Abstract
Background
The usefulness of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography for evaluating the treatment efficacy of breast cancers is well-established; however, the predictive values of parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) remain unknown.
Methods
This study examined 199 breast cancers treated with primary systemic chemotherapy (PSC) followed by operation, and determined the values of maximum standardized uptake value (SUVmax), peak SUV (SUVpeak), mean SUV (SUVmean), MTV, and TLG at baseline. Among these cases, data on early changes in these metabolic parameters in 70 breast cancers were also assessed.
Results
A pathological complete response (pCR) was achieved in 64 breast cancers. Breast cancers with low MTV at baseline had a significantly higher pCR rate than breast cancers with high MTV (47.9% vs. 23.4%; p = 0.0005). High reduction rates (∆) of SUVmax (p = 0.0001), SUVpeak (p = 0.0001), and SUVmean (p < 0.0001) resulted in an increased pCR compared with those for low ∆. The pCR rate was highest for the combination of low MTV and high ∆SUVmean (86.7%), and lowest for high MTV and low ∆SUVmean (15.4%); the remaining combinations were intermediate (58.6%; p < 0.0001). The combination of low MTV at baseline and high ∆SUVmean was a significant and independent predictor for pCR (odds ratio 28.63; 95% confidence interval 1.94–422.42; p = 0.0146) in multivariable analysis.
Conclusions
Low levels of MTV at baseline and a high reduction of SUVmean after PSC was significantly associated with pCR. These findings suggest the usefulness of these metabolic parameters for predicting the treatment efficacy of breast cancers.
Electronic supplementary material
The online version of this article (10.1245/s10434-019-07325-8) contains supplementary material, which is available to authorized users.
Pathological complete response (pCR), defined as the lack of residual cancer after neoadjuvant chemotherapy (NAC), has been established as a surrogate marker for excellent prognosis of operable breast cancers. Small tumor size, higher tumor grade, and high proliferative activity are significant predictors for a high rate of pCR.1 However, the sensitivity and specificity of predicting pCR using these clinical factors are not high2,3 and the identification of more precise predictive factors is a critical issue.
In addition to the diagnostic ability of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT),4 the usefulness of the maximum standardized uptake value (SUVmax) on FDG-PET for predicting the prognosis of operable breast cancers is well established.5–9 The value of SUVmax as a predictive tool for treatment efficacy was reported in metastatic10 and early breast cancers.11 In the NAC setting, a significant association between an increased SUVmax and a high rate of pCR has been reported.12,13 In addition to baseline values, changes in these values after treatment are significantly associated with the response to chemotherapy.14 Since altered glucose metabolism occurs earlier than tumor shrinkage,15 it may be possible to evaluate the treatment efficacy as early as after one or two treatment cycles.
SUVmax levels have been reported to be inaccurate compared with the actual uptake of FDG, due to the partial volume effect.16 Since volume-based parameters on FDG-PET, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG), evaluate not only metabolic activity but also total tumor burden, recent studies have focused on these metabolic parameters rather than on SUVmax for predicting the response to chemotherapy. Although early reductions of SUVmax are reportedly associated with improved response to NAC,17,18 which metabolic parameter offers the most precise prediction remains unknown. In addition, the superiority of baseline or early response to treatment PET data is also undetermined.
The present study explored the predictive values of SUVmax, SUVpeak, SUVmean, and volume-based parameters, including MTV and TLG, at baseline in breast cancers treated with primary systemic chemotherapy (PSC). Additionally, early changes in these parameters after treatment were also investigated in terms of their relationships with treatment efficacy.
Patients and Methods
Patient Recruitment
This retrospective study constitutively recruited a total of 267 breast cancer patients who underwent surgery after preoperative chemotherapy between October 2008 and May 2018. Among these participants, 194 patients who underwent FDG-PET/CT before starting PSC were selected. Since five patients had bilateral breast cancers, we analyzed a total of 199 breast cancers in 194 patients. We evaluated response in the primary sites (breast), and nine patients with stage IV who had PSC followed by operation were also included. We also obtained FDG-PET/CT data after the start of PSC for 69 patients (70 breast cancers).
The Ethics Committee of Hyogo College of Medicine approved the present study (numbers 1818 and 1708), and written informed consent was obtained from all 69 participants who underwent FDG-PET/CT after the start of PSC (number 1708, UMIN000030408). In the remaining patients, only baseline FDG-PET/CT data from clinical practice were used and offered no risk to participants; thus, written informed consent was not required (number 1818).
