Abstract
The TRP ion channel TRPM2 has an essential function in cell survival and protects the viability of a number of cell types after oxidative stress. It is highly expressed in many cancers including breast, prostate, and pancreatic cancer, melanoma, leukemia, and neuroblastoma, suggesting it promotes cancer cell survival. TRPM2 is activated by production of ADP-ribose (ADPR) following oxidative stress, which binds to the C-terminus of TRPM2, resulting in channel opening. In a number of cancers including neuroblastoma, TRPM2 has been shown to preserve viability and mechanisms have been identified. Activation of TRPM2 results in expression of transcription factors and kinases important in cell proliferation and survival including HIF-1/2α, CREB, nuclear factor (erythroid-derived 2)-related factor-2 (Nrf2), and Pyk2, and Src phosphorylation. Together, HIF-1/2α and CREB regulate expression of genes encoding proteins with roles in mitochondrial function including members of the electron transport complex involved in ATP production. These contribute to lower mitochondrial ROS production while expression of antioxidants regulated by HIF-1/2α, FOXO3a, CREB, and Nrf2 is maintained. CREB is also important in control of expression of key proteins involved in autophagy. When TRPM2-mediated calcium influx is inhibited, mitochondria are dysfunctional, cellular bioenergetics are reduced, production of ROS is increased, and autophagy and DNA repair are impaired, decreasing tumor growth and increasing chemotherapy sensitivity. Inhibition of TRPM2 expression or function results in decreased tumor proliferation and/or viability in many malignancies including breast, gastric, pancreatic, prostate, head and neck cancers, melanoma, neuroblastoma, and T-cell and acute myelogenous leukemia. However, in a small number of malignancies, activation of TRPM2 rather than inhibition has been reported to reduce tumor cell survival. Here, TRPM2-mediated Ca2+ signaling and mechanisms of regulation of cancer cell growth and survival are reviewed and controversies discussed. Evidence suggests that targeting TRPM2 may be a novel therapeutic approach in many cancers.
Keywords: TRPM2, HIF-1α, CREB, ROS, mitochondria, cancer
1. Introduction
Transient receptor potential (TRP) channels are a superfamily of ion channels involved in a large number of physiological functions [1, 2]. The TRPM (Melastatin) subfamily has a number of members which are involved in modulation of cell proliferation and survival [3–5]. One of these is TRPM2, the second member of the TRPM ion channel subfamily to be identified. Human TRPM2 is a 1503-amino acid channel permeable to Ca2+, Na2+, and K+ [6, 7]. It is widely expressed in many cell types including brain, hematopoietic cells, and heart [8]. Since its discovery, TRPM2 has been shown to play an important role in response to oxidative stress. The mechanisms through which TRPM2 modulates oxidative stress and regulates cell survival and the important role of TRPM2-mediated Ca2+ signaling in cancer will be reviewed.
1.1. Molecular Structure of TRPM2
The TRPM2 channel is a non-selective cation channel, and like other TRP channels, functions as a tetramer. For TRPM2, no physiological heterotetramers have been identified. The TRPM2 channel monomer contains an approximate 800 amino acid N-terminal region, six transmembrane domains (S1–6) leading to three extracellular loops, and a C-terminal coiled-coil loop and a NUDT9 ADP-ribose hydrolase domain which binds ADP-ribose (ADPR). The N-terminus has four TRPM subfamily homologous domains and a calmodulin-binding IQ-like motif [9, 10]. Both the N- and C-termini are intracellular, and the pore-forming loop is located between S5 and S6 [11]. Subunit composition is an important factor in regulation of channel function. A number of physiological TRPM2 splice variants have been identified including TRPM2-L (full-length or wild type), TRPM2-S (short) [12], TRPM2-ΔN [13], TRPM2-ΔC [13], and TRPM2-TE (tumor-enriched) [14]. TRPM2-S, a short isoform of 845 residues, is missing four of six transmembrane domains and the entire C-terminus and the Ca2+ pore. It functions as a dominant negative isoform, and can inhibit the function of the full length channel [12, 15, 16]. TRPM2-TE was identified in a search for antisense transcripts in melanoma to identify new tumor suppressor genes [14]. It is a 184 or 218 amino acid protein from the C-terminus of TRPM2 which is highly expressed in some tumor cells including melanoma and lung cancer compared to normal tissue and may protect cells from apoptosis. TRPM2-S and other splice variants are thought to affect channel function by participating in heterodimer formation and altering the tertiary structure of the TRPM2 tetramer required for ion permeability. Neither the mechanisms controlling splice variant or physiological TRPM2-L expression nor the physiological function and importance of TRPM2 isoform expression have been determined [12–14].
To better understand the gating mechanisms of TRPM2, its 3-dimensional structure has been elucidated. Using electron/cryo-electron microscopy, conformational changes in human TRPM2 upon ADPR and Ca2+ binding were reported which potentiate channel opening and explain the gating of TRPM2 by ADPR and Ca2+ [17, 18]. These findings were consistent with those reported for Nematostella vectensis TRPM2 [11] and zebrafish TRPM2 [19] analyzed with cryo-electron microscopy.
1.2. Activation of TRPM2
The extracellular signals which activate TRPM2 include oxidative stress, tumor necrosis factor α (TNFα), amyloid β-peptide, and concanavalin A [7, 13, 20, 21]. These signals stimulate production of ADPR in mitochondria [22] or through activation of poly (ADPR) polymerase (PARP) or poly (ADPR) glycohydrolase (PARG) [23, 24]. ADPR binds to the TRPM2 C-terminal NUDT9-H domain, activating the channel [8, 22, 25]. Although cyclic adenosine diphosphate ribose (cADPR) and pyridine dinucleotides have been reported to activate TRPM2 or to enhance activation by ADPR [25], when commercial preparations of these were purified with nucleotide pyrophosphatase or affinity-purified-specific ADPR hydrolase to eliminate contaminating ADPR, none of these stimulated TRPM2 binding, demonstrating that ADPR is activator of TRPM2 [26, 27]. An increase in intracellular Ca2+ also positively regulates TRPM2 [10, 28, 29]. Either initial calcium entry through ADPR-bound TRPM2 or an initiator Ca2+ spark from the cytosol activates the channel [11]. Ca2+-bound calmodulin then binds to IQ-motifs in the TRPM2 N-terminus, providing positive feedback for TRPM2 activation and increasing Ca2+ influx [10, 28, 29]. ADPR is ineffective in activating TRPM2 channels without either external or internal Ca2+ [11, 28]. The concentration of membrane phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to impact sensitivity of TRPM2 for activation by Ca2+ [30]. TRPM2 has also been reported to be temperature sensitive [31] and channel activity is inhibited by acidification [32–34].
1.3. Role of TRPM2 in Oxidative Stress
Oxidative stress results from an imbalance between the amount of reactive oxygen species (ROS) produced and antioxidant levels, depending on severity and duration. ROS are produced physiologically during respiration by the mitochondrial electron transport chain and pathologically by neutrophils and phagocytes in inflammation and infection. Low levels of ROS can modulate cellular survival and metabolic pathways to enhance cell proliferation, but as ROS levels rise, they damage tissues through protein oxidation, lipid peroxidation, and DNA oxidation and mutagenesis, activating cell death pathways [35, 36]. In most tissues, ischemic injury results in an increase in ROS. For example, in heart, following ischemic-reperfusion injury or doxorubicin exposure, ROS levels increase and myocytes are damaged [37]. Cancer cells produce more ROS than normal cells, and a number of chemotherapy agents including doxorubicin contribute to cell death by further increasing ROS [38, 39].
