p<0.01 vs. the vehicle-treated mice (BPP+NTX at 0+0 mg/kg) in the same sex; ++p<0.01 vs. male mice with the same treatment. Data shown are mean ± SEM.
| Treatment Dose (mg/kg) | Male | Female |
|---|---|---|
| Saline + saline 0 + 0 | 5.0 ± 0.27 | 6.5 ± 0.40 |
| BPP+NTX 5 + 0.5 | 4.6 ± 0.41 | 6.3 ± 0.50 |
| BPP+NTX 10 + 1 | 2.4 ± 0.49 * | 5.9 ± 0.61 ++ |
| BPP+NTX 20 + 2 | 2.2 ± 0.33 ** | 3.0 ± 0.56 ** |
3.2. Effects of single BPP+NTX (10+1 mg/kg) treatment on sucrose or saccharin DID in B6 mice.
3.2.1. Sucrose intake:
BPP+NTX significantly reduced sucrose intake in males [F(1,23)=4.6, p<0.05, post-hoc test p<0.05], but not in females [p=0.78] (Figure 2B). Two-way ANOVA also found a significant effect of sex [F(1,23)=8.5, p<0.01], and females displayed higher sucrose intakes than males after the BPP+NTX treatment [p<0.01].
3.2.2. Saccharin intake:
Two-way ANOVA revealed a significant effect of sex [F(1,20)=12.9, p<0.005], with no effect of treatment or interaction between sex and the treatment (Figure 2C). Females drank more saccharin than males after the vehicle [p<0.05].
3.3. Effect of selective MC4R antagonist HS014 and single BPP+NTX (10+1 mg/kg) treatment on alcohol DID in males.
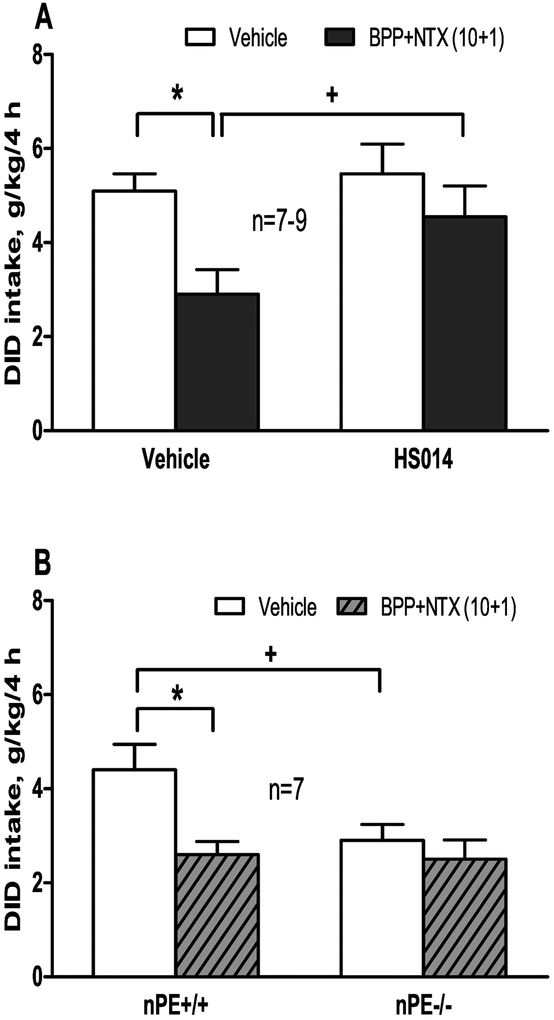
As shown in Figure 3A, HS014 pretreatment was only tested in males, as we did not find any effects of the BPP+NTX in females. Two-way ANOVA revealed a significant effect of BPP+NTX treatment [F(1,28)=6.37; p<0.05], without a significant effect of HS014 pretreatment [F(1,28)=3.5, p=0.07]. The BPP+NTX significantly reduced alcohol intake [post-hoc test p<0.05]. Though HS014 pretreatment at 0.5 μmol/kg alone had no effect per se, it abrogated the effect of the BPP+NTX on alcohol intake; there was a significant difference between the HS014 pretreatment and control vehicle groups followed by the BPP+NTX treatment [p<0.05].
