Abstract
Deficiency of C1q, the initiator of the complement classical pathway, is associated with the development of systemic lupus erythematosus (SLE). Explaining this association in terms of abnormalities in the classical pathway alone remains problematic because C3 deficiency does not predispose to SLE. Here, using a mouse model of SLE, we demonstrate that C1q, but not C3, restrains the response to self-antigens by modulating the mitochondrial metabolism of CD8+ T cells, which can themselves propagate autoimmunity. C1q deficiency also triggers an exuberant effector CD8+ T-cell response to chronic viral infection leading to lethal immunopathology. These data establish a link between C1q and CD8+ T-cell metabolism and may explain how C1q protects against lupus, with implications for the role of viral infections in the perpetuation of autoimmunity.
Systemic lupus erythematosus (SLE) is an autoimmune condition that develops as a result of complex genetic and environmental interactions. B- and CD4+ T-cell abnormalities are well known features of SLE (1), but the role of CD8+ T lymphocytes remains poorly understood. Transcriptomic data suggest that a CD8+ T-cell signature can predict disease outcome (2, 3).
There is evidence for a strong association between SLE and complement C1q deficiency (4). Previous work has shown that C1q deficiency leads to the ineffective clearance of apoptotic cells and consequently enhanced exposure to self-antigens, which facilitate autoimmunity (5). However, there are multiple pathways, including those mediated by C3, through which apoptotic cell clearance occurs (6). This suggests that the contribution of C1q is redundant and the “waste disposal” hypothesis (5) is inadequate to fully explain why C1q deficiency, and not C3 deficiency, is associated with autoimmunity. Alternative, but not mutually exclusive, hypotheses have been proposed (7). However, an explanation for this strong association in terms of classical complement pathway abnormalities alone remains unsatisfactory. Given that there is evidence that C1q has multiple roles that are independent of complement activation (8), we searched for an alternative function that could explain why C1q is so critical for maintaining self-tolerance.
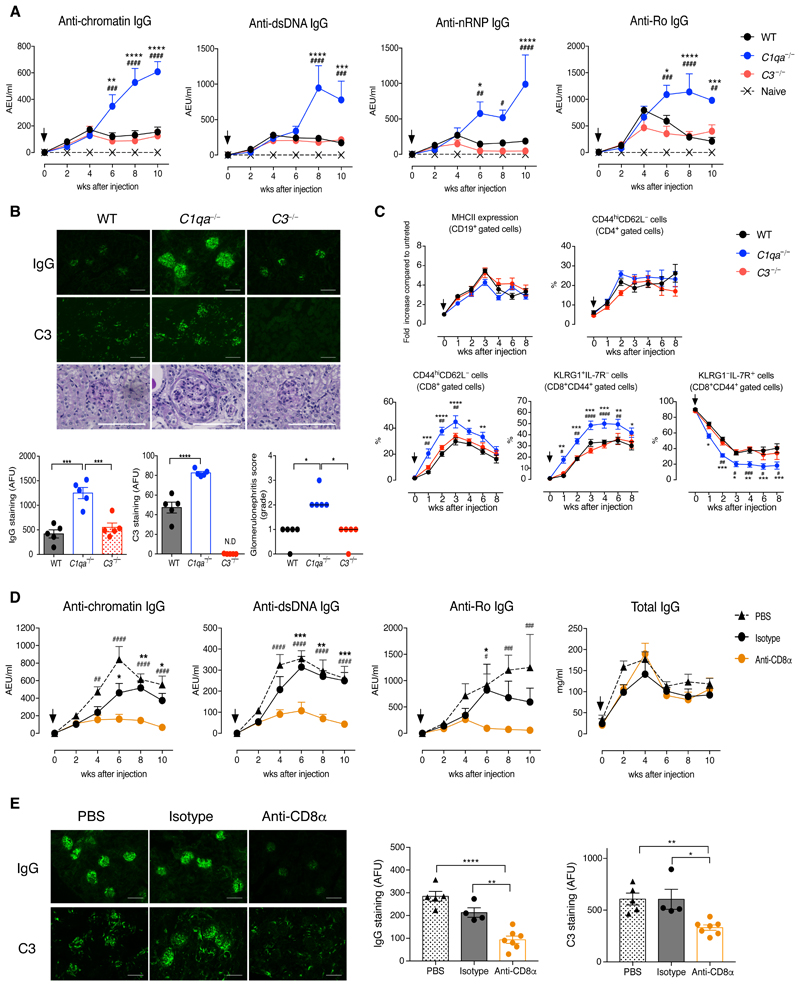
Chronic graft-versus-host-disease (cGvHD) is a well established inducible model of SLE. We used the bm12-cGvHD model (9) and injected splenocytes from B6(C)-H2-Ab1bm12/KhEgJ (bm12) mice into co-isogenic C57BL/6 (B6) recipients lacking C1q (C1qa−/−) or C3 (C3−/−). Lupus autoantibody levels were similar at disease onset (weeks 0-4), but increased at later time points only in the C1qa−/− mice (Fig. 1A). At week 10, C1qa−/− mice displayed more severe glomerulonephritis and increased glomerular deposition of IgG and C3 (Fig. 1B). They also had splenomegaly with higher