TRPM2 has been implicated in a number of physiological and pathological pathways involving oxidative stress [40, 41]. Early research supported the classical paradigm, that after TRPM2 is activated by oxidative stress resulting in ADPR production, a sustained increase in intracellular calcium may occur leading to cell death [7, 42], which may be enhanced by cytokine production aggravating inflammation and tissue injury [43, 44]. However, a number of more recent reports suggest a different paradigm, that Ca2+ entry via TRPM2 channels can be protective rather than deleterious. For wild type mice subjected to intraperitoneal injection of endotoxin, survival was five times better than for TRPM2 KO mice [45]. Cation entry via TRPM2 channels resulted in plasma membrane depolarization and decreased NOX-mediated ROS production in wild type phagocytes, preventing endotoxin-induced lung inflammation. TRPM2 also protected the hearts of wild type mice from cardiac dysfunction after ischemia/reperfusion [41, 46]. Cardiac myocytes from TRPM2 KO mice had significantly higher ROS, and TRPM2 was required for bioenergetics maintenance and mitochondrial oxidant homeostasis through a Ca2+ dependent process [37, 47]. In humans, a TRPM2 mutant (P1018L) was found in a subset of Guamanian amyotrophic lateral sclerosis and Parkinson dementia patients. Unlike wild type TRPM2 which does not inactivate, the P1018L mutant inactivates after channel opening by ADPR, limiting calcium entry and strongly suggesting that TRPM2 is required for normal neuronal function [48]. Furthermore, in pyramidal neutrons subjected to oxidant injury, TRPM2 protected against cellular damage from oxidative stress [49]. Together, data suggest that TRPM2 channels in disease states can act either as friend (reducing ROS production) [45, 46, 49, 50] or foe (when Ca2+ influx significantly increases) [12, 44, 51, 52] depending on the experimental model and conditions. Similar to this, physiological levels of ROS can activate transcription factors and signaling kinases, but excessive increases in ROS result in damage to mitochondria, DNA, proteins, and lipids, leading to cell loss and organ failure. The interactions of Ca2+ and ROS associated with cell injury are not completely understood. In some models, primarily nonmalignant, TRPM2 expression can enhance cell death through elevated intracellular Ca2+ or Zn2+ [52–56], but the predominance of data in cancer models support the conclusion that TRPM2 expression and function have an important role in preserving cancer cell viability and survival.
1.4. Role of TRPM2 in Autophagy
Autophagy has been shown to have important roles in cancer. It may have a protective role in early stages of cancer development, eliminating aggregated proteins or damaged organelles, reducing oxidative stress, local inflammation, and chromosomal instability, and functioning as a suppressor of tumor development. However, in later stages, autophagy can promote tumor growth and survival, contributing to chemotherapy resistance by providing nutrients and essential amino acids and nucleotides to cells, and by removing damaged DNA, mitochondria and ROS [57–60]. TRPM2 has been shown to regulate autophagy through several pathways. In TRPM2-inhibited neuroblastoma cells, a defect in autophagy/mitophagy was demonstrated which resulted in accumulation of ROS and damaged mitochondria and may contribute to reduced cell viability. When TRPM2-L was inhibited by TRPM2-S, accumulation of two proteins usually removed by mitophagy, Hsp60 and Tom20, a mitochondrial translocase receptor, was demonstrated [16]. Expression of HIF-1/2α was reduced in these cells, associated with reduced expression of the downstream mitochondrial target BNIP3, which has an important role in autophagy. TRPM2-S expressing cells showed abundant dysmorphic mitochondria, which were swollen and had degenerated cristae, compared to TRPM2 wild type expressing cells, which had mitochondria with normal cristae [16]. Cells in which TRPM2 was depleted with CRISPR also demonstrated reduced autophagy [61]. These results were confirmed by Almasi et al in gastric cancer cells [62], who also found that TRPM2 knockdown was associated with a decrease in autophagy and mitophagy and impaired mitochondrial metabolism and ATP production. Reduced levels of autophagy and mitophagy-associated proteins including the ATGs and BNIP3 were also found in TRPM2-depleted gastric cancer cell lines. In contrast, in HeLa cells, TRPM2-mediated Ca2+ influx induced by oxidative stress resulted in phosphorylation and activation of calcium/calmodulin dependent protein kinase II (CAMKII), which then phosphorylated BECN1/Beclin 1. BECN1 dissociated from PIK3C3 to bind Bcl-2 and inhibit autophagy, rendering cells more susceptible to death [63].
2. TRPM2 is Highly Expressed in Neuroblastoma and Modulates Mitochondrial Function and Cellular Bioenergetics.
TRPM2 has been found to be highly expressed in many cancers including bladder [64], breast [64], head and neck [64, 65], lung [64], pancreatic [66], and prostate cancer [67], melanoma [14], and neuroblastoma [16], suggesting it promotes cancer growth and enhances cell survival. Neuroblastoma is the most frequent non-CNS tumor of childhood. TRPM2 has been shown to be important in neuroblastoma proliferation and chemotherapy sensitivity. TRPM2 was first inhibited by expression of the dominant negative short splice variant TRPM2-S in neuroblastoma cell lines. TRPM2 inhibition resulted in significantly increased susceptibility to cell death induced by low concentrations of H2O2 (50–100 μM) [15] as well as doxorubicin [16]. In cells expressing full length (wild type) TRPM2 compared to TRPM2-S, expression of FOXO3a and MnSOD (SOD2) were significantly increased, and ROS levels were decreased, demonstrating that TRPM2 confers protection against cell death through reduction of oxidative stress, critically important in cancer cells because of frequently higher ROS levels. The ability of TRPM2 to enhance growth of neuroblastoma tumors was confirmed in mouse xenografts using human neuroblastoma cells expressing TRPM2-L or TRPM2-S [16]. In TRPM2-S expressing tumor cells, HIF-1/2α was significantly reduced, as was expression of proteins encoded by target genes regulated by HIF-1/2α including those involved in glycolysis (lactate dehydrogenase A and enolase 2), oxidant stress (FOXO3a), angiogenesis (VEGF), mitophagy and mitochondrial function (BNIP3, NDUFA4L2), and mitochondrial electron transport chain activity. The reduction in HIF-1/2α was associated with reduced HIF-1/2α mRNA and increased von Hippel-Lindau E3 ligase in TRPM2-S expressing cells. Associated with reduction in expression of mitochondrial proteins was a decrease in mitochondrial membrane potential, mitochondrial Ca2+ uptake, basal and maximal oxygen consumption rates, and ATP production [16]. As discussed in 1.4, reduced HIF-1/2α contributed to decreased autophagy/mitophagy through lower BNIP3, resulting in accumulation of dysfunctional mitochondria with reduced bioenergetic capacity including lower OCR and ATP generation, and increased ROS [16, 61]. Similar experiments were performed with a second model in which TRPM2 was depleted with CRISPR/Cas9 technology [61]. In neuroblastoma cells in which TRPM2 was depleted, tumor growth of xenografts was significantly reduced (Figure 1A) and doxorubicin sensitivity increased. Similar to TRPM2-S expressing cells, in TRPM2 depleted cells, HIF-1/2α and proteins in the downstream signaling cascade were reduced, mitochondrial function including oxygen consumption rate was impaired, mitochondrial superoxide production was significantly increased (Figure 1B), and ATP production reduced. Expression of wild type TRPM2 but not the TRPM2 pore mutant E960D in depleted cells restored cell viability, mitochondrial function and reduced ROS. This demonstrated the critical role of TRPM2-mediated calcium entry in modulation of tumor growth, mitochondrial function, cellular bioenergetics, and susceptibility to chemotherapeutic agents (Figure 2) [61]. The precise mechanisms through which TRPM2 modulates HIF-1/2α expression are not known. One mechanism may be activation of calcineurin by calcium influx, dephosphorylating RACK1, blocking RACK1 dimerization, and increasing HIF-1/2α levels by impeding its ubiquitination and degradation [68]. Expression of superoxide dismutase 2 (SOD2) reduced ROS levels in TRPM2-depleted cells but failed to restore ATP production, demonstrating that regulation of ROS is one of several key metabolic pathways regulated by TRPM2.