Figure 3.
(A) In male B6 mice (n=7–9), pretreatment with selective MC4R antagonist HS014 (0.5 μmol/kg) blocks the effect of single BPP+ NTX (10+1 mg/kg) on reducing 15% alcohol intake in a 5-day drinking-in-the-dark (DID) model. *p<0.05 vs. the vehicle-treated mice; +p<0.05 vs. the BPP+NTX without HS014 pretreatment; (B) Genotype differences in the effects of single administration of BPP+NTX (10+1 mg/kg) on 15% alcohol intake in a 5-day drinking-in-the-dark (DID) model in male nPE mice (n=7). *p<0.05 vs. the vehicle-treated mice within genotype; +p<0.05 vs. the nPE+/+ mice with the same vehicle treatment. Data shown are mean + SEM.
3.4. Effect of single BPP+NTX (10+1 mg/kg) treatment on alcohol DID in male nPE+/+ and nPE−/− mice (Figure 3B).
Two-way ANOVA revealed significant effects of genotype [F(1,24)=5.0, p<0.05] and treatment [F(1,24)=6.1, p<0.05], but no significant genotype × treatment interaction [p=0.095]. Between genotypes, nPE−/− had less intake than nPE+/+ [p<0.05]. To test our a priori hypothesis that there were genotype differences after the BPP+NTX treatments, we included post-hoc analysis which revealed that BPP+NTX at 10+1 mg/kg reduced alcohol intake in nPE+/+ [p<0.05] only.
3.5. Effects of single BPP+NTX (10+1 mg/kg) treatment on alcohol IA in B6 mice.
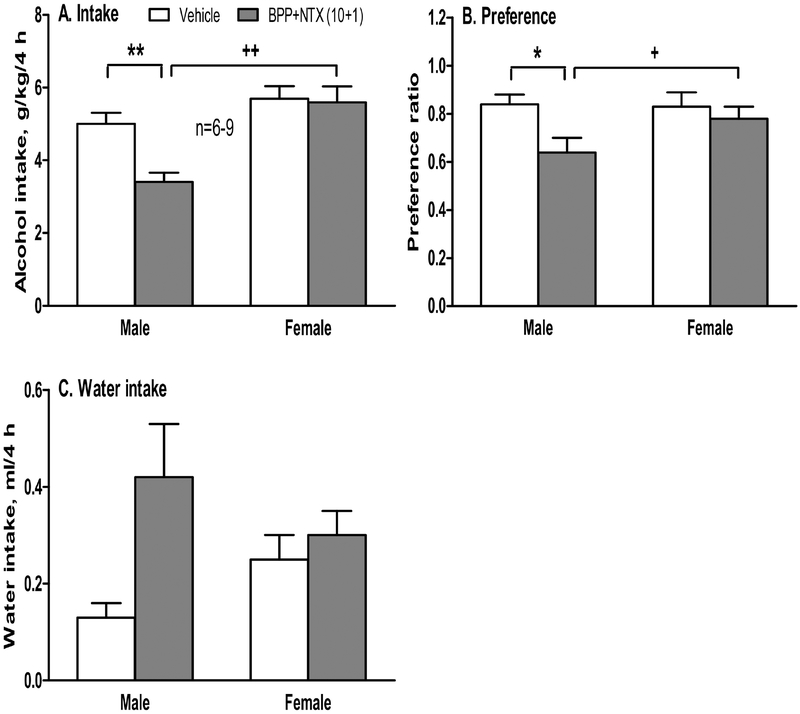
For alcohol intake at 4 hours, two-way ANOVA showed a significant effect of BPP+NTX treatment [F(1,23)=6.67, p<0.05], a significant effect of sex [F(1,23)=18.7, p<0.005]; and a significant interaction between BPP+NTX treatment and sex [F(1,23)=4.30, p<0.05]. Post-hoc tests found that: (1) BPP+NTX significantly reduced alcohol intake in males [p<0.01], but not in females [p=0.72] (Figure 4A); and (2) female mice had more alcohol intakes than male mice after the BPP+NTX [p<0.01]. At 4 hours, single BPP+NTX also significantly reduced alcohol preference ratio in male mice [F(1,23)=12.7, p<0.005 for the treatment; post-hoc test p<0.05 between the vehicle and BPP+NTX groups in males] (Figure 4B). In contrast, BPP+NTX had no effect on female preference [p=0.37], and female mice had higher alcohol preference than male mice after the BPP+NTX [F(1,23)=6.89, p<0.05 for sex; post-hoc test p<0.05 between the males and females after BPP+NTX]. As represented in Figure 4C, there was no significant effect on water intake of BPP+NTX, sex or their interaction after 4 hours of alcohol access.