Chemotherapy Regimen and Evaluation of Pathological Response
Preoperative chemotherapies involving anthracycline-containing, taxane-based, sequential use of anthracycline-containing and taxane, and unspecified regimens were administered in 6, 48, 137, and 3 patients, respectively. Concurrent use of trastuzumab with chemotherapy was administered to 64 patients. Pathological examinations of the whole area of pre-existing breast cancer lesions were performed, and pCR was defined as complete absence of invasive cancer cells in the breast.19
18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT) Procedure
FDG-PET/CT was performed using a Gemini GXL16 or Gemini TF64 PET/CT scanner (Philips Medical Systems, Eindhoven, The Netherlands) following injection of 4.0 or 3.0 MBq/kg body weight FDG for the GXL16 and TF64, respectively. Scanning images were obtained approximately 60 min after injection, as described previously.20 The 194 patients underwent FDG-PET/CT examination before starting PSC, of whom 69 (70 breast cancers) underwent a repeat FDG-PET/CT examination after starting chemotherapy. We obtained FDG-PET/CT data after one cycle of PSC (2–3 weeks after the start of chemotherapy), except for one patient whose data were obtained after two cycles (electronic supplementary data).
Imaging Analyses
To quantify 18F-FDG uptake, the SUVs were measured. We set the volume of interest (VOI) as the area in which FDG accumulated in the breast, along the margin of tumor uptake. The SUV was calculated as the regional radioactivity concentration (Bq/mL)/[injected dose (Bq)/patient weight (g)] in the most intense area of 18F-FDG accumulation (a region of interest [ROI]). We selected the region containing the tumor in which the FDG in the breast was accumulated, as observed on the image, and set a target VOI manually in the breast cancer primary lesion with FDG accumulation. The maximum value of SUV in the VOI was defined as the SUVmax, and the volume of voxels of ≥ 40% of the SUVmax in the VOI was defined as the MTV.21–23 The SUVpeak was defined as the average activity concentration within a 1 cm3 spherical VOI centered on the ‘hottest focus’ within the primary tumor. The average SUV value in the voxel that showed ≥ 40% was defined as the SUVmean and TLG was defined as MTV × SUVmean. These parameters were all automatically calculated by the computer software package GI-PET (AZE Co., Ltd, Tokyo, Japan). Harmonization of data in different PET/CT systems was performed using phantom data.
The percentage changes (∆%) of PET data at baseline and after the start of PSC in each of the five parameters were calculated as follows: percentage change (∆%) = (delayed parameter − baseline parameter)/baseline parameter × 100.
Statistical Analysis
The associations of clinicopathological characteristics between breast cancers that achieved pCR and those that did not were analyzed using the Fisher’s exact or Wilcoxon rank-sum tests. The relationships between pCR and levels of each metabolic parameter were calculated using Fisher’s exact tests, and logistic regression was used to obtain odds ratios (ORs) and 95% confidence intervals (CIs) by univariable and multivariable analyses of clinical factors or metabolic parameters and pCR. Statistical significance was set at p < 0.05. All statistical calculations were performed using JMP Pro 13 (SAS Institute Inc., Cary, NC, USA).
Results
Relationships Between Clinicopathological Factors and Pathological Response
We defined pCR and non-pCR in 64 and 135 breast cancers, respectively. The pCR rates were significantly higher in those with small tumor size, higher nuclear grade, estrogen receptor (ER)-negative/human epidermal growth factor receptor 2 (HER2)-negative (triple-negative [TN]) and HER2-positive subtypes, high levels of Ki67, and an anthracycline and taxane regimen (electronic supplementary Table 1 and data). The SUVmax, SUVpeak, and SUVmean were significantly associated with nuclear grade and Ki67 expression levels. There were significant associations between tumor size and all parameters except SUVmax, and the subtypes were significantly associated with all parameters except TLG. Lymph node metastasis was significantly associated with MTV and TLG (electronic supplementary Table 2).
Determination of the Optimal Cut-Off Values for Pathological Complete Response (pCR) of Metabolic Parameters by PET/CT at Baseline and During Primary Systemic Chemotherapy
Representative cases of PET imaging are shown in Fig. 1. The FDG uptake detected in the left breast at baseline was diminished after one cycle of chemotherapy (Fig. 1a, b) in patients who achieved pCR; however, the uptake of FDG at baseline in the right breast remained after one cycle of chemotherapy (Fig. 1c, d). The cut-off values of SUVmax, SUVpeak, SUVmean, MTV, and TLG at baseline for pCR were determined using receiver operating characteristic (ROC) curves calculated using the Youden index for the areas under the curve (AUC) (electronic supplementary Fig. 1). Similar methods were used to determine the cut-off values of the reduction rate in each metabolic parameter for pCR (electronic supplementary Fig. 2).
Fig. 1.