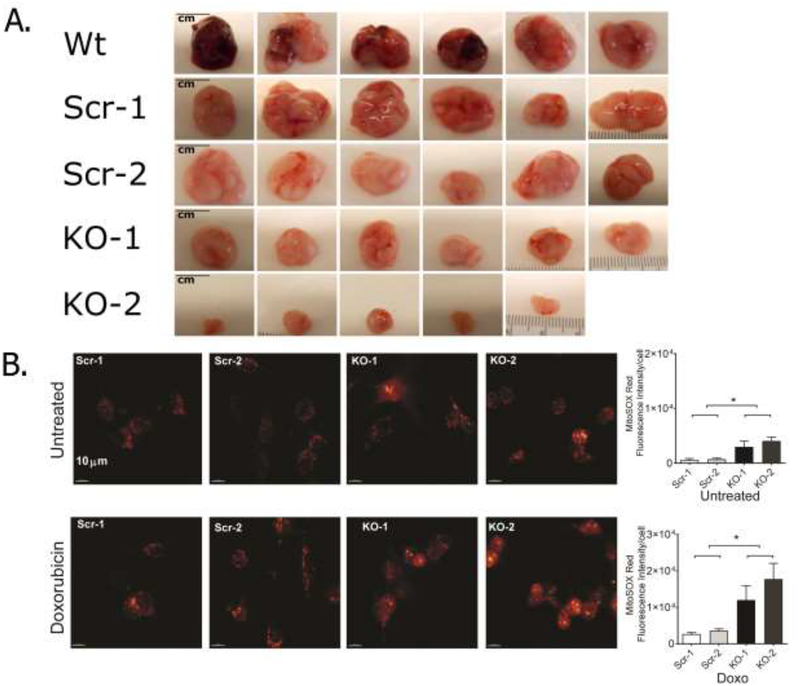
Figure 1. TRPM2 promotes growth of neuroblastoma xenografts and reduces ROS.
A. Athymic mice were injected in the flank with parental SH-SY5Y cells (Wild type, Wt), cells in which TRPM2 was deleted with CRISPR (two clones, KO-1, KO-2), or scrambled control cells (Scr-1, Scr-2). Representative photographs of tumors removed at 6 weeks after cell injection are shown. In two experiments (n=13–14), p≤0.01 for differences in Scr vs KO tumor volumes and weights. B. ROS levels were measured in SH-SY5Y neuroblastoma cells in which TRPM2 was depleted with CRISPR (two clones, KO-1, KO-2), or scrambled control cells (Scr-1, Scr-2). Cells were loaded with MitoSOX Red and intensity of fluorescence measured with confocal microscopy at baseline or 24 hours after treatment with 0.3 μM doxorubicin. A representative field of cells from each group and statistical analysis from a representative experiment are shown. Mitochondrial ROS were increased in TRPM2 depleted cells at baseline and after doxorubicin treatment. *p<0.05.
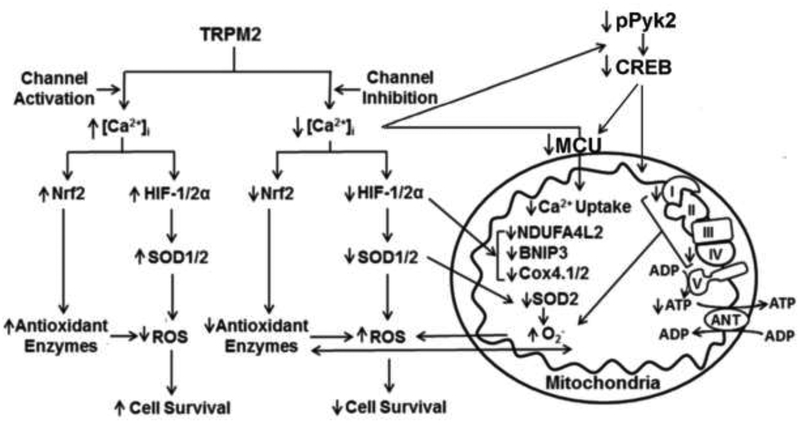
Figure 2. TRPM2 Maintains Cell Survival through Modulation of HIF-1/2α, CREB, Nrf2, and Pyk2.
TRPM2 mediates expression of HIF-1/2α, CREB, and Nrf2 in neuroblastoma cells. Pyk2 phosphorylation and activation by TRPM2 through a Ca2+-dependent process is involved in modulating CREB expression. These factors contribute to maintenance of mitochondrial function and ATP production, and lower ROS levels through reduced ROS production and preservation of the antioxidant response. When TRPM2 is inhibited or depleted, Ca2+ influx into the cell and into mitochondria through the mitochondrial calcium uniporter (MCU) is reduced, expression of key transcription factors HIF-1/2α, CREB, and Nrf2 is decreased, mitochondrial function, cellular bioenergetics, and autophagy/mitophagy are impaired, activation of kinases including Pyk2 and Src and cell viability are reduced, and ROS levels and sensitivity to chemotherapy are increased.
To further explore signaling pathways regulated by TRPM2 in cancer, the roles of Pyk2 and CREB were examined. Pyk2 is overexpressed in and important for survival of many cancers, through pathways including activation of the cAMP-responsive element binding protein CREB. CREB is a key transcription factor which regulates genes involved in oncogenesis and cell survival including in the antioxidant response, mitochondrial metabolism, and autophagy [69]. Previous reports that TRPM2 activates Pyk2 [44] and Src [70] were confirmed and extended to demonstrate that TRPM2 is required to maintain expression and phosphorylation of Pyk2 and CREB and Src phosphorylation (Figure 2) [69]. Src is an activator of Pyk2. Inhibition of TRPM2 reduced phosphorylation of Src and Pyk2, and expression of total Pyk2 and CREB in the mitochondria, and phosphorylated Src, CREB, and total CREB in the nucleus, impacting expression of a number of cellular and mitochondrial genes involved in cell survival. Reduction in both expression of the mitochondrial calcium uniporter (MCU), a CREB transcriptional target, and its function, regulated by Pyk2 phosphorylation, may be responsible for reduced mitochondrial calcium uptake and contribute to reduced mitochondrial function and bioenergetics [69]. Reconstitution with TRPM2 but not the TRPM2 pore mutant E960D restored expression and phosphorylation of Pyk2 and CREB, particularly after doxorubicin. In addition to regulation of MCU, CREB expression plays an important role in preserving cell survival through regulation of expression of genes required for maintenance of mitochondrial function, autophagy/mitophagy, ROS production, and the antioxidant response.
To protect themselves from cytotoxic levels of ROS, cancer cells frequently increase their anti-oxidant capacity. The transcription factor nuclear factor (erythroid-derived 2)-related factor-2 (Nrf2) modulates expression of more than 200 genes, many important in regulation of enzymes or cofactors involved in the anti-oxidant response [71]. Nrf2 is highly expressed in many malignant cells, where it functions to protect them from oxidative stress and cytotoxic chemotherapy, which can increase ROS above a cytotoxic threshold and cause irreversible cell damage. Nrf2 stability is regulated by Ketch-like ECH-associated protein 1 (Keap1), which targets Nrf2 for ubiquitination and proteosomal degradation [35, 72], and by the IQ motif containing GTPase activating protein 1 (IQGAP1), which plays a critical role in Nrf2 stability through a Ca2+ dependent process [73]. Nrf2 induces expression of expression of many antioxidant enzymes which are critical in generation of the antioxidant cofactors GSH, NADPH, and NADH [35, 74]. TRPM2 has recently been demonstrated to have a role in modulation of Nrf2 expression (B. Miller, unpublished data). Together, these studies in neuroblastoma show the important role TRPM2 plays in cell survival through modulation of both ROS production and the antioxidant response. When TRPM2 is inhibited or depleted, ROS are significantly increased by both mitochondrial dysfunction and reduced antioxidants, enhancing the likelihood that a cytotoxic threshold will be reached resulting in cell death, particularly following treatment with agents like doxorubicin. The role TRPM2 plays in regulation of transcription factors which support malignant growth including HIF-1/2α, CREB, and Nrf2 is under investigation. Many pathways modulated by TRPM2 in neuroblastoma including those involving oxidative stress, mitochondrial function, bioenergetics, and autophagy have been recently confirmed in acute myeloid leukemia cells (B. Miller, unpublished data).
3. TRPM2 Preserves Viability of Many Cancers
The increased expression of TRPM2 in many malignancies [14, 64], its roles in cancer growth and cell survival, and the potential of modulation, particularly inhibition, as a therapeutic modality is becoming well recognized. Different mechanisms influencing cell death and intracellular localization of TRPM2 in different types of cancer have been reported and unifying themes are emerging, which are reviewed in detail below and summarized in Table 1.
Table 1.