Figure 4.
Effect of single administration of BPP+NTX (10+1 mg/kg) on 15% alcohol intake, water intake, and preference ratio in male and female B6 mice (n=6–8) after 3-week intermittent access (IA) alcohol drinking. On the test day, alcohol and water intake values were recorded after 4 hours of alcohol access. *p<0.05 or **p<0.01 vs. the vehicle-treated mice in the same sex; +p<0.05 or ++p<0.01 vs. male mice with the same treatment. Data shown are mean + SEM.
In males, single administration of BPP+NTX (10+1 mg/kg) did not have any significant effects after either 8 or 24 hours or over the entire 24 hour-period (see Table S5).
3.6. Effects of two and four repeated treatments with BPP+NTX (10+1 mg/kg) on alcohol IA in B6 mice.
For alcohol intake at 4 hours, two repeated administrations of BPP+NTX (10+1 mg/kg) reduced alcohol intake in males [F(1,24)=9.7, p<0.005; post-hoc test p<0.05], but not in females [p=0.41] (Table 3A). There was also a significant effect of sex [F(1,24)=6.9, p<0.05], and females drank more alcohol than males after BPP+NTX [post-hoc test p<0.05]. For alcohol preference, there was a significant effect of the treatment [F(1,24)=6.3, p<0.05], without a significant reduction in males [post-hoc test p=0.07] or females [p=0.49].
Table 3.
Effect of two (A) and four (B) repeat administrations of BPP+NTX (10+1 mg/kg) on 15% alcohol intake, water and preference ratio in male and female B6 mice after 3-week intermittent access (IA) alcohol drinking. On the test day, alcohol and water intake values were recorded after 4 hours of alcohol access. *p<0.05 vs. the vehicle-treated mice in the same sex; +p<0.05 vs. male mice with the same treatment. Data shown are mean ± SEM.
| A. Two repeats | Male (n=7) | Female (n=7) | ||
|---|---|---|---|---|
| Treatment | Vehicle | BPP+NTX | Vehicle | BPP+NTX |
| Alcohol intake (g/kg/4h) | 5.1 ± 0.33 | 3.4 ± 0.46 * | 5.6 ± 0.23 | 5.0 ± 0.48 + |
| Preference ratio | 0.80 ± 0.05 | 0.64 ± 0.05 | 0.85 ± 0.03 | 0.70 ± 0.10 |
| Water intake (ml/4h) | 0.11 ± 0.06 | 0.31 ± 0.09 | 0.26 ± 0.06 | 0.30 ± 0.09 |
| B. Four repeats | Male (n=7) | Female (n=7) | ||
| Treatment | Vehicle | BPP+NTX | Vehicle | BPP+NTX |
| Alcohol intake (g/kg/4h) | 4.8 ± 0.47 | 3.5 ± 0.40 * | 5.4 ± 0.34 | 4.9 ± 0.17 + |
| Preference ratio | 0.85 ± 0.03 | 0.70 ± 0.11 | 0.81 ± 0.04 | 0.70 ± 0.10 |
| Water intake (ml/4h) | 0.14 ± 0.09 | 0.39 ± 0.16 | 0.28 ± 0.10 | 0.49 ± 0.17 |
Similarly, after four repeated administrations of BPP+NTX (Table 3B), there was a decrease in alcohol intake in males [F(1,24)=9.0, p<0.01; post-hoc test p<0.05], but not in females [p=0.29]. This sex difference was still significant [F(1,24)=10.5, p<0.005], and females had more alcohol intake than males after 4 repeated BPP+NTX [post-hoc test p<0.05].