percentages of germinal center B cells, follicular helper T cells (TFH), activated CD4+ and CD8+ T cells than the WT and C3−/− counterparts (Fig. S1). During the course of the disease there were no differences in blood B- and CD4+ T-cell activation between experimental groups, but the proportion of activated CD44hiCD62L−CD8+ T cells in the C1qa−/− mice was increased with a relative expansion of KLRG1+IL-7R− short-lived effector cells (SLECs) and a corresponding reduction in KLRG1−IL-7R+ memory precursor effector cells (MPECs) (Fig. 1C) (10). Consistent with the alterations in blood, cGvH-treated C1qa−/− mice had early (from week 1) splenic CD8+ T-cell abnormalities, whereas the initial B- and CD4+ T-cell responses were similar to WT and C3−/− animals (Fig. S1). Furthermore, the in vitro restimulation of C1qa−/−CD8+ T cells resulted in increased IFN-γ and granzyme B expression and fewer IL-2+ cells (Fig. S2), indicating that the lack of C1q, but not of C3, resulted in CD8+ T-cell responses skewed towards an effector phenotype. To determine whether bystander inflammation or self-antigen stimulation promoted CD8+ T-cell activation during bm12-cGvH induction, naïve CD8+ T cells from B6.CD45.1+ and OVA-specific TCR transgenic (OT-I) mice were co-transferred into B6.CD45.2+ animals, which were challenged with bm12 splenocytes. Donor CD45.1+CD8+ T cells expanded and became activated like the host CD45.2+CD8+ T cells, whereas pentamer+OT-I cells remained quiescent (Fig. S3), suggesting that TCR engagement by self-antigen was required.
Fig. 1. Autoimmune features and CD8+ T-cell response in C1qa−/− mice after bm12-cGvH induction.
(A) Autoantibody levels after bm12 injection (arrows) (n = 5 mice/group). (B) IgG, C3, and PAS staining of kidney sections at week 10. Quantification of glomerular IgG and C3 deposition expressed as arbitrary fluorescence units (AFU). ND: not detectable. Glomerulonephritis score: 0-4, bars indicate the median; *p<0.05; Kruskal–Wallis test. (C) Flow cytometric analysis of blood cells after cGvH induction (arrows) (n = 8-10 mice/group). (D to E) C1qa−/− mice were administrated PBS, anti-CD8α, or isotype-matched IgG2b antibody (n = 4-7 mice/group). (D) Autoantibody and IgG levels after cGvH induction (arrows). (E) Images and quantification of glomerular IgG and C3 deposition at week 10. *p<0.05, **p<0.01, ****p<0.001; One-way ANOVA (B and E); *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001 (WT vs C1qa−/−), #p<0.05, ##p<0.01, ###p<0.005, ####p<0.0001 (C1qa−/− vs C3−/−) two-way ANOVA (A and C); *p<0.05, **p<0.01, ***p<0.005 (isotype vs anti-CD8α), ##p<0.01, ###p<0.005, ####p<0.0001 (PBS vs anti-CD8α) two-way ANOVA (D). Data are mean ± SEM unless indicated otherwise; pooled results of two experiments (C); representative of two (D and E) or three (A and B) experiments. Scale bars, 100 μm (B and E).