TRPM2 Function in Human Cancer
| Cancer Type | Study System | Activity | Mechanism | References |
|---|---|---|---|---|
| Breast Cancer | Breast adenocarcinoma cell lines | TRPM2 inhibition reduced cell proliferation TRPM2 inhibition increased cell death after doxorubicin and tamoxifen |
TRPM2 expression in the nucleus was increased in cancer cells TRPM2 inhibition increased DNA damage |
76,77 |
| Gastric Cancer | Gastric cancer cell lines | TRPM2 inhibition reduced cell proliferation TRPM2 down-regulation increased cell death after doxorubicin and paclitaxel |
TRPM2 inhibition decreased mitochondrial function, autophagy, bioenergetics, and JNK signaling TRPM2 inhibition increased ROS and apoptosis. |
62 |
| Lung Cancer | NSCLC patient samples Human lung cancer cell lines |
Novel long non-coding RNA (TRPM2-AS) widely expressed High expression of TRPM2-AS correlated with larger tumor size, advanced TNM stage, and poor patient survival |
Long non-coding RNA TRPM2-AS was associated with increased proliferation and reduced apoptosis Down regulation of TRPM2-AS reduced cell proliferation and increased apoptosis |
85 |
| Human alveolar epithelial adenocarcinoma cells | TRPM2 inhibition reduced cell survival after irradiation | TRPM2 inhibition suppressed the DNA damage response | 90 | |
| Neuroblastoma | Neuroblastoma patient samples Human neuroblastoma cell lines Xenografts |
TRPM2 inhibition reduced cell viability, tumor growth in xenografts TRPM2 inhibition increased chemotherapy sensitivity |
TRPM2 inhibition reduced mitochondrial function, autophagy, antioxidant response, bioenergetics, HIF-1/2a, CREB, MCU level and activity TRPM2 inhibition increased ROS levels |
8,12,15,16, 61,69 |
| Pancreatic Cancer | Patient samples Pancreatic cancer cell lines | High TRPM2 expression correlated with shorter patient survival | Higher TRPM2 expression promoted increased cell proliferation, migration, and invasion | 66,89 |
| Prostate Cancer | Pati ent Tumor Tissue Human prostate cell lines | TRPM2 inhibition reduced cancer cell proliferation | Significant TRPM2 localization in nucleus in cancer cells | 67 |
| Long non-coding antisense TRPM2 transcripts (TRPM2-AS) are overexpressed and linked to poor patient outcome | When TRPM2-AS was reduced, a large increase in TRPM2 expression was detected, associated with increased cell apoptosis | 86,87 | ||
| Squamous Cell Carcinoma | Human tongue carcinoma samples Tongue carcinoma cell lines |
TRPM2 inhibition reduced tumor viability | TRPM2 inhibition increased apoptosis, reduced tongue cancer cell survival, and inhibited cancer cell migration Significant TRPM2 localization to the nucleus was found in cancer cells |
64,65 |
| T-Cell Leukemia | Human leukemia cell lines | TRPM2 had a pro-survival effect and inhibition increased irradiation-induced cell death | TRPM2 inhibition decreased phosphorylation of CAMKII, reduced cells in G2/M cell cycle arrest, blocked inactivation of cdc2, and increased cell death | 84 |
3.1. TRPM2 Promotes Survival of Triple-Negative and Estrogen Receptor Positive Breast Cancer
Of the three major molecular subtypes of breast cancer, triple-negative is the most aggressive and has the worst outcome, but a significant percentage of patients with the most common type, estrogen-receptor-positive breast cancer, also fail therapy. 2-Aminoethoxydiphenyl borate (2-APB) is a nonspecific inhibitor of many plasma membrane and organellar ion channels including TRPM2 [75]. In human breast adenocarcinoma cell lines, TRPM2 showed a protective effect in minimizing DNA damage, whereas pharmacological inhibition of TRPM2 with 2-APB or TRPM2 mRNA silencing decreased cell proliferation and significantly increased DNA damage [76]. In both triple negative and estrogen-receptor positive breast cancer, TRPM2 inhibition resulted in increased DNA damage and cytotoxicity, similar to that seen in neuroblastoma [77]. TRPM2 was present in the nuclear fraction of breast adenocarcinoma but not exclusive to that location; 40–45% of TRPM2 was located in nuclear fractions and the rest in other subcellular fractions including the cytoplasm. Mechanisms of TRPM2 activity in the nucleus were hypothesized to include facilitation of DNA repair by nuclear TRPM2 or promotion of nuclear calcium influx, which need to be explored further. ROS levels were not examined, but the high levels of oxidative stress found in TRPM2-depleted neuroblastoma cells suggest that this could be a potential mechanism to explain the increased DNA damage found in breast cancer when TRPM2 is inhibited. In contrast, in noncancerous MCF-10A mammary epithelial cells, TRPM2 neither localized to the nucleus nor was TRPM2 inhibition observed to have an effect on proliferation. These data suggest that targeting of TRPM2 could be a synergistic approach to enhance treatment of breast cancer patients including those with chemotherapy resistance, similar to that proposed in neuroblastoma. Other TRPM channels have also been found to have roles in breast cancer proliferation, migration, and invasion including TRPM7 and TRPM8 [78–83]. How TRPM channels mediate their individual effects and whether or not their activities overlap or integrate with each other or TRPM2 are areas for future exploration.
3.2. TRPM2 Preserves Gastric Cancer Cell Survival through Regulation of JNK
Gastric cancer is the fifth most common cancer and the five year survival rate is approximately 30%. TRPM2 expression in tumors negatively correlated with overall survival of gastric cancer patients. When TRPM2 was downregulated with shRNA in two gastric cancer cell lines, AGS and MKN-45, cells grew slower and the percentage of apoptotic cells increased [62]. Mitochondrial function characterized by oxygen consumption rates and ATP production was significantly decreased in TRPM2 depleted cells and expression of COX 4.1 and 4.2 and BNIP3 were reduced, as was reported in neuroblastoma [16]. Autophagy was also decreased, with reduced levels of autophagy proteins including ATG3, ATG5, ATG6, ATG7, and ATG12 and decreased conversion of LC3-I to LC3-II. Impairment of autophagy contributed to accumulation of damaged mitochondria, reduced cellular bioenergetics, and increased ROS, leading to cell death. TRPM2 regulated autophagy through an mTOR-independent but JNK-signaling dependent pathway, mediated through modulation of ATGs, BNIP3, and JNK activation. Apoptotic effects of both paclitaxel and doxorubicin were increased in TRPM2 depleted cells, demonstrating that TRPM2 preserves cell survival whereas inhibition increases chemotherapy sensitivity, also suggesting this may be a therapeutic approach to enhance gastric tumor cell death.
3.3. Targeting TRPM2 Enhances Cell Death in T Cell Leukemia in a Bcl-2 Dependent Manner
In Jurkat cells stably expressing Bcl-2 or empty vector, inhibition of TRPM2 with N-(p-amylcinnamoyl)anthranilic acid (ACA) followed by irradiation decreased phosphorylation of CAMKII and blocked radiation-induced phosphorylation-dependent inactivation of cdc2 [84]. Both the nonspecific TRPM2 inhibitors ACA and chlotrimazole increased cell death. TRPM2 knockdown also significantly decreased the number of cells arrested in G2/M and reduced viability. This evidence suggests that irradiation stimulates Ca2+ entry through TRPM2, which is higher in Bcl-2 overexpressing T Cell leukemia cells, and contributes to inactivation of cdc2, G2/M cell cycle arrest and cell survival. TRPM2 inhibition released cells from G2/M arrest and resulted in cell death. These data suggest that TRPM2 inhibition may be a therapeutic approach to increase radiation sensitivity in T-cell leukemia.
3.4. Role of TRPM2 in Prostate and Lung Cancer Proliferation
TRPM2 is essential for prostate cancer cell proliferation [67]. When TRPM2 was depleted with siRNA, the growth of cancerous prostate cells but not non-cancerous cells was reduced. In non-cancerous cells, TRPM2 localized in the plasma membrane and cytoplasm but was absent in the nucleus, whereas in prostate cancer cells a significant amount of TRPM2 localized in nucleus as well as in the non-nuclear fraction. The function of nuclear TRPM2 in cancer cells is not known. These findings suggest that depletion of TRPM2 may be a therapeutic approach to control prostate cancer growth. However, an additional finding was that a long non-coding RNA which is an antisense transcript of TRPM2 (TRPM2-AS) is overexpressed in prostate cancer and linked to poor clinical outcome. When TRPM2-AS is knocked down, cell apoptosis increased coupled to cell cycle arrest and a large increase in TRPM2 expression was detected [85, 86]. These results appear to be in conflict, because in the first report knockdown of TRPM2 reduced cell proliferation, whereas in the second expression of a long noncoding TRPM2 antisense transcript was linked to poor patient outcome and knockdown of the antisense transcript was associated with increased TRPM2 expression, increased cell cycle arrest and apoptosis [87]. However, how long non-coding RNAs function in cancer is complex and not completely understood. It has been suggested that TRPM2-AS may function to prevent over-activation of TRPM2 rather than to completely abolish function [86].