3.7. Effect of single BPP+NTX (10+1 mg/kg) treatment on locomotor activity in B6 mice without alcohol exposure.
BPP+NTX had no effect on locomotor activity in either sex (Table S6).
4. DISCUSSION
To explore the effectiveness of BPP+NTX (Contrave®) as a possible treatment for alcohol-related disorders, the current experiments employed male and female mice to investigate the effects of the BPP, NTX and their combinations on limited alcohol drinking in the DID model and excessive alcohol drinking in the IA model. In male mice only, single administration of BPP+NTX (10+1 mg/kg) significantly reduced alcohol intake when available for 4 hours after 5-day DID (Figure 2A), and decreased excessive alcohol intake after 4 hours of alcohol access after 3-week IA (Figure 4A). Similar to the effect of single treatment with BPP+NTX (10+1 mg/kg) on reducing excessive drinking after IA, the repeated treatments with the combination efficiently decreased IA drinking in male mice, without tolerance development yet after this short multiple-dosing paradigm (Table 3).
When compared the effects of BPP alone (10 mg/kg) with those of the BPP+NTX (10+1 mg/kg), we found that BPP alone at the same dose (even a higher dose at 20 mg/kg) did not alter alcohol drinking in male mice (Table S2), which is consistent with the observation that BPP alone was minimally effective in reducing alcohol intake in alcohol-preferring male rats [Nicholson et al., 2018]. Similarly, NTX at 1 mg/kg low dose showed minimal effect on alcohol intake in either male or female mice as presented in the present (Table S3A) and previous studies [Rhodes et al., 2005; Zhou et al., 2017a, 2018]. The highest dose of BPP+NTX (20+2 mg/kg) reached the significant effects in both male and female mice that were comparable to those induced by 2 mg/kg NTX alone (Tables S3B and S4), suggesting the effect of 20+2 mg/kg combination was mainly resulting from NTX at 2 mg/kg. Of interest, both the studies in alcohol-preferring male rats [Nicholson et al., 2018] and B6 male mice [the current one] in different drinking models suggest that the combination of BPP with NTX was more efficacious in reducing alcohol drinking (when effective) than either drug alone, suggesting a potential synergistic effect by the BPP+NTX combination (Figure 1). Finally, the marked reductions of alcohol drinking in male mice could not be attributed to alterations of locomotor activity as the BPP+NTX (10+1 mg/kg) did not alter locomotion in male mice (Table S6).
On all behavioral measures explored in this study with both male and female mice side by side, it was notable and unexpected that there was no clear evidence of BPP+NTX effect in female mice on alcohol intake in either the DID or IA model. BPP+NTX (10+1 mg/kg) did not show any effect on sucrose intake in female mice either. Our dose-response experiment also tested the other BPP+NTX combinations: 5+0.5, 20+1 or 20+2 mg/kg in female mice. Unexpectedly, we failed again to observe any effect of the BPP+NTX combinations at 5+0.5 mg/kg or 20+1 mg/kg in females (Figure 1 and Table 2). Though the highest dose of BPP+NTX (20+2 mg/kg) reached the significant effects in females, it was likely contributed by NTX at 2 mg/kg alone. Consistent with previous studies in mice (including our own reports [e.g., Hwa et al., 2011; Zhou et al., 2017b, 2018]), the present study confirmed sex differences in alcohol drinking, with relatively higher alcohol intake in female mice [recent review by Becker and Koob 2016]. As suggested by a recent publication [Satta et al., 2018], female rodents may consume more alcohol than males as they metabolize alcohol at a faster rate. No significant pharmacokinetic interactions have been found in men or rodents receiving combinations of alcohol with BPP [Posner et al., 1984; Tartara et al., 1985]. However, the current study did not measure the BAC levels in either male or female B6 mice and could not provide any information on whether the BPP+NTX treatments alter alcohol metabolic rates in a sex-dependent manner or not, which may contribute to the sex differences in the sensitivity to the BPP+NTX combinations. It has been previously reported that in the rodent hypothalamus, there was no sex difference in the expression levels of several feeding peptides and their receptors, including POMC and melanocortin 3 receptors [Lensing et al., 2016]. However, MC4R and agouti-related peptide have been reported to be expressed in the hypothalamus and other brain areas in a sex-dependent manner in mice [Qu et al., 2014; Lensing et al., 2016]. With very limited information on comparison of sex differences, at this time we cannot provide a clear explanation on the sex differences in response to BPP+NTX on alcohol and sucrose intake. Our observed behavioral action of BPP+NTX supports the potential use of this combination to reduce alcohol consumption, with clear consideration of sex differences and a relatively short duration (about 4 hours) of its effect.