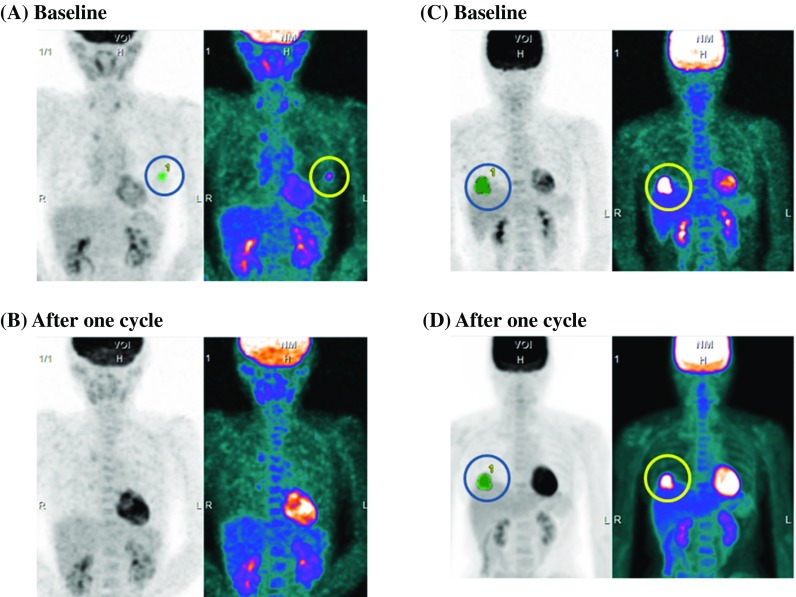
Representative cases of FDG accumulation in breast cancers at baseline and after the start of primary systemic chemotherapy. a Baseline and b after one treatment cycle in a case of breast cancer that achieved a pCR. c Baseline and d after one treatment cycle of a case of breast cancer that did not achieve a pCR. FDG fluorodeoxyglucose, pCR pathological complete response
Associations Between pCR After PSC and Each Metabolic Parameter
Breast cancers with high baseline levels of SUVmax, SUVpeak, and SUVmean had a significantly higher rate of pCR than breast cancers with low levels. However, the frequency of pCR was significantly higher for low baseline levels of MTV and TLG (electronic supplementary Fig. 3). Of these baseline parameters, MTV was the most significant predictor for pCR (47.9% vs. 23.4%; p = 0.0005).
Similarly, breast cancers with high reduction rates had significantly higher frequencies of pCR for ∆SUVmax, ∆SUVpeak, ∆SUVmean, and ∆TLG, but not ∆MTV, in which the significance was marginal (electronic supplementary Fig. 4). The difference in pCR rates between the two groups was most significant for the ∆SUVmean (72.2% vs. 23.5%; p < 0.0001).
Univariable and Multivariable Analyses of pCR, Including Metabolic Parameters at Baseline and During Treatment
First, we analyzed data of all 199 breast cancers, including metabolic parameters at baseline. Tumor size, nuclear grade, Ki67 expression levels, subtypes, chemotherapy regimen, and all metabolic parameters at baseline, including SUVmax, SUVpeak, SUVmean, MTV, and TLG, were significantly associated with pCR in univariable analysis (Table 1). The multivariable analyses included these clinical factors with one of the metabolic parameters. As shown in Table 1, baseline MTV was significantly associated with pCR in multivariable analysis (OR 0.30, 95% CI 0.11–0.84; p = 0.0212).
Table 1.
Univariable and multivariable analyses of the clinicopathological characteristics and metabolic parameters at baseline for pathological complete response in 199 breast cancers
| n | Univariable | p value | Multivariable | p value | |
|---|---|---|---|---|---|
| [OR (95% CI)] | [OR (95% CI)] | ||||
| Menopausal status | |||||
| Premenopausal | 80 | 1.00 | |||
| Postmenopausal | 119 | 1.59 (0.85–2.96) | 0.1447 | ||
| T size (cm) | |||||
| ≤ 2.0 | 43 | 1.00 | 1.00 | ||
| > 2 | 156 | 0.40 (0.20–0.80) | 0.0093 | 0.40 (0.13–1.20) | 0.1024 |
| Lymph node metastasis | |||||
| Negative | 104 | 1.00 | |||
| Positive | 95 | 0.65 (0.36–1.20) | 0.1677 | ||
| Nuclear grade | |||||
| 1 | 74 | 1.00 | 1.00 | ||
| 2 + 3 | 115 | 5.76 (2.62–12.66) | < 0.0001 | 3.14 (1.09–9.04) | 0.0342 |
| Ki67 expression levelsa | |||||
| Low | 50 | 1.00 | 1.00 | ||
| High | 139 | 9.74 (2.89–32.89) | 0.0002 | 20.58 (1.55–237.07) | 0.0219 |
| Subtypesb | |||||
| TN | 48 | 1.00 | 1.00 | ||
| Luminal A | 31 | 0.04 (0.01–0.34) | 0.0029 | 2.23 (0.08–65.78) | 0.6418 |