TRPM2 is highly expressed in lung cancer [64]. In non-small cell lung cancer (NSCLC), expression of novel long non-coding antisense RNA, TRPM2-AS, was found to be widely upregulated and higher expression levels correlated with larger tumor size, advanced TNM stage, and poor patient survival [85]. Silencing of TRPM2-AS with siRNA significantly reduced cell proliferation and increased apoptosis. Further work will be necessary to understand the role of long non-coding RNAs involving TRPM2 in cell proliferation and patient survival, and the impact on TRPM2 expression and function.
3.5. TRPM2 in Squamous Cell Carcinoma
TRPM2 expression is enhanced in human tongue carcinoma specimens and in tongue carcinoma cell lines [64, 65]. In tongue carcinoma SCC9 cells, treatment with 0.5 or 1 mM H2O2 increased apoptosis. Knockdown of TRPM2 with siRNA also increased apoptosis, reduced survival and inhibited migration of tongue carcinoma SCC9 cells. Subcellular localization of TRPM2 was different between cancerous and non-cancerous cells, and a significant amount of TRPM2 protein localized to the nucleus in cancer cells. Although the mechanisms of cell death in TRPM2 KO cells were not explored in detail, it was independent of the p53-p21 pathway. The conclusion of this work is that TRPM2 contributes to survival and migration of SCC cancer cells and may be a potential therapeutic target in head and neck cancers [65].
4. Other Mechanisms through which TRPM2 Modulates Cancer Progression
4.1. Role of TRPM2 in Cell Migration and Pancreatic Cancer
Activation of TRPM2 by H2O2 has been shown to induce filopodia formation, loss of actin stress fibers, and disassembly of focal adhesions, leading to increased migration of both HeLa cells and prostate cancer cells [88]. TRPM2 activation increased intracellular levels of both Ca2+ and zinc, but zinc and Ca2+ regulated actin cytoskeleton and focal adhesion dynamics reciprocally. Zinc enriched lysosomes migrated toward the leading edge of the cell, suggesting that zinc played a dominant role in promoting cell migration. These data suggest that either TRPM2 inhibition or chelators of free Zn2+ may reduce or prevent metastatic progression.
High TRPM2 expression was recently associated with shorter survival time in patient with pancreatic cancer [66]. In vitro, higher expression of TRPM2 enhanced pancreatic cell proliferation, migratory ability in a scratch wound healing assay, and invasive ability in a Transwell assay using Matrigel. High expression of TRPM2 correlated strongly with expression of proteins including probable phospholipid-transporting ATP-ase IM (ATP8B4), γ-parvin (PARVG), tudor domain-containing protein 9 (TDRD9), Toll-like receptor 7 (TLR7), and Scm-like with four MBT domains protein 2 (SFMBT2), which may provide indications of the mechanisms of TRPM2 signaling [66]. In addition, in pancreatic ductal adenocarcinoma cells, the sirtuin SIRT6 promoted expression of inflammatory cytokines IL-8 and TNF and enhanced the migratory capacity of these cells thorough a mechanism that involved ADPR production and activation of TRPM2 [89]. Together, these reports suggest that TRPM2 not only promotes cell proliferation in pancreatic cancer but also migration and invasion which could enhance metastatic potential.
4.2. Role of TRPM2 in Cell Survival after Radiation
TRPM2 is involved in repair of DNA damage induced by radiation. Gamma irradiation induces DNA damage, resulting in activation of PARP and generation of ADPR, which activates TRPM2. To examine the role of TRPM2 in radiation-induced DNA damage and cell death, human alveolar epithelial adenocarcinoma cells depleted of TRPM2 expression were exposed to gamma-rays or UVB irradiation. Signaling pathways associated with DNA repair were suppressed by TRPM2 knockdown. These included ATM activation, 53BP1 (p53-binding protein) accumulation at sites of DNA damage, EGFR (epithelial growth factor receptor) nuclear translocation, and release of ATP [90]. ATM is a serine/threonine kinase activated by DNA double-strand breaks. ATM phosphorylates/activates proteins that initiate the DNA damage checkpoint, leading to cell cycle arrest, DNA repair and/or apoptosis. 53BP1 is a well-known DNA damage response factor and depletion contributes to genomic instability. Radiation-induced EGFR nuclear translocation has a key role in DNA repair. Data support the conclusion that TRPM2 has an important role in the response to DNA damage induced by both ionizing and non-ionizing radiation. Another report showed that following ionizing radiation, TRPM2 activation mediated ATP production and release. ATP activates the purinergic P2Y6 and P2Y12 receptors, which induce the DNA damage response [91]. Activation of the P2Y6 receptor is involved in nuclear translocation of EGFR, which is suppressed in TRPM2 depletion, and in induction of antioxidants [92]. In T-cell leukemia, after irradiation TRPM2 inhibition or knockdown decreased the number of cell accumulating in G2/M and increased the number of dead cells [84]. These studies indicate that TRPM2 is a radiation sensor protein mediating ATP release and DNA repair to enhance cell survival.
4.3. Other mechanisms through which TRPM2 Modulates Cell Survival in Cancer
In some types of cancer, rather than promotion of cell survival, TRPM2 expression in cancer cells correlates with increased susceptibility to oxidative stress and improved patient outcome. TRPM2 is highly expressed in bladder cancer [64]. In T24 bladder cancer cells, overexpression of TRPM2 promoted apoptosis and TRPM2 depletion antagonized histone deacetylase inhibition induced apoptosis. Evidence supported the mechanism that inhibition of histone deacetylase was associated with increased H3K9 acetylation in the TRPM2 promoter and increased TRPM2 expression, which promoted increased apoptosis [93]. In a study of 33 patients with glioblastoma multiforme, TRPM2 was highly expressed compared to controls. Survival time was significantly longer in patients with higher TRPM2 levels and survival shorter in patients with lower TRPM2 levels [94]. These data suggested that TRPM2 expression increases responsiveness to chemotherapy and radiation in glioblastoma. However, in another study, high TRPM2 expression in glioblastoma was associated with worse patient survival (unpublished results, Miller laboratory). Wild type glioblastoma A172 cells were found to have undetectable levels of endogenous TRPM2 [95]. When TRPM2 was heterologously expressed in these cells, an H2O2 dose dependent increase in intracellular calcium was observed as well as increased susceptibility to cell death at H2O2 doses of 100 and 250 μM. However, in this study performed with a glioblastoma cell line that did not endogenously express TRPM2, increased expression of TRPM2 did not affect proliferation, invasion, or migration. These studies suggest that in glioblastoma, TRPM2 may be able to enhance cell death through increased Ca2+ entry after H2O2 exposure, but more work needs to be done to clarify its role.
The role of TRPM2 in the cellular immune response against cancer cells is currently being investigated. Neutrophils have been shown to play a critical role, but they are a heterogeneous population and both protumor and antitumor neutrophil subpopulations exist. Whereas antitumor neutrophils can kill tumor cells and reduce metastasis, not all tumor cells are susceptible to neutrophil cytotoxicity. In a recent report, TRPM2 supported primary tumor growth but increased susceptibility to neutrophil cytotoxicity [96]. TRPM2 non-expressing cells were H2O2 and neutrophil resistant. Whereas tumors with lower expression of TRPM2 were significantly smaller, they generated significantly more metastatic tumors [96]. These data suggested that while TRPM2 inhibition reduces primary tumor growth and increases chemotherapy sensitivity, it may reduce neutrophil cytotoxicity and has the potential to increase metastatic spread in some settings which need to be better understood.
5. Conclusions
TRPM2 is highly expressed in many types of cancer, suggesting TRPM2 promotes tumor survival. Inhibition of TRPM2 has been demonstrated to enhance cell death and increase sensitivity to doxorubicin and other chemotherapeutic agents in a number of malignancies including neuroblastoma [16, 61, 69], T cell leukemia [84], gastric cancer [62], and triple-negative and estrogen-receptor positive breast cancer cell lines [77]. The preponderance of data in cancer models support the concept that TRPM2 expression and function have an important role in preserving cancer cell viability, and TRPM2 inhibition may be a novel therapeutic approach (Table 1). However, in some studies reviewed here, TRPM2 expression correlated with increased cancer susceptibility to chemotherapy, and depletion increased metastasis and/or had an adverse effect on patient outcome. Mechanisms responsible for these differences require further investigation.