In rats and mice, many groups have observed a reduction of food consumption and a decrease of operant self-administration of palatable foods by BPP+NTX treatments [Greenway et al., 2010; Wright and Rogers, 2013; Billes et al., 2014; Levy et al., 2018]. In the present experiment, therefore, it was not surprising to find that BPP+NTX (10+1 mg/kg) significantly reduced sucrose (caloric reinforcer) intake in male mice in the DID paradigm (Figure 2B). Though BPP has reward enhancing effects [Dwoskin et al., 2006], the reduction of alcohol or sucrose intake after single administration of BPP+NTX could not be attributed to the replacement of the rewarding effects of alcohol or sucrose with those of BPP, as BPP+NTX did not change saccharin intake (palatable non-caloric reinforcer) (Figure 2C). Alternatively, BPP+NTX may enhance satiety [Greenway et al., 2009] and the regulatory mechanisms of satiety, presumably, would be at play during chronic excessive IA drinking in males and even when alcohol or sucrose availability is limited, such as in the DID model. Though the precise mechanisms are not known, it is very possible that BPP+NTX had a selective effect on caloric reinforcers (alcohol and sucrose), but not non-caloric reinforcers such as saccharin, in our mouse models with sex differences.
It is well known that POMC activity and expression in the hypothalamus are inhibited by POMC-derived peptide beta-endorphin via MOP-r and involved in alcohol drinking behaviors [Zhou and Kreek, 2015; Zhou et al., 2017b; Levy et al., 2018]. Of interest, exposure to BPP+NTX (10+1 mg/kg) was associated with increased hypothalamic POMC neuronal activity [Greenway et al,. 2009] and enhanced POMC gene expression in the male rat hypothalamus [Levy et al., 2018]. A functional magnetic resonance imaging study further demonstrated that BPP+NTX blunted hypothalamic reactivity to food cues in obese individuals [Wang et al., 2014]. Therefore, the potential interaction between BPP+NTX and hypothalamic POMC neurons was also explored in the present study. Specifically, we investigated whether BPP+NTX (10+1 mg/kg) could affect alcohol drinking in the nPE knockout males with hypothalamic-specific deletion of POMC gene expression. It was found that nPE−/− males had a lack of reduction in alcohol intake after single BPP+NTX, indicating that a blunted effect of the BPP+NTX was due to hypothalamic POMC depletion (Figure 3B). Consistent with our previous report [Zhou et al., 2017b], the present study replicated our observation that nPE−/− male mice had reduced alcohol intake, probably due to central beta-endorphin deficiency after hypothalamic POMC deletion. The lack of significant effect of BPP+NTX (10+1 mg/kg) in nPE−/− males is not likely to a floor effect due to their lowered basal alcohol intake because acute administration of V1b antagonist still further reduced alcohol intake in nPE−/− males [Zhou et al., 2018]. As the nPE−/− mice had significantly lower BAC levels than the nPE+/+ mice (Table S1A), which appeared related to their differences in alcohol intake between the genotypes (Table S1B), it is possible that the BPP+NTX may only work to encounter alcohol’s effects when mice had sufficiently high BAC levels. Besides beta-endorphin, melanocortins (also derived from POMC) are well-known hypothalamic feeding peptides modulating food and alcohol intake [Cowley et al., 2001; Tolle and Low, 2008; Olney et al., 2014]. As beta-endorphin/MOP-r is blocked by NTX in the BPP+NTX combination, we further tested whether MC4R and melanocortins could play a role in the DID model. Although pretreatment with selective MC4R antagonist HS014 alone had no effect on alcohol drinking per se, it blunted the effect of BPP+NTX (10+1 mg/kg) on reducing DID intake in males, suggesting a MC4R-mediated mechanism (Figure 3A). Taken together, the results of the genetic and pharmacological analyses using nPE knockout mice and MC4R antagonists provide new information about the psychopharmacology of BPP+NTX.