| Luminal B | 49 | 0.18 (0.06–0.50) | 0.0010 | 0.20 (0.06–0.67) | 0.0114 |
| Luminal-HER2 | 36 | 0.57 (0.23–1.41) | 0.2198 | 1.18 (0.34–4.09) | 0.7908 |
| HER2 | 33 | 3.43 (1.32–8.91) | 0.0114 | 5.45 (1.53–19.41) | 0.0089 |
| Chemotherapy regimen | |||||
| Taxane | 48 | 1.00 | 1.00 | ||
| Anthracycline and taxane | 142 | 3.26 (1.42–7.47) | 0.0053 | 2.83 (0.92–8.77) | 0.0707 |
| SUVcmax | |||||
| Low | 60 | 1.00 | |||
| High | 139 | 2.10 (1.04–4.24) | 0.0396 | Not included | |
| SUVdpeak | |||||
| Low | 62 | 1.00 | |||
| High | 137 | 2.24 (1.11–4.51) | 0.0249 | Not included | |
| SUVemean | |||||
| Low | 47 | 1.00 | |||
| High | 152 | 2.84 (1.24–6.52) | 0.0135 | Not included | |
| MTVf | |||||
| Low | 71 | 1.00 | |||
| High | 128 | 0.33 (0.18–0.62) | 0.0005 | 0.30 (0.11–0.84) | 0.0212 |
| TLGg | |||||
| Low | 110 | 1.00 | |||
| High | 89 | 0.43 (0.23–0.81) | 0.0092 | Not included | |
OR odds ratio, CI confidence interval, TN triple-negative, ER estrogen-receptor, HER2 human epidermal growth factor receptor 2, SUVmax maximum standardized uptake value, SUVpeak peak standardized uptake value, SUVmean mean standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis
aLow < 20%, high ≥ 20%
bTN, ER-negative/HER2-negative; Luminal A, ER-positive/HER2-negative with Ki67 < 20%; Luminal B, ER-positive/HER2-negative with Ki67 ≥ 20%; Luminal-HER2, ER-positive/HER2-positive; HER2, ER-negative/HER2-positive
cLow < 3.664, high ≥ 3.664
dLow < 3.279, high ≥ 3.279
eLow < 1.782, high ≥ 1.782
fLow < 4.416, high ≥ 4.416
gLow < 20.138, high ≥ 20.138
Data of metabolic parameters during treatment in 70 breast cancers were further analyzed. All of ∆SUVmax, ∆SUVpeak, ∆SUVmean, and ∆TLG were significant predictive factors for pCR in the univariable analysis (Table 2). Since the association between pCR and ∆SUVmean was most significant, we performed multivariable analysis, including only ∆SUVmean as a metabolic parameter, and identified ∆SUVmean as a significant and independent factor, as shown in Table 2 (OR 8.05, 95% CI 1.45–44.80; p = 0.0173).
Table 2.
Univariable and multivariable analyses of the clinicopathological characteristics and the reduction of metabolic parameters after the start of chemotherapy for pathological complete response in 70 breast cancers
| n | Univariable | p value | Multivariable | p value | |
|---|---|---|---|---|---|
| [OR (95% CI)] | [OR (95% CI)] | ||||
| Menopausal status | |||||
| Premenopausal | 31 | 1.00 | |||
| Postmenopausal | 39 | 1.28 (0.50–3.29) | 0.6110 | ||
| T size (cm) | |||||
| ≤ 2.0 | 14 | 1.00 | 1.00 | ||
| > 2 | 56 | 0.11 (0.02–0.53) | 0.0060 | 0.07 (0.004–1.03) | 0.0522 |
| Lymph node metastasis | |||||
| Negative | 29 | 1.00 | |||
| Positive | 41 | 0.64 (0.24–1.66) | 0.3538 | ||
| Nuclear grade | |||||
| 1 | 16 | 1.00 | 1.00 | ||
| 2 + 3 | 50 | 11.42 (2.33–55.88) | 0.0026 | 14.89 (1.07–207.75) | 0.0446 |
| Ki67 expression levelsa | |||||
| Low | 9 | 1.00 | |||