CD8α+ DCs cross-present apoptotic cell-associated antigens to CD8+ T cells (11). However, the cross-priming by CD8α+ DCs in C1qa−/− animals was not impaired (Fig. S4A to S4C). Furthermore, after cGvH induction the number and phenotype of CD8α+ DCs was unaffected by C1q deficiency (Fig. S4D, S4E). We then depleted CD8+ T cells to demonstrate their direct contribution to the autoimmune response in cGvHD. Although similar autoantibody levels were initially detected in all groups (Fig. 1D), from week 4 onwards, CD8+ T cell-depleted C1qa−/− animals displayed a progressive decline in lupus-associated autoantibodies, whereas total IgG levels remained unaffected (Fig. 1D). CD8+ T cell-depleted C1qa−/− mice also showed reduced glomerular deposition of IgG and C3 compared with non-depleted mice (Fig. 1E). Thus these data suggest that CD8+ T cells are responsible for perpetuating the lupus-like disease observed in cGvH-treated C1qa−/− mice.
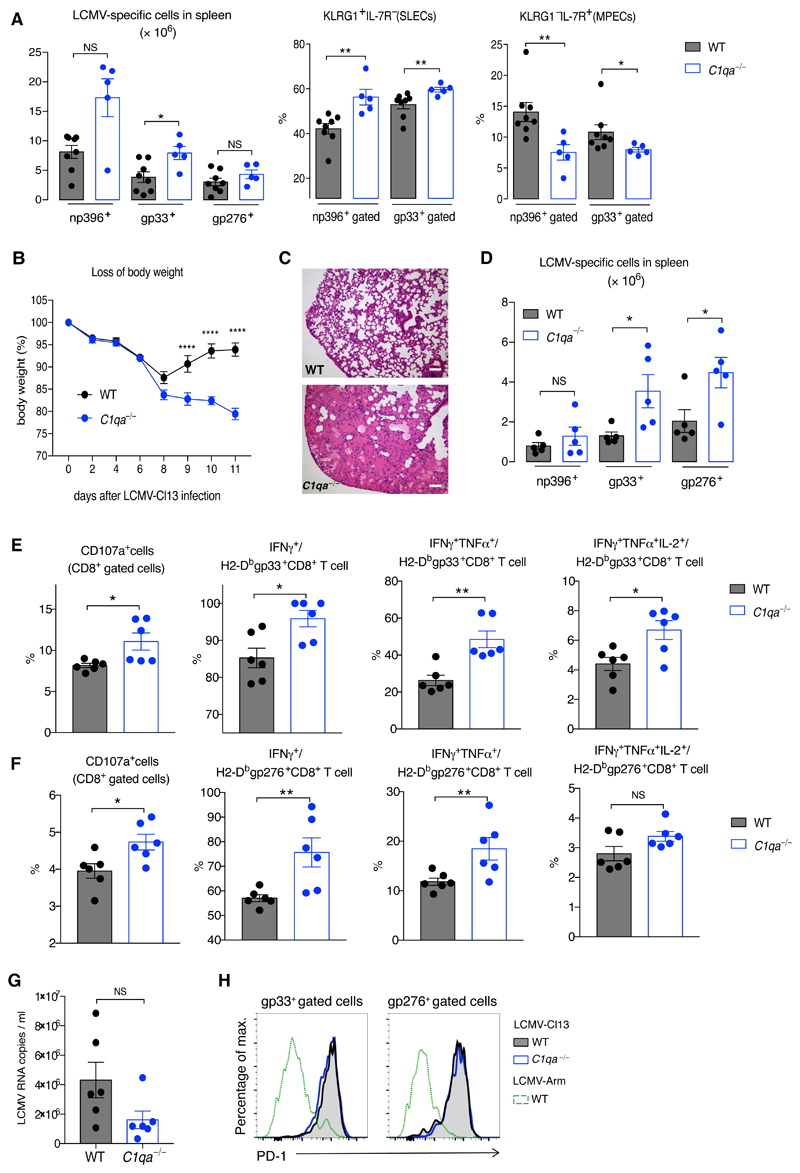
To explore whether C1q also modulates CD8+ T-cell immunity during infections we used lymphocytic choriomeningitis virus (LCMV) models. C1qa−/− mice, subjected to acute LCVM-Armstrong (Arm) infection, had an aberrant effector LCMV-specific CD8+ T-cell response at day 8 (Fig. 2A and Fig. S5A), but did not show markedly different memory and recall responses (Fig. S5B to Fig S5D). We next used the chronic LCMV-clone 13 (Cl13) model where an exaggerated effector immune response can cause lethal lung immunopathology (12). When compared to WT mice, Cl13-infected C1qa−/− mice experienced greater body weight loss and had to be culled at day 11 (Fig. 2B). Examination of C1qa−/− lung tissue showed edema that was absent in the controls (Fig. 2C and FigS6A). Consistent with a vigorous CD8+ T-cell response, C1qa−/− mice showed increased LCMV-specific gp33+ and gp276+CD8+ T-cell populations (Fig. 2D and Fig. S6B). On day 8, when C1qa−/− mice still had numbers of LCMV-specific CD8+ T cells comparable to WT animals, virus-specific C1qa−/−CD8+ T cells were functionally over-reactive, with enhanced degranulation and cytokine production (Fig. 2E, 2F). Consistent with an enhanced CD8+ T-cell response, serum viral loads were lower in Cl13-infected C1qa−/− mice compared to WT mice (Fig. 2G). Moreover, the upregulation of PD-1 expression was similar in WT and C1qa−/−LCMV-specific CD8+ T cells, indicating that C1q deficiency did not impair the PD-1 signaling pathway (Fig. 2H). These findings demonstrate that C1q plays a pivotal role in regulating effector CD8+ T-cell responses in both autoimmunity and viral infection.