Many groups have demonstrated that levels of alcohol intake in female mice or rats do not vary with different phases of the estrous cycle, suggesting that hormonal fluctuations during the estrous cycle may not play a critical role on alcohol intake in either the DID or IA model [Hwa et al., 2011; Priddy et al., 2017; Satta et al., 2018]. However, the main circulating form of estrogen 17beta-estradiol has been found to regulate the amount of alcohol consumption in female rodents, as both increases and decreases of alcohol intake have been reported in female rodents with estrogen administration [reviewed in Satta et al. 2018]. As a decreased alcohol intake in mice with lack of beta-endorphin, MOP-r or hypothalamic POMC gene was modestly greater in female mice [Hall et al., 2001; Racz et al., 2008; Zhou et al., 2017b], one potential pathway is the interaction between POMC/MOP-r and estrogens. Indeed, it has been found that estrogens altered the levels of POMC expression and its coding peptides (melanocortins and endorphin) in the hypothalamus of mice [Desjardins et al., 1993; Gao et al., 2007; de Souza et al., 2011] and altered alcohol-induced rewards [Pastor et al., 2011]. Estrogens may also modulate the effects of alcohol on MOP-r binding in specific brain regions [Carter and Soliman, 1998], which could be involved in alcohol drinking in a sex-sensitive manner [Hall et al., 2001]. Therefore, it is very possible that in male mice, the BPP+NTX treatment promoted hypothalamic POMC neuronal activity, POMC expression or MC4R activity, which may be blunted by estrogens in female mice. The possible mechanism behind the observed BPP+NTX effect in a sex-dependent manner is currently under investigation in our laboratory. Additionally, there may be many physiological differences between sexes, such as endocrine systems that could underlie the sex dimorphism in the alcohol-related behaviors [Becker and Koob 2016; Nieto et al., 2018].
In rodents, BPP is self-administered, increases dopamine transmission, and has reward-enhancing effects, suggesting that BPP has addictive potential [Dwoskin et al., 2006; Barrett et al., 2017]. Limited research in humans has suggested that BPP at high doses has a low potential for abuse [Reeves and Ladner 2013]. Our recent research has used a rodent model of excessive alcohol consumption to test potential compounds and to identify those that can decrease alcohol drinking. One interesting approach is to select compounds that have the potential to decrease alcohol drinking when used at relatively lower doses that have fewer side effects [Zhou et al., 2017a, 2018]. Indeed, our current pharmacological study found that combining low doses of BPP with NTX showed the potential to reduce alcohol intake, with avoiding the abuse potential. The present results also provided clear data that many neurotransmitter systems, including dopamine, norepinephrine, beta-endorphin/MOP-r, and melanocortin/MC4R, are involved in the regulation of alcohol consumption. Therefore, combination medications modulating multiple neuronal systems may have enhanced efficacy over monotherapy strategies.
Drug abuse is affected by gender [Chartoff and McHugh 2016], and there is a need to consider gender differences in alcohol research because men and women respond differently to potential treatments [Lovallo et al., 2012; Clayton and Collins 2014; O’Malley et al., 2018]. For an example, naltrexone treatment has been found to reduce alcohol consumption with or without gender differences [Pettinati et al., 2008; Krishnan-Sarin et al., 2007; Garbutt et al., 2005; Hernandez-Avila, 2006; Greenfield et al., 2010]. This is especially important for the current and coming experiments because our preclinical research is testing the two pharmacologically active components of the FDA approved drug Contrave® for its potential use in alcohol treatment. Our results support the potential use of the combinations of BPP+NTX to reduce alcohol consumption in a phase II clinical trial experiment [Navarro et al., 2019], with clear consideration of gender differences. A similar reduction observed with sucrose intake may indicate an off-target effect of the combinations.
Supplementary Material
Highlights:
A combination of bupropion with naltrexone;
Synergistic blockade of alcohol drinking by the combination;
Sex differences in the effect of the combination;
Hypothalamic POMC- and MC4R- dependent mechanisms
Acknowledgement
This work was supported by NIH grants AA021970 (YZ), Robertson Therapeutic Discover Fund at the Rockefeller University (YZ), Natural Sciences and Engineering Research Council of Canada (FL), DK066604 (MJL), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors declare that they have no conflicts of interest.