| High | 58 | 8.57 (1.01–72.98) | 0.0493 | Not calculated | |
| Subtypesb | |||||
| TN | 19 | 1.00 | 1.00 | ||
| Luminal A | 7 | Not calculated | Not calculated | ||
| Luminal B | 18 | 0.09 (0.02–0.45) | 0.0030 | 0.11 (0.01–0.84) | 0.0335 |
| Luminal-HER2 | 11 | 0.55 (0.12–2.56) | 0.4494 | 0.34 (0.04–3.01) | 0.3352 |
| HER2 | 14 | 1.69 (0.34–8.40) | 0.5197 | 0.71 (0.07–6.79) | 0.7692 |
| Chemotherapy regimen | |||||
| Taxane | 6 | 1.00 | |||
| Anthracycline and taxane | 63 | 5.50 (0.61–49.80) | 0.1294 | ||
| ∆SUVcmax | |||||
| Low | 33 | 1.00 | |||
| High | 37 | 7.39 (2.55–21.39) | 0.0002 | Not included | |
| ∆SUVdpeak | |||||
| Low | 33 | 1.00 | |||
| High | 37 | 7.39 (2.55–21.39) | 0.0002 | Not included | |
| ∆SUVemean | |||||
| Low | 34 | 1.00 | |||
| High | 36 | 8.45 (2.88–24.81) | 0.0001 | 8.05 (1.45–44.80) | 0.0173 |
| ∆MTVf | |||||
| Low | 26 | 1.00 | |||
| High | 44 | 2.49 (0.91–6.79) | 0.0756 | Not included | |
| ∆TLGg | |||||
| Low | 32 | 1.00 | |||
| High | 38 | 6.50 (2.27–18.62) | 0.0005 | Not included | |
OR odds ratio, CI confidence interval, TN triple-negative, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, SUVmax maximum standardized uptake value, SUVpeak peak standardized uptake value, SUVmean mean standardized uptake value, MTV metabolic tumor volume, TLG total lesion glycolysis, ∆ reduction rate
aLow < 20%, high ≥ 20%
bTN, ER-negative/HER2-negative; Luminal A, ER-positive/HER2-negative with Ki67 < 20%; Luminal B, ER-positive/HER2-negative with Ki67 ≥ 20%; Luminal-HER2, ER-positive/HER2-positive; HER2, ER-negative/HER2-positive
cLow < − 56.3, high ≥ − 56.3
dLow < − 55.1, high ≥ − 55.1
eLow < − 55.8, high ≥ − 55.8
fLow < − 22.2, high ≥ − 22.2
gLow < − 65.0, high ≥ − 65.0
Predictive Ability of pCR for the Combination of Baseline Metabolic Tumor Volume (MTV) and ∆SUVmean Parameters
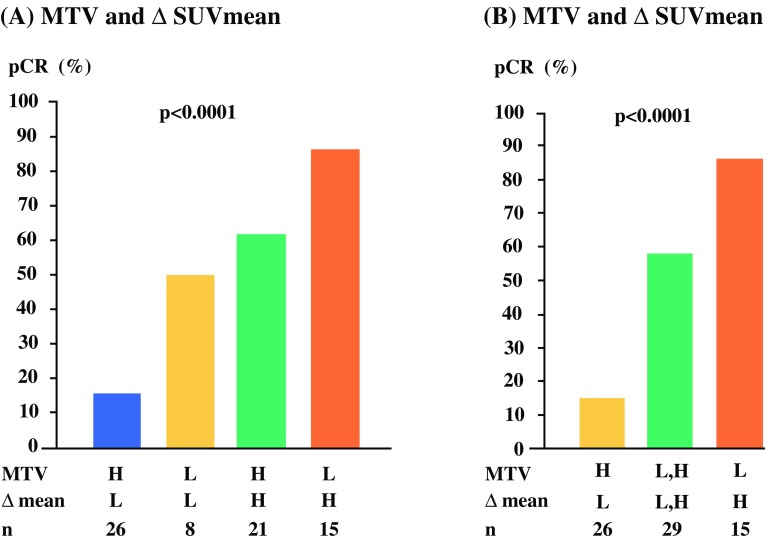
Since both baseline MTV and ∆SUVmean were significantly associated with pCR, we further analyzed the combination of these parameters. The pCR rate was highest for low baseline MTV and high ∆SUVmean (86.7%), and lowest for high baseline MTV and low ∆SUVmean (15.4%) [Fig. 2a]. Since breast cancers with high baseline MTV and high ∆SUVmean, as well as those with low baseline MTV and low ∆SUVmean, showed intermediate pCR rates (61.9% and 50%, respectively), we further combined these two intermediate groups in Fig. 2b (pCR rate 58.6%). In multivariable analysis, the combination of baseline MTV and ∆SUVmean was a significant and independent predictor of pCR (OR 28.63, 95% CI 1.94–422.42; p = 0.0146 for low baseline MTV and high ∆SUVmean) (Table 3).
Fig. 2.
pCR rates according to the combination of MTV at baseline and ∆SUVmean. The pCR rates of a four groups and b three groups after combining both high and both low MTV and ∆SUVmean. pCR pathological complete response, MTV metabolic tumor volume, SUVmean mean standardized uptake value, H high, L low, ∆ reduction rate
Table 3.