TRPM2 preserves cancer cell viability through maintenance of mitochondrial function, cellular bioenergetics, ATP production, autophagy, reduction in cellular ROS levels, and DNA repair [15, 16, 61, 69, 76, 90]. Elevated levels of ROS are found in the majority of cancers and promote tumorigenesis through activation of transcription factors and signaling pathways and DNA damage [35]. Cancer cells simultaneously require increased levels of antioxidants to detoxify ROS and protect viability [71], maintenance of mitochondrial function and bioenergetics to meet energy demands, repair of DNA to maintain its integrity, and access to nutrients and building material through autophagy. TRPM2 promotes expression of transcription factors including HIF-1/2α, CREB, and Nrf2 and activation of kinases and other signaling pathways to address these requirements [16, 61, 97]. Mechanisms though which TRPM2 mediates these effects are under investigation. TRPM2 is an exciting potential target in therapy of many cancers as further evidence of its important function in cell survival is forthcoming and controversies in its essential roles are clarified.
Highlights.
TRPM2 is highly expressed in many human cancers.
It increases cell survival and inhibition reduces viability of malignant cells.
-TRPM2 inhibition impairs mitochondrial function, bioenergetics and autophagy.
TRPM2 inhibition reduces antioxidant defenses and increases ROS levels.
Targeting TRPM2 may be a novel therapeutics approach in treatment of many cancers.
Funding:
This work was supported in part by the National Institutes of Health Grants R01-GM117014; Hyundai Hope on Wheels Scholar Grant; and the Four Diamonds Fund of the Pennsylvania State University.
Abbreviations:
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- ADPR
adenosine diphosphate ribose
- cADPR
Cyclic adenosine diphosphate ribose
- 2-APB
2-aminoethoxydiphenyl borate
- ATGs
autophagy-related proteins
- ATM
ataxia-telangiectasia mutated serine/threonine protein kinase
- BECN1/Beclin 1
BECN1 gene encoding beclin-1 protein which plays a key role in autophagy
- BNIP3
BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3
- CAMKII
calcium/calmodulin dependent protein kinase II
- COX4.1/4.2
cyclooxygenase 4.1/4.2
- CREB
cAMP responsive element-binding protein
- EGFR
epidermal growth factor receptor
- FOXO3a
Forkhead box O3 transcription factor
- GSH
Glutathione
- HIF-1α
Hypoxia-inducible factor – alpha
- HSP60
Heat shock protein 60
- Keap1
Ketch-like ECH-associated protein 1
- IQGAP1
IQ motif containing GTPase activating protein 1
- MCU
Mitochondrial Calcium Uniporter
- NADH
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidase
- Nrf2
nuclear factor (erythroid-derived 2)-related factor-2
- PARG
poly (ADPR) glycohydrolase
- PARP
poly (ADPR) polymerase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- Pyk2
proline-rich tyrosine kinase 2
- ROS
reactive oxygen species
- Src
proto-oncogene tyrosine-protein kinase
- TNF-α
Tumor Necrosis Factor alpha
- Tom20
translocase of the outer mitochondrial membrane 20
- TRPM
Transient Receptor Potential Melastatin
- TRPM2-S
Transient Receptor Potential Melastatin Channel 2- short isoform
- TRPM2-TE
Transient Receptor Potential Melastatin Channel 2- tumor-enriched isoform
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Akopian A, Role of TRP ion channels in physiology and pathology, Semin Immunopathol, 38 (2016) 275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nilius B, Flockerzi V, Mammalian transient receptor potential (TRP) cation channels. Preface, Handb Exp Pharmacol, 223 (2014) v–vi. [PubMed] [Google Scholar]
- [3].Nilius B, Owsianik G, Voets T, Peters JA, Transient receptor potential cation channels in disease, Physiol Rev, 87 (2007) 165–217. [DOI] [PubMed] [Google Scholar]
- [4].Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW, Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis, Cancer Res, 58 (1998) 1515–1520. [PubMed] [Google Scholar]
- [5].Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M, A key role for TRPM7 channels in anoxic neuronal death, Cell, 115 (2003) 863–877. [DOI] [PubMed] [Google Scholar]
- [6].Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N, Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain, Genomics, 54 (1998) 124–131. [DOI] [PubMed] [Google Scholar]
- [7].Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y, LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death, Mol Cell, 9 (2002) 163–173. [DOI] [PubMed] [Google Scholar]
- [8].Miller BA, Zhang W, TRP Channels as Mediators of Oxidative Stress, Adv Exp Med Biol, 704 (2011) 531–544. [DOI] [PubMed] [Google Scholar]
- [9].Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM, ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology, Nature, 411 (2001) 595–599. [DOI] [PubMed] [Google Scholar]
- [10].Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, Miller BA, Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin, J Biol Chem, 281 (2006) 9076–9085. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Z, Toth B, Szollosi A, Chen J, Csanady L, Structure of a TRPM2 channel in complex with Ca(2+) explains unique gating regulation, Elife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA, A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death, J Biol Chem, 278 (2003) 16222–16229. [DOI] [PubMed] [Google Scholar]
- [13].Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A, Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose, J Biol Chem, 277 (2002) 23150–23156. [DOI] [PubMed] [Google Scholar]
- [14].Orfanelli U, Wenke AK, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G, Identification of novel sense and antisense transcription at the TRPM2 locus in cancer, Cell Res, 18 (2008) 1128–1140. [DOI] [PubMed] [Google Scholar]
- [15].Chen SJ, Zhang W, Tong Q, Conrad K, Hirschler-Laszkiewicz I, Bayerl M, Kim JK, Cheung JY, Miller BA, Role of TRPM2 in cell proliferation and susceptibility to oxidative stress, Am J Physiol Cell Physiol, 304 (2013) C548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen SJ, Hoffman NE, Shanmughapriya S, Bao L, Keefer K, Conrad K, Merali S, Takahashi Y, Abraham T, Hirschler-Laszkiewicz I, Wang J, Zhang XQ, Song J, Barrero C, Shi Y, Kawasawa YI, Bayerl M, Sun T, Barbour M, Wang HG, Madesh M, Cheung JY, Miller BA, A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2alpha, J Biol Chem, 289 (2014) 36284–36302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maruyama Y, Ogura T, Mio K, Kiyonaka S, Kato K, Mori Y, Sato C, Three-dimensional reconstruction using transmission electron microscopy reveals a swollen, bell-shaped structure of transient receptor potential melastatin type 2 cation channel, J Biol Chem, 282 (2007) 36961–36970. [DOI] [PubMed] [Google Scholar]
- [18].Wang L, Fu TM, Zhou Y, Xia S, Greka A, Wu H, Structures and gating mechanism of human TRPM2, Science, 362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang Y, Winkler PA, Sun W, Lu W, Du J, Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium, Nature, 562 (2018) 145–149. [DOI] [PubMed] [Google Scholar]
- [20].Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, Zhang W, Miller BA, Benham CD, McNulty S, Amyloid beta-peptide(1–42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures, J Neurochem, 95 (2005) 715–723. [DOI] [PubMed] [Google Scholar]
- [21].Gasser A, Glassmeier G, Fliegert R, Langhorst MF, Meinke S, Hein D, Kruger S, Weber K, Heiner I, Oppenheimer N, Schwarz JR, Guse AH, Activation of T cell calcium influx by the second messenger adp-ribose, J Biol Chem, 281 (2006) 2489–2496. [DOI] [PubMed] [Google Scholar]
- [22].Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM, Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels, J Biol Chem, 280 (2005) 6138–6148. [DOI] [PubMed] [Google Scholar]
- [23].Fonfria E, Marshall ICB, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, Scharenberg AM, McNulty S, TRPM2 Channel Opening in Response to Oxidative Stress is Dependent on Activation of Poly (ADP-Ribose) Polymerase, Br.J. Pharmacol, 143 (2004) 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buelow B, Song Y, Scharenberg AM, The Poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes, J Biol Chem, 283 (2008) 24571–24583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kolisek M, Beck A, Fleig A, Penner R, Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels, Mol Cell, 18 (2005) 61–69. [DOI] [PubMed] [Google Scholar]