Fig. 2. Essential role for C1q in chronic LCMV infection.
(A) Numbers of splenic np396+, gp33+, and gp276+ CD8+ T cells and proportions of SLECs and MPECs among LCMV-specific CD8+ T cells in LCMV-Arm-infected WT and C1qa−/− mice at day 8 (n = 5-8 mice/group). (B to G) Analysis of WT and C1qa−/− mice infected with LCMV-Cl13. (B) Percentage of body weight loss (n = 5 mice/group). (C) Representative lung histology on day 11. Scale bars, 100 μm. (D) Numbers of splenic LCMV-specific CD8+ T cells at day 11 (n = 5 mice/group). (E to F) Percentages of CD8+ T cells positive for CD107a and the proportion of LCMV-specific CD8+ T cells producing IFN-γ, TNF-α, and IL-2 after incubation with LCMV gp33 peptide (E) or gp276 peptide (F) at day 8 (n = 6 mice/group). (G) Serum viral load measured using quantitative PCR. (H) PD-1 expression on LCMV-specific CD8+ T cells at day 8 after LCMV-Cl13 and LCMV-Arm. NS: not significant; *p<0.05, **p<0.01, ****p<0.0001; unpaired Student’s t-test (A, D to F); two-way ANOVA (B). Data are mean ± SEM and representative of two experiments.
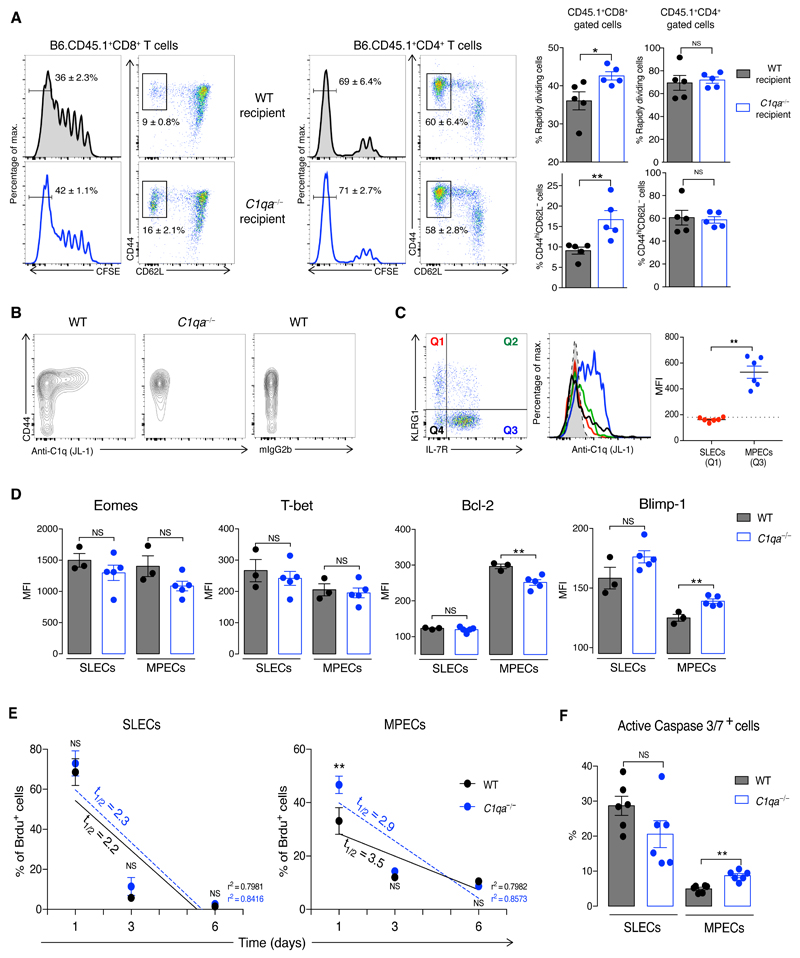
Complement can mediate its cellular effects via both extracellular and intracellular pathways (13). To explore how C1q affect CD8+ T cells, we co-transferred naïve CD8+ T cells, isolated from B6.CD45.1+ and C1qa−/−.CD45.2+ mice, into B6.CD45.1+.CD45.2+ mice, which were challenged with bm12 CD4+ T cells one day later. C1q-sufficient and C1q-deficient donor CD8+ T cells showed similar expansion and activation, suggesting a cell-extrinsic effect of C1q (Fig. S7). We corroborated this using the lymphopenia-induced proliferation model by co-transferring CFSE-labelled CD8+ and CD4+ T cells from B6.CD45.1+ mice into irradiated B6.CD45.2+ and C1qa−/−.CD45.2+ mice. Fourteen days later, donor B6.CD45.1+CD8+ T cells showed greater proliferation and activation in C1qa−/−.CD45.2+ recipients, whereas the co-transferred B6.CD45.1+CD4+ T cells were unaffected (Fig. 3A and Fig. S8A). As in the bm12-cGvHD model, C1q operated