Univariable and multivariable analyses of the clinicopathological characteristics and the combination of MTV at baseline with the reduction of SUVmean in 70 breast cancers
| n | Univariable analysis | p value | Multivariable analysisa | p value | |
|---|---|---|---|---|---|
| [OR (95% CI)] | [OR (95% CI)] | ||||
| MTVb | |||||
| Low | 47 | 1.00 | 1.00 | ||
| High | 23 | 0.20 (0.07–0.60) | 0.0043 | 0.18 (0.03–1.19) | 0.0749 |
| ∆SUVcmean | |||||
| Low | 34 | 1.00 | |||
| High | 36 | 8.45 (2.88–24.81) | 0.0001 | 8.05 (1.45–44.80) | 0.0173 |
| MTV and ∆SUVmean | |||||
| High and low | 26 | 1.00 | 1.00 | ||
| Both high, or both low | 29 | 7.79 (2.13–28.49) | 0.0019 | 6.89 (1.10–43.24) | 0.0394 |
| Low and high | 15 | 35.75 (5.73–223.00) | 0.0001 | 28.63 (1.94–422.42) | 0.0146 |
MTV metabolic tumor volume, SUVmean mean standardized uptake value, OR odds ratio, CI confidence interval, pCR pathological complete response, ∆ reduction rate
aAdjusted for tumor size, nuclear grade, and subtypes that were significantly associated with pCR by univariable analysis in Table 2
bLow < 4.416, high ≥ 4.416
cLow < − 55.8, high ≥ − 55.8
Discussion
The results of the present study demonstrated that low levels of baseline MTV and early reduction of SUVmean after the start of treatment were significant and independent predictive factors for a higher rate of pCR in breast cancers treated with PSC. The combination of both parameters predicts pCR more precisely compared with that of baseline MTV or ∆SUVmean alone. The SUVmax was a significant predictor of pCR after NAC in 273 breast cancers (OR per one-unit increase 1.09, 95% CI 1.02–1.16; p = 0.008).24 However, consistent with our study, SUVmax, SUVpeak, and SUVmean at baseline were not associated with pathological response in previous studies.21,25–29 Contrary to SUVs, MTV is a volume-based metabolic parameter that represents both metabolic activity and total tumor burden in each tumor. However, Cho et al. reported no significant association between pCR and baseline TLG or MTV values.27 Although Cheng et al. reported no correlation between TLG and pCR,21 baseline MTV was marginally associated with pCR in the HER2-negative group (n = 30; p = 0.081). The reason for this discrepancy may be the smaller number of participants (n = 26 and n = 30) compared with our study (n = 199).
Although SUVmax levels at baseline and after one cycle were not correlated with pCR in 50 TN breast cancers, higher ∆SUVmax values were significantly associated with increased pCR in multivariable analysis (OR 7.1; p = 0.014).30 Similarly, early changes in SUVmax corrected for lean body mass (SULmax) values between those achieving pCR and those not achieving pCR differed significantly in 59 HER2-negative breast cancers (63.0% vs. 32.9%; p = 0.003).31 Further studies demonstrated that not at baseline, but rather ∆SUVmax after the start of NAC, was significantly associated with pCR.25,28,29,32,33 In addition, the mean percentage of ∆TLG30% (p = 0.005), but not ∆MTV30% (p = 0.262), was significantly greater in the pCR group27; however, neither ∆TLG nor ∆MTV were significantly associated with pCR in the report by Cheng et al.21 Despite the significant correlation between tumor size reduction rate and the reduction rates of MTV (p = 0.0004) or TLG (p = 0.002), but not SUVmax (p = 0.07),34 the ∆MTV and ∆TLG might be less useful than ∆SUVmax when considering their pCR predicting ability.
Groheux et al. reported the AUC of pCR prediction increased from 0.63 to 0.76 when combined ∆SUVmax with genomic grade index (GGI; p = 0.016) in TN breast cancer patients.28 We identified the significance of the combination of baseline MTV and ∆SUVmean in terms of pCR prediction. Interestingly, MTV was significantly associated with tumor size, but not with grade. Conversely, SUVmax was significantly associated with grade, but not with tumor size.33 Thus, MTV and ∆SUV may be a useful combination for predicting pCR mediating through different mechanisms. Small metabolic tumor size evaluable by MTV and high reduction rate of metabolic activity evaluable by SUVmean may be linked to achieving a pCR. We obtained data regarding metabolic parameters after treatment in 22 breast cancers; all six breast cancers that retained FDG uptake had non-pCR. Even though FDG uptake diminished after treatment, 5 of 16 (31.3%) breast cancers were defined as non-pCR (electronic supplementary data). Thus, the data obtained after treatment may not improve the ability to predict pCR.
We set the optimal cut-off values of ∆SUVmax, ∆SUVpeak, and ∆SUVmean at 56.3%, 55.1%, and 55.8%, respectively, and the predictive values of these metabolic parameters were similar. Previous studies reported ∆SUVmax cut-off values ranging from 50 to 82.2%.35 In addition, we used the average SUV value in the voxel that showed ≥ 40% of SUVmax as the SUVmean, as used in previous studies.21–23 In other studies, thresholds of VOI were set to values between 30 and 50%.27 Although the best threshold was unknown, we obtained similar results when calculated with other cut-off values and the reproducibility of SUVmean measurement was confirmed by a coauthor (data not shown). Issues regarding which parameter of SUV is most useful, and the best optimal cut-off value or threshold, require confirmation in future studies. In addition, we concluded, based on 70 breast cancers, that the sample size was not enough. Further studies involving large numbers of participants are needed. To our knowledge, this is the first study to demonstrate the useful combination of metabolic parameters obtained by PET for pCR in breast cancers treated with preoperative chemotherapy.