- [26].Toth B, Csanady L, Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel, J Biol Chem, 285 (2010) 30091–30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Toth B, Iordanov I, Csanady L, Ruling out pyridine dinucleotides as true TRPM2 channel activators reveals novel direct agonist ADP-ribose-2’-phosphate, J Gen Physiol, 145 (2015) 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ, Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation, J Biol Chem, 278 (2003) 11002–11006. [DOI] [PubMed] [Google Scholar]
- [29].Du J, Xie J, Yue L, Intracellular calcium activates TRPM2 and its alternative spliced isoforms, Proc Natl Acad Sci U S A, 106 (2009) 7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Toth B, Csanady L, Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents, Proc Natl Acad Sci U S A, 109 (2012) 13440–13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M, TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion, Embo J, 25 (2006) 1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Du J, Xie J, Yue L, Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity, J Gen Physiol, 134 (2009) 471–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Starkus JG, Fleig A, Penner R, The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification, J Physiol, 588 (2010) 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Csanady L, Permeating proton found guilty in compromising TRPM2 channel activity, J Physiol, 588 (2010) 1661–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DeBerardinis RJ, Chandel NS, Fundamentals of cancer metabolism, Sci Adv, 2 (2016) e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hole PS, Darley RL, Tonks A, Do reactive oxygen species play a role in myeloid leukemias?, Blood, 117 (2011) 5816–5826. [DOI] [PubMed] [Google Scholar]
- [37].Hoffman NE, Miller BA, Wang J, Elrod JW, Rajan S, Gao E, Song J, Zhang XQ, Hirschler-Laszkiewicz I, Shanmughapriya S, Koch WJ, Feldman AM, Madesh M, Cheung JY, Ca(2)(+) entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance, Am J Physiol Heart Circ Physiol, 308 (2015) H637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schumacker PT, Reactive oxygen species in cancer cells: live by the sword, die by the sword, Cancer Cell, 10 (2006) 175–176. [DOI] [PubMed] [Google Scholar]
- [39].Trachootham D, Alexandre J, Huang P, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?, Nat Rev Drug Discov, 8 (2009) 579–591. [DOI] [PubMed] [Google Scholar]
- [40].Simon F, Varela D, Cabello-Verrugio C, Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans, Cell Signal, 25 (2013) 1614–1624. [DOI] [PubMed] [Google Scholar]
- [41].Miller BA, Cheung JY, TRPM2 protects against tissue damage following oxidative stress and ischaemia-reperfusion, J Physiol, 594 (2016) 4181–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sumoza-Toledo A, Penner R, TRPM2: a multifunctional ion channel for calcium signalling, J Physiol, 589 (2011) 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Knowles H, Li Y, Perraud AL, The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation, Immunol Res, 55 (2013) 241–248. [DOI] [PubMed] [Google Scholar]
- [44].Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y, TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration, Nat Med, 14 (2008) 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, Malik AB, The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation, Nat Immunol, 13 (2012) 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miller BA, Wang J, Hirschler-Laszkiewicz I, Gao E, Song J, Zhang XQ, Koch WJ, Madesh M, Mallilankaraman K, Gu T, Chen SJ, Keefer K, Conrad K, Feldman AM, Cheung JY, The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury, Am J Physiol Heart Circ Physiol, 304 (2013) H1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miller BA, Hoffman NE, Merali S, Zhang XQ, Wang J, Rajan S, Shanmughapriya S, Gao E, Barrero CA, Mallilankaraman K, Song J, Gu T, Hirschler-Laszkiewicz I, Koch WJ, Feldman AM, Madesh M, Cheung JY, TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria, J Biol Chem, 289 (2014) 7615–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hermosura MC, Cui AM, Go RC, Davenport B, Shetler CM, Heizer JW, Schmitz C, Mocz G, Garruto RM, Perraud AL, Altered functional properties of a TRPM2 variant in Guamanian ALS and PD, Proc Natl Acad Sci U S A, 105 (2008) 18029–18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bai JZ, Lipski J, Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture, Neurotoxicology, 31 (2010) 204–214. [DOI] [PubMed] [Google Scholar]
- [50].Cheung JY, Miller BA, Transient Receptor Potential-Melastatin Channel Family Member 2: Friend or Foe, Trans Am Clin Climatol Assoc, 128 (2017) 308–329. [PMC free article] [PubMed] [Google Scholar]
- [51].Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML, Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death, Cell Death Differ, 13 (2006) 1815–1826. [DOI] [PubMed] [Google Scholar]
- [52].Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB, TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1, J Clin Invest, 124 (2014) 4989–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Manna PT, Munsey TS, Abuarab N, Li F, Asipu A, Howell G, Sedo A, Yang W, Naylor J, Beech DJ, Jiang LH, Sivaprasadarao A, TRPM2-mediated intracellular Zn2+ release triggers pancreatic beta-cell death, Biochem J, 466 (2015) 537–546. [DOI] [PubMed] [Google Scholar]
- [54].Mortadza SS, Sim JA, Stacey M, Jiang LH, Signalling mechanisms mediating Zn2+-induced TRPM2 channel activation and cell death in microglial cells, Sci Rep, 7 (2017) 45032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Miyanohara J, Kakae M, Nagayasu K, Nakagawa T, Mori Y, Arai K, Shirakawa H, Kaneko S, TRPM2 Channel Aggravates CNS Inflammation and Cognitive Impairment via Activation of Microglia in Chronic Cerebral Hypoperfusion, J Neurosci, 38 (2018) 3520–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li X, Jiang LH, Multiple molecular mechanisms form a positive feedback loop driving amyloid beta42 peptide-induced neurotoxicity via activation of the TRPM2 channel in hippocampal neurons, Cell Death Dis, 9 (2018) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Auberger P, Puissant A, Autophagy, a key mechanism of oncogenesis and resistance in leukemia, Blood, 129 (2017) 547–552. [DOI] [PubMed] [Google Scholar]
- [58].Piya S, Andreeff M, Borthakur G, Targeting autophagy to overcome chemoresistance in acute myleogenous leukemia, Autophagy, 13 (2017) 214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kania E, Pajak B, Orzechowski A, Calcium homeostasis and ER stress in control of autophagy in cancer cells, Biomed Res Int, 2015 (2015) 352794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Evangelisti C, Evangelisti C, Chiarini F, Lonetti A, Buontempo F, Neri LM, McCubrey JA, Martelli AM, Autophagy in acute leukemias: a double-edged sword with important therapeutic implications, Biochim Biophys Acta, 1853 (2015) 14–26. [DOI] [PubMed] [Google Scholar]
- [61].Bao L, Chen SJ, Conrad K, Keefer K, Abraham T, Lee JP, Wang J, Zhang XQ, Hirschler-Laszkiewicz I, Wang HG, Dovat S, Gans B, Madesh M, Cheung JY, Miller BA, Depletion of the Human Ion Channel TRPM2 in Neuroblastoma Demonstrates Its Key Role in Cell Survival through Modulation of Mitochondrial Reactive Oxygen Species and Bioenergetics, J Biol Chem, 291 (2016) 24449–24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Almasi S, Kennedy BE, El-Aghil M, Sterea AM, Gujar S, Partida-Sanchez S, El Hiani Y, TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway, J Biol Chem, 293 (2018) 3637–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Q, Guo W, Hao B, Shi X, Lu Y, Wong CW, Ma VW, Yip TT, Au JS, Hao Q, Cheung KH, Wu W, Li GR, Yue J, Mechanistic study of TRPM2-Ca(2+)-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition, Autophagy, 12 (2016) 1340–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Park YR, Chun JN, So I, Kim HJ, Baek S, Jeon JH, Shin SY, Data-driven Analysis of TRP Channels in Cancer: Linking Variation in Gene Expression to Clinical Significance, Cancer Genomics Proteomics, 13 (2016) 83–90. [PubMed] [Google Scholar]