independently of C3 (Fig. S8B). Lymphopenia-induced T-cell expansion is triggered by low-affinity interactions (14). Analysis of OT-I proliferation with OVA peptides of different affinities showed that C1q had an inhibitory effect only in response to partial (T4) and weak (G4) agonists, but not to a strong (N4) ligand (Fig. S8C). Similarly, C1q inhibited human CD8+ T-cell activation, proliferation, and cytotoxic functions under suboptimal stimulation (Fig. S9). C1q was detected mainly on activated CD8+ T cells (mouse and human) (Fig. 3B and Fig. S10A) and almost exclusively on MPECs (Fig. 3C). Pre-incubation with the globular C1q region, but not with the collagen tail, inhibited C1q binding in a dose-dependent manner (Fig. S10B and S10C), indicating that C1q recognizes activated CD8+ T cells via its globular domain. Correspondingly, expression of the globular C1q receptor (p32/gC1qR) (15), a mitochondrial molecule present on the surface of several immune cells (Fig. S11A), was increased on activated mouse and human CD8+ T cells (Fig. S11B, S11C). Consistent with the preferential binding of C1q to MPECs (Fig. 3C), cGvH-treated C1qa−/−MPECs expressed lower levels of the anti-apoptotic factor Bcl-2 and higher levels of Blimp-1, a repressor that promotes cytotoxic T functions (16), than WT MPECs (Fig. 3D, Fig. S12A and S12B). Furthermore, the proportion of C1qa−/−MPECs, but not SLECs, secreting Granzyme B was higher compared to WT cells (Fig. S12C). Abnormal Bcl-2 expression in C1qa−/− mice suggests that C1q may influence MPEC viability. In cGvH-treated C1qa−/− mice, MPECs, but not SLECs, displayed a higher rate of BrdU decay compared to WT animals (Fig. 3E), indicating a more rapid turnover of this subpopulation. Moreover, the percentage of C1qa−/−MPECs expressing active Caspase 3/7 was higher (Fig. 3F and Fig. S12D). Altogether these findings suggest that C1q controls the programming and survival of MPECs through its globular domain.
Fig. 3. C1q selectively regulates MPEC programming and survival.