Conclusions
The combination of baseline MTV and ∆SUVmean precisely predicted the pCR in breast cancers treated with chemotherapy. The predictive value of this combination was independent and strong compared with that of other clinical factors, including tumor size, tumor grade, Ki67 levels, and subtypes. Small metabolic tumor size evaluable by MTV, and a high metabolic activity reduction as determined by SUVmean, might be useful for predicting improved pCR in breast cancers treated with preoperative chemotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 26461963).
Disclosure
Tomoko Higuchi, Yukie Fujimoto, Hiromi Ozawa, Ayako Bun, Reiko Fukui, Yoshimasa Miyagawa, Michiko Imamura, Kazuhiro Kitajima, Koichiro Yamakado, and Yasuo Miyoshi declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mazouni C, Kau SW, Frye D, et al. Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Annals of Oncology. 2007;18:874–880. doi: 10.1093/annonc/mdm008. [DOI] [PubMed] [Google Scholar]
- 2.Munkacsy G, Szasz MA, Menyhart O. Gene expression-based prognostic and predictive tools in breast cancer. Breast Cancer. 2015;22:245–252. doi: 10.1007/s12282-015-0594-y. [DOI] [PubMed] [Google Scholar]
- 3.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CY, Lin CL, Kao CH. Staging/restaging performance of F18-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in breast cancer: a review and meta-analysis. Eur J Radiol. 2018;107:158–165. doi: 10.1016/j.ejrad.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat. 2013;141:269–275. doi: 10.1007/s10549-013-2687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn SG, Park JT, Lee HM, et al. Standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography for prediction of tumor recurrence in breast cancer beyond tumor burden. Breast Cancer Res. 2014;16:502. doi: 10.1186/s13058-014-0502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JS, Lee J, Kim HJ, et al. (18)F-FDG/PET may help to identify a subgroup of patients with T1-T2 breast cancer and 1-3 positive lymph nodes who are at a high risk of recurrence after mastectomy. Cancer Res Treat. 2016;48:508–517. doi: 10.4143/crt.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evangelista L, Cervino AR, Ghiotto C, et al. Could semiquantitative FDG analysis add information to the prognosis in patients with stage II/III breast cancer undergoing neoadjuvant treatment? Eur J Nucl Med Mol Imaging. 2015;42:1648–1655. doi: 10.1007/s00259-015-3088-4. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi T, Nishimukai A, Ozawa H, et al. Prognostic significance of preoperative 18F-FDG PET/CT for breast cancer subtypes. Breast. 2016;30:5–12. doi: 10.1016/j.breast.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Goulon D, Necib H, Henaff B, Rousseau C, Carlier T, Kraeber-Bodere F. Quantitative Evaluation of Therapeutic Response by FDG-PET-CT in Metastatic Breast Cancer. Front Med (Lausanne) 2016;3:19. doi: 10.3389/fmed.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade WP, Lima EN, Osório CA, et al. Can FDG-PET/CT predict early response to neoadjuvant chemotherapy in breast cancer? Eur J Surg Oncol. 2013;39:1358–1363. doi: 10.1016/j.ejso.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Avril S, Muzic RF, Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. 18F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer. J Nucl Med. 2016;57:34S–39S. doi: 10.2967/jnumed.115.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groheux D, Mankoff D, Espie M, Hindie E. 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43:983–993. doi: 10.1007/s00259-015-3295-z. [DOI] [PubMed] [Google Scholar]
- 14.Akimoto E, Kadoya T, Kajitani K, et al. Role of 18F-PET/CT in Predicting Prognosis of Patients With Breast Cancer After Neoadjuvant Chemotherapy. Clin Breast Cancer. 2018;18:45–52. doi: 10.1016/j.clbc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 17.Groheux D, Sanna A, Majdoub M, et al. Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER+/HER2- breast cancer. J Nucl Med. 2015;56:824–831. doi: 10.2967/jnumed.115.154138. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Lee HM, Choi SE, et al. The Prognostic Impact of Early Change in 18F-FDG PET SUV After Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer. J Nucl Med. 2016;57:1183–1188. doi: 10.2967/jnumed.115.166322. [DOI] [PubMed] [Google Scholar]
- 19.The Japanese Breast Cancer Society . General rules for clinical and pathological recording of breast cancer. 18. Tokyo: Kanehara Co., Ltd; 2018. [Google Scholar]