- [65].Zhao LY, Xu WL, Xu ZQ, Qi C, Li Y, Cheng J, Liu LK, Wu YN, Gao J, Ye JH, The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma, Sci Rep, 6 (2016) 38471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lin R, Wang Y, Chen Q, Liu Z, Xiao S, Wang B, Shi B, TRPM2 promotes the proliferation and invasion of pancreatic ductal adenocarcinoma, Mol Med Rep, 17 (2018) 7537–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zeng X, Sikka SC, Huang L, Sun C, Xu C, Jia D, Abdel-Mageed AB, Pottle JE, Taylor JT, Li M, Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation, Prostate Cancer Prostatic Dis, 13 (2010) 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu YV, Hubbi ME, Pan F, McDonald KR, Mansharamani M, Cole RN, Liu JO, Semenza GL, Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization, J Biol Chem, 282 (2007) 37064–37073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hirschler-Laszkiewicz I, Chen SJ, Bao L, Wang J, Zhang XQ, Shanmughapriya S, Keefer K, Madesh M, Cheung JY, Miller BA, The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation, Am J Physiol Cell Physiol, 315 (2018) C571–C586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mittal M, Urao N, Hecquet CM, Zhang M, Sudhahar V, Gao XP, Komarova Y, Ushio-Fukai M, Malik AB, Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization, Arterioscler Thromb Vasc Biol, 35 (2015) 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Abdul-Aziz A, MacEwan DJ, Bowles KM, Rushworth SA, Oxidative stress responses and NRF2 in human leukaemia, Oxid Med Cell Longev, 2015 (2015) 454659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jaramillo MC, Zhang DD, The emerging role of the Nrf2-Keap1 signaling pathway in cancer, Genes Dev, 27 (2013) 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim JH, Xu EY, Sacks DB, Lee J, Shu L, Xia B, Kong AN, Identification and functional studies of a new Nrf2 partner IQGAP1: a critical role in the stability and transactivation of Nrf2, Antioxid Redox Signal, 19 (2013) 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gorrini C, Harris IS, Mak TW, Modulation of oxidative stress as an anticancer strategy, Nat Rev Drug Discov, 12 (2013) 931–947. [DOI] [PubMed] [Google Scholar]
- [75].Togashi K, Inada H, Tominaga M, Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB), Br J Pharmacol, 153 (2008) 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hopkins MM, Feng X, Liu M, Parker LP, Koh DW, Inhibition of the transient receptor potential melastatin-2 channel causes increased DNA damage and decreased proliferation in breast adenocarcinoma cells, Int J Oncol, 46 (2015) 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Koh DW, Powell DP, Blake SD, Hoffman JL, Hopkins MM, Feng X, Enhanced cytotoxicity in triple-negative and estrogen receptorpositive breast adenocarcinoma cells due to inhibition of the transient receptor potential melastatin-2 channel, Oncol Rep, 34 (2015) 1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liu J, Chen Y, Shuai S, Ding D, Li R, Luo R, TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3beta pathway, Tumour Biol, 35 (2014) 8969–8977. [DOI] [PubMed] [Google Scholar]
- [79].Zhou W, Guo S, Xiong Z, Liu M, Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy, Expert Opin Ther Targets, 18 (2014) 1177–1196. [DOI] [PubMed] [Google Scholar]
- [80].Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, Yang Z, Zeng H, Shen Q, Zou F, TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway, Cancer Lett, 333 (2013) 96–102. [DOI] [PubMed] [Google Scholar]
- [81].Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, Wieringa B, Canisius SV, Zwart W, Wessels LF, Sweep FC, Bult P, Span PN, van Leeuwen FN, Jalink K, TRPM7 is required for breast tumor cell metastasis, Cancer Res, 72 (2012) 4250–4261. [DOI] [PubMed] [Google Scholar]
- [82].Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, Ouadid-Ahidouch H, Estrogen regulation of TRPM8 expression in breast cancer cells, BMC Cancer, 10 (2010) 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Guilbert A, Gautier M, Dhennin-Duthille I, Rybarczyk P, Sahni J, Sevestre H, Scharenberg AM, Ouadid-Ahidouch H, Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain, Eur J Cancer, 49 (2013) 3694–3707. [DOI] [PubMed] [Google Scholar]
- [84].Klumpp D, Misovic M, Szteyn K, Shumilina E, Rudner J, Huber SM, Targeting TRPM2 Channels Impairs Radiation-Induced Cell Cycle Arrest and Fosters Cell Death of T Cell Leukemia Cells in a Bcl-2-Dependent Manner, Oxid Med Cell Longev, 2016 (2016) 8026702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Huang C, Qin Y, Liu H, Liang N, Chen Y, Ma D, Han Z, Xu X, Zhou X, He J, Li S, Downregulation of a novel long noncoding RNA TRPM2-AS promotes apoptosis in non-small cell lung cancer, Tumour Biol, 39 (2017) 1010428317691191. [DOI] [PubMed] [Google Scholar]
- [86].Orfanelli U, Jachetti E, Chiacchiera F, Grioni M, Brambilla P, Briganti A, Freschi M, Martinelli-Boneschi F, Doglioni C, Montorsi F, Bellone M, Casari G, Pasini D, Lavorgna G, Antisense transcription at the TRPM2 locus as a novel prognostic marker and therapeutic target in prostate cancer, Oncogene, 34 (2015) 2094–2102. [DOI] [PubMed] [Google Scholar]
- [87].Mouraviev V, Lee B, Patel V, Albala D, Johansen TE, Partin A, Ross A, Perera RJ, Clinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancer, Prostate Cancer Prostatic Dis, 19 (2016) 14–20. [DOI] [PubMed] [Google Scholar]
- [88].Li F, Abuarab N, Sivaprasadarao A, Reciprocal regulation of actin cytoskeleton remodelling and cell migration by Ca2+ and Zn2+: role of TRPM2 channels, J Cell Sci, 129 (2016) 2016–2029. [DOI] [PubMed] [Google Scholar]
- [89].Bauer I, Grozio A, Lasiglie D, Basile G, Sturla L, Magnone M, Sociali G, Soncini D, Caffa I, Poggi A, Zoppoli G, Cea M, Feldmann G, Mostoslavsky R, Ballestrero A, Patrone F, Bruzzone S, Nencioni A, The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses, J Biol Chem, 287 (2012) 40924–40937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Masumoto K, Tsukimoto M, Kojima S, Role of TRPM2 and TRPV1 cation channels in cellular responses to radiation-induced DNA damage, Biochim Biophys Acta, 1830 (2013) 3382–3390. [DOI] [PubMed] [Google Scholar]
- [91].Tsukimoto M, Purinergic Signaling Is a Novel Mechanism of the Cellular Response to Ionizing Radiation, Biol Pharm Bull, 38 (2015) 951–959. [DOI] [PubMed] [Google Scholar]
- [92].Tamaishi N, Tsukimoto M, Kitami A, Kojima S, P2Y6 receptors and ADAM17 mediate low-dose gamma-ray-induced focus formation (activation) of EGF receptor, Radiat Res, 175 (2011) 193–200. [DOI] [PubMed] [Google Scholar]
- [93].Cao QF, Qian SB, Wang N, Zhang L, Wang WM, Shen HB, TRPM2 mediates histone deacetylase inhibition-induced apoptosis in bladder cancer cells, Cancer Biother Radiopharm, 30 (2015) 87–93. [DOI] [PubMed] [Google Scholar]
- [94].Alptekin M, Eroglu S, Tutar E, Sencan S, Geyik MA, Ulasli M, Demiryurek AT, Camci C, Gene expressions of TRP channels in glioblastoma multiforme and relation with survival, Tumour Biol, 36 (2015) 9209–9213. [DOI] [PubMed] [Google Scholar]
- [95].Ishii M, Oyama A, Hagiwara T, Miyazaki A, Mori Y, Kiuchi Y, Shimizu S, Facilitation of H2O2-induced A172 human glioblastoma cell death by insertion of oxidative stress-sensitive TRPM2 channels, Anticancer Res, 27 (2007) 3987–3992. [PubMed] [Google Scholar]
- [96].Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, Lev S, Polyansky L, Shaul ME, Sionov RV, Cohen-Daniel L, Aqeilan RI, Shaul YD, Mori Y, Karni R, Fridlender ZG, Binshtok AM, Granot Z, TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells, Cancer Res, 78 (2018) 2680–2690. [DOI] [PubMed] [Google Scholar]
- [97].Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL, HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells, Cell, 129 (2007) 111–122. [DOI] [PubMed] [Google Scholar]