(A) Analysis of CFSE+CD45.1+CD8+ and CD45.1+CD4+T cells co-transferred in sub-lethally irradiated CD45.2+ WT or C1qa−/− hosts (n = 5 mice/group). Percentages of activated and fast proliferating donor T-cell subsets in spleen on day 14. (B) Flow cytometric gating of C1q staining on blood CD8+ T cells at day 14 after cGvHD. (C) KLRG1 and IL-7R expression in WT CD44+CD8+ T cells (left); histogram of C1q staining (middle) and quantification (right) on MPECs and SLECs. Dotted line indicates MFI of isotype control. Each symbol represents a mouse. (D) Expression of transcription factors in splenic SLECs and MPECs from WT and C1qa−/− mice two weeks after cGvH induction (n = 3-5 mice/group). (E) Decay of Brdu+SLECs and Brdu+MPECs over 6 days (from day 11 after cGvH induction) in WT and C1qa−/− mice. Half-life times (t1/2) of the decay and the R-squared value (r2) of the linear regressions are indicated (n = 6 mice/group). (F) Fractions of splenic SLECs and MPECs Caspase 3/7+ at week 3 after cGvHD (n = 6 mice/group). NS: not significant; *p<0.05, **p<0.01; unpaired Student’s t-test (A,C,D,F); two-way ANOVA (E). Data are mean ± SEM and representative of three experiments.
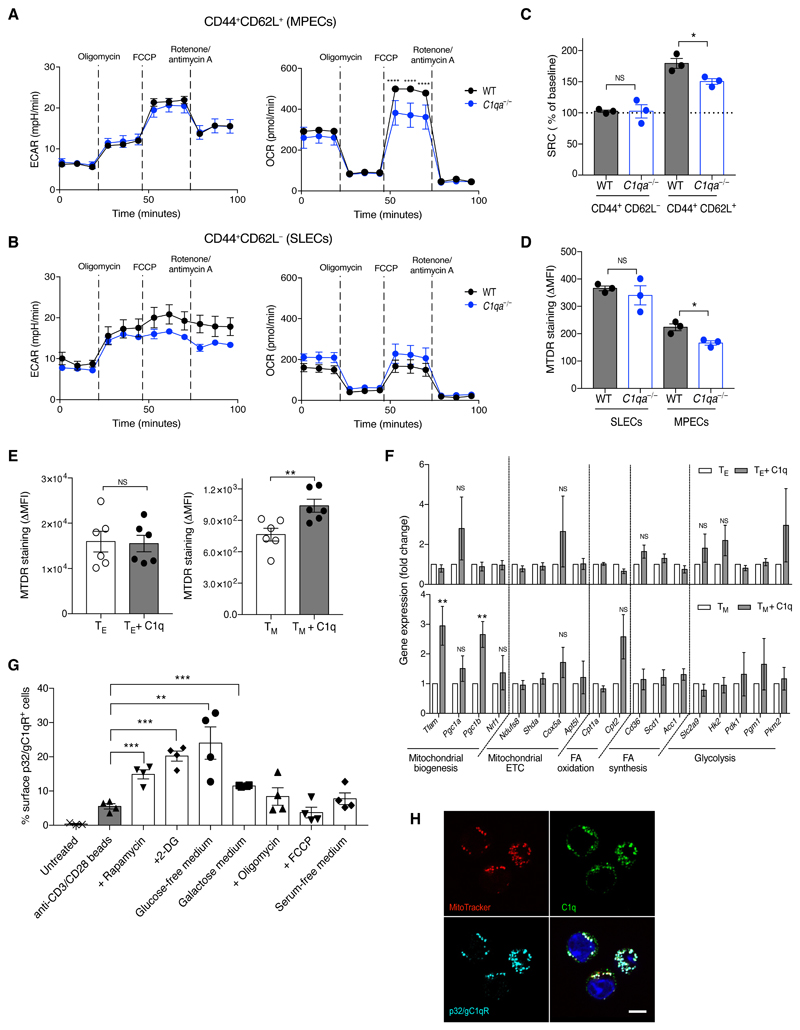
CD8+ T cells undergo major metabolic changes upon activation (17). CD44+CD62L+CD8+ (MPECs) and CD44+CD62L−CD8+ (SLECs) T cells from cGvH-treated C1qa−/− animals exhibited similar extracellular acidification rate (ECAR) and basal oxygen consumption rate (OCR) when compared to WT cells. However, C1qa−/−MPECs, but not SLECs, had impaired mitochondrial spare respiratory capacity (SRC) (Fig. 4A to 4C). SRC has been shown to correlate with mitochondrial mass (17) and MitoTracker staining showed reduced mitochondrial content in C1qa−/−MPECs compared to WT MPECs (Fig. 4D). The addition of C1q increased the MitoTracker staining (Fig. 4E) and upregulated the expression of mitochondrial biogenesis genes such as Tfam and Ppargc1b in IL-15-differentiated memory-committed OT-I cells, but not in IL-2-differentiated effector-like cells (Fig. 4F). Consistent with a C1q-dependent pathway regulating MPEC mitochondrial biogenesis, in vitro metabolic conditions favoring a MPEC molecular profile (18) promoted p32/gC1qR surface expression on activated CD8+ T cells (Fig. 4G and Fig. S13). The internalization of surface-bound C1q occurred via an endocytic pathway (Fig. S14) and C1q co-localised with p32/gC1qR in the mitochondria (Fig. 4H).