- 20.Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med. 2018;32:44–53. doi: 10.1007/s12149-017-1217-9. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Zhang J, Wang Y, et al. Textural features of 18F-FDG PET after two cycles of neoadjuvant chemotherapy can predict pCR in patients with locally advanced breast cancer. Ann Nucl Med. 2017;31:544–552. doi: 10.1007/s12149-017-1184-1. [DOI] [PubMed] [Google Scholar]
- 22.Jena A, Taneja S, Singh A, et al. Association of pharmacokinetic and metabolic parameters derived using simultaneous PET/MRI: initial findings and impact on response evaluation in breast cancer. Eur J Radiol. 2017;92:30–36. doi: 10.1016/j.ejrad.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Vicente AM, Pérez-Beteta J, Pérez-García VM, Molina D, Jiménez-Londoño GA, et al. Metabolic Tumor Burden Assessed by Dual Time Point [18F]FDG PET/CT in Locally Advanced Breast Cancer: Relation with Tumor Biology. Mol Imaging Biol. 2017;19:636–644. doi: 10.1007/s11307-016-1034-x. [DOI] [PubMed] [Google Scholar]
- 24.Jin S, Kim SB, Ahn JH, et al. 18 F-fluorodeoxyglucose uptake predicts pathological complete response after neoadjuvant chemotherapy for breast cancer: a retrospective cohort study. J Surg Oncol. 2013;107:180–187. doi: 10.1002/jso.23255. [DOI] [PubMed] [Google Scholar]
- 25.Pahk K, Rhee S, Cho J, et al. The role of interim 18F-FDG PET/CT in predicting early response to neoadjuvant chemotherapy in breast cancer. Anticancer Res. 2014;34:4447–4455. [PubMed] [Google Scholar]
- 26.Groheux D, Majdoub M, Sanna A, et al. Early Metabolic Response to Neoadjuvant Treatment: FDG PET/CT Criteria according to Breast Cancer Subtype. Radiology. 2015;277:358–371. doi: 10.1148/radiol.2015141638. [DOI] [PubMed] [Google Scholar]
- 27.Cho N, Im SA, Cheon GJ, et al. Integrated 18F-FDG PET/MRI in breast cancer: early prediction of response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:328–339. doi: 10.1007/s00259-017-3849-3. [DOI] [PubMed] [Google Scholar]
- 28.Groheux D, Biard L, Lehmann-Che J, et al. Tumor metabolism assessed by FDG-PET/CT and tumor proliferation assessed by genomic grade index to predict response to neoadjuvant chemotherapy in triple negative breast cancer. Eur J Nucl Med Mol Imaging. 2018;45:1279–1288. doi: 10.1007/s00259-018-3998-z. [DOI] [PubMed] [Google Scholar]
- 29.de Cremoux P, Biard L, Poirot B, et al. 18FDG-PET/CT and molecular markers to predict response to neoadjuvant chemotherapy and outcome in HER2-negative advanced luminal breast cancers patients. Oncotarget. 2018;9:16343–16353. doi: 10.18632/oncotarget.24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humbert O, Riedinger JM, Charon-Barra C, et al. Identification of Biomarkers Including 18FDG-PET/CT for Early Prediction of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin Cancer Res. 2015;21:5460–5468. doi: 10.1158/1078-0432.CCR-15-0384. [DOI] [PubMed] [Google Scholar]
- 31.Connolly RM, Leal JP, Goetz MP, et al. TBCRC 008: Early Change in 18F-FDG Uptake on PET Predicts Response to Preoperative Systemic Therapy in Human Epidermal Growth Factor Receptor 2–Negative Primary Operable Breast Cancer. J Nucl Med. 2015;56:31–37. doi: 10.2967/jnumed.114.144741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiyoto S, Sugawara Y, Hosokawa K, Nishimura R, Yamashita N, Ohsumi S, et al. Predictive Ability of 18F-fluorodeoxyglucose Positron Emission Tomography/computed Tomography for Pathological Complete Response and Prognosis after Neoadjuvant Chemotherapy in Triple-negative Breast Cancer Patients. Asia Ocean J Nucl Med Biol. 2016;4:3–11. doi: 10.7508/aojnmb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemarignier C, Martineau A, Teixeira L, Vercellino L, Espié M, Merlet P, et al. Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:1145–1154. doi: 10.1007/s00259-017-3641-4. [DOI] [PubMed] [Google Scholar]
- 34.Im HJ, Kim YK, Kim YI, Lee JJ, Lee WW, Kim SE. Usefulness of Combined Metabolic-Volumetric Indices of (18)F-FDG PET/CT for the Early Prediction of Neoadjuvant Chemotherapy Outcomes in Breast Cancer. Nucl Med Mol Imaging. 2013;47:36–43. doi: 10.1007/s13139-012-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Yao L, Jin P, Hu L, Li X, Guo T, Yang K. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast. 2018;40:106–115. doi: 10.1016/j.breast.2018.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.