REFERENCES
- Barrett ST, Geary TN, Steiner AN, Bevins RA (2017) Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology 234: 187–198. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacol Rev 68: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billes SK, Sinnayah P, Cowley MA (2014) Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res 84: 1–11. [DOI] [PubMed] [Google Scholar]
- Bumaschny VF, Yamashita M, Casas-Cordero R, Otero-Corchón V, de Souza FS, Rubinstein M, Low MJ (2012) Obesity-programmed mice are rescued by early genetic intervention. J Clin Invest 122: 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A, Soliman MR (1998) Estradiol and progesterone alter ethanol-induced effects on mu-opioid receptors in specific brain regions of ovariectomized rats. Life Sci 62: 93–101. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, McHugh RK (2016) Translational studies of sex differences in sensitivity to opioid addiction. Neuropsychopharmacology 41: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P, Herxheimer A (2011) Unexpected aggressive behaviour: interaction of bupropion and alcohol. Int J Risk Saf Med. 23: 133–137. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484. [DOI] [PubMed] [Google Scholar]
- Desjardins GC, Brawer JR, Beaudet A (1993) Estradiol is selectively neurotoxic to hypothalamic beta-endorphin neurons. Endocrinology 132: 86–93. [DOI] [PubMed] [Google Scholar]
- de Souza FS, Nasif S, López-Leal R, Levi DH, Low MJ, Rubinsten M (2011) The estrogen receptor α colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol 660: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT (2006) Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12: 178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Ho AM, Winham SJ, Karpyak VM (2019) Sex hormones in alcohol consumption: a systematic review of evidence. Addict Biol. 24:157–169. doi: 10.1111/adb.12589. Epub 2017 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD (2013) Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res 37: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Fischer SM, Dilley JE, Nicholson ER, Smith TN, Filosa NJ, Rademacher LC (2016) Combining varenicline (Chantix) with naltrexone decreases alcohol drinking more effectively than does either drug alone in a rodent model of alcoholism. Alcohol Clin Exp Res 40: 1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Robert L, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL (2007) Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13: 89–94. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Group VS (2005) Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 293: 1617–1625. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, O’Malley S, Randall PK, Randall CL (2010) Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res 34: 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O’Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA (2009) Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 17: 30–49. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E (2010) Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376: 595–605. [DOI] [PubMed] [Google Scholar]
- Greig SL, Keating GM (2015) Naltrexone ER/Bupropion ER: A Review in Obesity Management. Drugs 75: 1269–1280. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR (2001) Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology 154: 43–49. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR (2006) Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin Exp Res 30: 860–865. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF (2003) Effects of naltrexone alone and in combination with acamprosate on alcohol deprivation effect in rats. Neuropsychopharmacology 28: 1463–1471. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% alcohol. Alcohol Clin Exp Res 35: 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, YorkWilliams SL, Hutchison KE (2015) Clinical neuroscience of addiction: similarities and differences between alcohol and other drugs. Alcohol Clin Exp Res 39: 2073–2084. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS (2007) Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry 62: 694–697. [DOI] [PubMed] [Google Scholar]
- Lam DD, de Souza FS, Nasif S, Yamashita M, López-Leal R, Otero-Corchon V, Meece K, Sampath H, Mercer AJ, Wardlaw SL, Rubinstein M, Low MJ (2015) Partially redundant enhancers cooperatively maintain Mammalian Pomc expression above a critical functional threshold. PLoS Genet 11: e1004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensing CJ, Adank DN, Doering SR, Wilber SL, Andreasen A, Schaub JW, Xiang Z, Haskell-Luevano C (2016) Ac-Trp-DPhe(p-I)-Arg-Trp-NH2, a 250-Fold Selective Melanocortin-4 Receptor (MC4R) Antagonist over the Melanocortin-3 Receptor (MC3R), Affects Energy Homeostasis in Male and Female Mice Differently. ACS Chem Neurosci 7: 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Daniels S, Hudson R, Horman T, Flynn A, Zhou Y, Leri F (2018) Bupropion and naltrexone combination alters high fructose corn syrup self-administration and gene expression in rats. Neuropharmacology 135: 547–554. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS (2012) Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology 37: 1922–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Luhn KL, Kampov-Polevoy AB, Garbutt JC, Thiele TE (2019) Bupropion, Alone and in Combination with Naltrexone, Blunts Binge-Like Ethanol Drinking and Intake Following Chronic Intermittent Access to Ethanol in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2019 February 28. doi: 10.1111/acer.13992. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson ER, Dilley JE, Froehlich JC (2018) Co-administration of low-dose naltrexone and bupropion reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res 42: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto SJ, Quave CB, Kosten TA (2018) Naltrexone alters alcohol self-administration behaviors and hypothalamic-pituitary-adrenal axis activity in a sex-dependent manner in rats. Pharmacol Biochem Behav 167: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2014) Targeting central melanocortin receptors: a promising novel approach for treating alcohol abuse disorders. Front Neurosci 8: 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe A, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ (1992) Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry 49: 881–887. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis. Psychopharmacology 160: 19–29. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P, Gueorguieva R (2018) Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry 75:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Font L, Miquel M, Phillips TJ, Aragon CM (2011) Involvement of the beta-endorphin neurons of the hypothalamic arcuate nucleus in ethanol-induced place preference conditioning in mice. Alcohol Clin Exp Res 35: 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O’Brien CP (2008) Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat 34: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Bye A, Jeal S, Peck AW, Whiteman P (1984) Alcohol and bupropion pharmacokinetics in healthy male volunteers. Eur J Clin Pharmacol 26: 627–630. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HE, Li JP, Chen W, Li YM, Jiang Q, Jiang HZ, Huo JL, Zhao ZH, Liu B, Zhang QL (2014) Differential expression of the melanocortin-4 receptor in male and female C57BL/6J mice. Mol Biol Rep 41: 3245–3256. [DOI] [PubMed] [Google Scholar]
- Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Osby U, Gunnar A, Maier W, Bilkei-Gorzo A, Nothen M, Zimmer A (2008) The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry 64: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RR, Ladner ME (2013) Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol 33: 584–585. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84: 53–63. [DOI] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, Lasek AW (2018) Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcohol Clin Exp Res 42: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M, Ugureanu S, Rey J (2016) Naltrexone/Bupropion ER (Contrave) Newly approved treatment option for chronic weight management in obese adults. PT 41: 164–172. [PMC free article] [PubMed] [Google Scholar]
- Tartara A, Formigli L, Crema F, Maurelli M, Perucca E, Marchioni E, Manzo L, Savoldi F (1985) Alcohol interactions with typical and atypical antidepressants. Neurobehav Toxicol Teratol 7: 139–141. [PubMed] [Google Scholar]
- Toll BA, Leary V, Wu R, Salovey P, Meandzija B, O’Malley SS (2008) A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addict Behav 33: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle V, Low MJ (2008) In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes 57: 86–94. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Volkow ND, Wang R, Telang F, Caparelli EC, Dunayevich E (2014) Effect of combined naltrexone and bupropion therapy on the brain’s reactivity to food cues. Int J Obes (Lond) 38: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright FL, Rodgers RJ (2013) Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash. Psychopharmacology 228: 291–307. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ (2015) Persistent increase in rat hypothalamic POMC gene expression following chronic withdrawal from chronic “binge” pattern escalating-dose, but not steady-dose, cocaine. Neuroscience 289: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Crowley RS, Ben K, Prisinzano TE, Kreek MJ (2017a) Synergistic blockade of alcohol escalation drinking in mice by a combination of novel kappa opioid receptor agonist Mesyl Salvinorin B and naltrexone. Brain Res 1662: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rubinstein M, Low MJ, Kreek MJ (2017b) Hypothalamic-specific proopiomelanocortin deficiency reduces alcohol drinking in male and female mice. Genes Brain Behav 16: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rubinstein M, Low M, Kreek MJ (2018) V1b receptor antagonist SSR149415 and naltrexone synergistically decrease excessive alcohol drinking in male and female mice. Alcohol Clin Exp Res 42: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.