Fig. 4. C1q regulates mitochondrial metabolism in MPECs.
(A to B) ECAR and OCR under basal conditions and after mitochondrial inhibitors in sorted splenic CD8+ T cells from WT and C1qa−/− mice two weeks after cGvHD. Curve showing mean ± SEM of four technical replicates of pooled samples from four animals. Data representative of three experiments. (C) SRC of the CD8+ T-cell subsets as in A and B. Each symbol represents a biological replicate. (D) MTDR staining in SLECs and MPECs two weeks after cGvHD (n = 3 mice/group). (E) MTDR staining of in vitro IL-2- (TE) and IL-15- (TM) differentiated OT-I cells with and without hC1q (n = 6 mice/group). (F) Mitochondrial gene expression in TE and TM cells with and without hC1q (n = 5 mice/group). (G) Percentages of activated CD8+ T cells expressing p32/gC1qR under different metabolic conditions (n = 4 mice/group). (H) Confocal images of TM cells cultured with hC1q, stained with MitoTracker (red), anti-C1q (green), anti-p32/gC1qR (cyan) and DAPI (blue). Scale bar: 5 μm. NS: not significant; *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001; two-way ANOVA (A and B), unpaired Student’s t-test (C to G). Data are mean ± SEM and representative of three experiments (D to G). FCCP: fluorocarbonylcyanide phenylhydrazone; ∆MFI = MFI–FMO; MTDR: MitoTracker deep red; 2DG: 2-deoxyglucose.
Altogether these data link C1q to the metabolic reprogramming and regulation of activated CD8+ T cells and lead us to propose a new paradigm for the protective role of C1q in SLE: C1q limits tissue damage and autoimmunity by acting as a “metabolic rheostat” for effector CD8+ T cells that are capable of propagating autoimmunity via the generation of unique autoantigen fragments by granzyme B (19, 20) (Fig. S15). The role of CD8+ T cells in SLE has been largely overlooked and remains poorly characterized, with conflicting findings in human and animal studies (20–24), perhaps reflecting a changing role of these cells at different stages of the disease. By uncovering the role of effector CD8+ T cells in a lupus-like disease associated with C1q deficiency, our data demonstrate that an aberrant effector CD8+ T-cell response to viral infection may auto-amplify the breakdown of self-tolerance. This is in addition to molecular mimicry and the bystander activation of autoreactive T cells (25). Furthermore, very little is known about the metabolic profile of CD8+ T cells in SLE. As a CD8+ T-cell transcriptional signature can predict the clinical outcome (2, 3), it is conceivable that metabolic abnormalities in these cells play a key role. Our study showing that C1q, a key lupus susceptibility gene in humans, can influence the mitochondrial metabolism of CD8+ T cells demonstrates this link. As p32/gC1qR is ubiquitously present in mitochondria and is indispensable for mitochondrial function (26) one hypothesizes that the surface expression of p32/gC1qR coupled with another receptor may determine the specificity of the cellular effect(s) mediated by C1q. Thus, our findings describe a new paradigm to explain how C1q may prevent lupus flares and highlight the importance of the interplay between complement and immunometabolism in autoimmunity.
Supplementary Materials
One Sentence Summary.
C1q regulates the development of balanced effector CD8+ T-cell responses by acting as a rheostat of mitochondrial metabolism.
Acknowledgments
We thank the staff of the Imperial Central Biomedical Services for the care of the animals. We thank L Lawrence for histological processing of the samples, HT Cook for histological analysis, N Shaikh for technical support, D Carling and G Chennell for Seahorse analysis, C. Reis e Sousa for providing the OVA-MEFs and E Simpson for critical reading of the paper.
Funding: This work was supported by the Wellcome Trust (Grant reference number: 108008/Z/15/Z) (to M.B.). J.A.H. is recipient of a Wellcome Trust and Royal Society Sir Henry Dale Fellowship (101372/Z/13/Z). We also acknowledge the contribution from the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/.
The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Authors contributions: G.S.L. performed the experiments with the assistance from G.C., I.B., Z.B., K.T., S.R.; N.M.T., I.B., P.A-R. provided key reagents; J.A.H. and J.S. assisted with data analysis and interpretation; M.B. supervised and conceived the project. M.B. and G.S.L. wrote the paper. All authors commented on the manuscript.
Competing interests: Authors declare no competing interests.
All data are available in the main text or the supplementary materials
References
- 1.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 2.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinney EF, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–591. doi: 10.1038/nm.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ. Lupus, DNase and defective disposal of cellular debris. Nat Genet. 2000;25:135–136. doi: 10.1038/75963. [DOI] [PubMed] [Google Scholar]
- 6.Martin M, Blom AM. Complement in removal of the dead - balancing inflammation. Immunol Rev. 2016;274:218–232. doi: 10.1111/imr.12462. [DOI] [PubMed] [Google Scholar]
- 7.Elkon KB, Santer DM. Complement, interferon and lupus. Curr Opin Immunol. 2012;24:665–670. doi: 10.1016/j.coi.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Thielens NM, Tedesco F, Bohlson SS, Gaboriaud C, Tenner AJ. C1q: a fresh look upon an old molecule. Mol Immunol. 2017;89:73–83. doi: 10.1016/j.molimm.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg RA, Via CS. T cells, murine chronic graft-versus-host disease and autoimmunity. J Autoimmun. 2012;39:240–247. doi: 10.1016/j.jaut.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelenay S, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122:1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 13.Freeley S, Kemper C, Le Friec G. The "ins and outs" of complement-driven immune responses. Immunol Rev. 2016;274:16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multiligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Welsh RM. Blimp hovers over T cell immunity. Immunity. 2009;31:178–180. doi: 10.1016/j.immuni.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukumar M, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco P, et al. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 21.Kis-Toth K, et al. Selective Loss of Signaling Lymphocytic Activation Molecule Family Member 4-Positive CD8+ T Cells Contributes to the Decreased Cytotoxic Cell Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016;68:164–173. doi: 10.1002/art.39410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couzi L, et al. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 2007;56:2362–2370. doi: 10.1002/art.22654. [DOI] [PubMed] [Google Scholar]
- 23.Mozes E, Kohn LD, Hakim F, Singer DS. Resistance of MHC class I-deficient mice to experimental systemic lupus erythematosus. Science. 1993;261:91–93. doi: 10.1126/science.8316860. [DOI] [PubMed] [Google Scholar]
- 24.Mozes E, Lovchik J, Zinger H, Singer DS. MHC class I expression regulates susceptibility to spontaneous autoimmune disease in (NZBxNZW)F1 mice. Lupus. 2005;14:308–314. doi: 10.1191/0961203305lu2079oa. [DOI] [PubMed] [Google Scholar]
- 25.Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255:197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi M, et al. p32/gC1qR is indispensable for fetal development and mitochondrial translation: importance of its RNA-binding ability. Nucleic Acids Res. 2012;40:9717–9737. doi: 10.1093/nar/gks774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 28.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman RH, et al. Skin allograft rejection is suppressed in mice lacking the antiviral enzyme, 2',5'-oligoadenylate-dependent RNase L. Viral Immunol. 2002;15:77–83. doi: 10.1089/088282402317340242. [DOI] [PubMed] [Google Scholar]
- 30.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Maldonado MA, Madaio M, Eisenberg RA. The role of host (endogenous) T cells in chronic graft-versus-host autoimmune disease. J Immunol. 1998;161:5880–5885. [PubMed] [Google Scholar]
- 32.McCausland MM, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J Virol Methods. 2008;147:167–176. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harker JA, Dolgoter A, Zuniga EI. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4(+) T cell responses and viral control during chronic infection. Immunity. 2013;39:548–559. doi: 10.1016/j.immuni.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering MC, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 35.Zafirova B, et al. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31:270–282. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CH, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacnet-Delorme P, Chevallier S, Arlaud GJ. Beta-amyloid fibrils activate the C1 complex of complement under physiological conditions: evidence for a binding site for A beta on the C1q globular regions. J Immunol. 2001;167:6374–6381. doi: 10.4049/jimmunol.167.11.6374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.