Abstract
We report a case of ureteral stump carcinoma following a radical nephrectomy for renal cell carcinoma. A 76-year-old man was diagnosed as having ascending colon cancer and a right renal carcinoma. He was treated with partial colon resection and radical nephrectomy without lymphadenectomy. The histology was renal cell carcinoma. Three years after that surgery, he complained of intermittent macrohematuria. Abdominal computed tomography (CT) suggested a solid mass in the pelvis. We then performed a biopsy with CT guidance. An epithelial tumor was suspected by immunohistochemistry. A total excision of ureter was then performed. The histology showed the features of urothelial carcinoma, G3, v(+), pT3. He received adjuvant chemotherapy with gemcitabine and cisplatin. He was free of disease for the following 11 months.
Keywords: Ureteral stump carcinoma, Renal cell carcinoma
Introduction
A primary carcinoma of the ureteral stump following a radical nephrectomy for renal cell carcinoma is quite rare. Only nine cases have been reported previously. Herein, we report the case of a patient with UC arising from the ureteral stump 3 years after an ipsilateral nephrectomy for RCC.
Case report
A 76-year-old man presented with asymptomatic intermittent macroscopic hematuria during a follow-up for localized RCC that had been treated by a right radical nephrectomy (Fig. 1). A pathological examination of the material obtained from the nephrectomy revealed that the tumor was RCC, pT1a, stage I, clear cell type, ly0, v0 (Fig. 2), with no evidence of extracapsular extension or regional lymph node metastasis and the positive margin of the resected ureter. The patient had a background of colon cancer which was treated by colon surgery at the same time as the removal of the RCC. At that time, he had no complaints of asymptomatic macroscopic hematuria. Cytology of urine was normal and abdominal CT did not reveal the signs of malignancy at the right ureter (Fig. 1). He then underwent systemic chemotherapy with Tegafur/Uracil to the ascending colon cancer for 6 months. Three years later, the patient complained of intermittent macrohematuria. A solid mass (5 cm × 7 cm) in the pelvis was found on abdominal CT (Fig. 3). Although a primary retroperitoneal tumor was highly suspected, differentiation between primary ureteral tumor and retroperitoneal tumor was not possible. The result of urinary cytology showed negative. A biopsy with CT guidance was thus undertaken. Immunohistochemistry revealed that only CK7 was positive; CD20 was negative (Fig. 4). We then performed a total excision of the right ureteral stump. The histology was UC, high grade, v(+), pT3 (Fig. 5). As adjuvant chemotherapy, we administered systemic chemotherapy with gemcitabine and cisplatin. The patient was free of disease in the following 11 months.
Fig. 1.
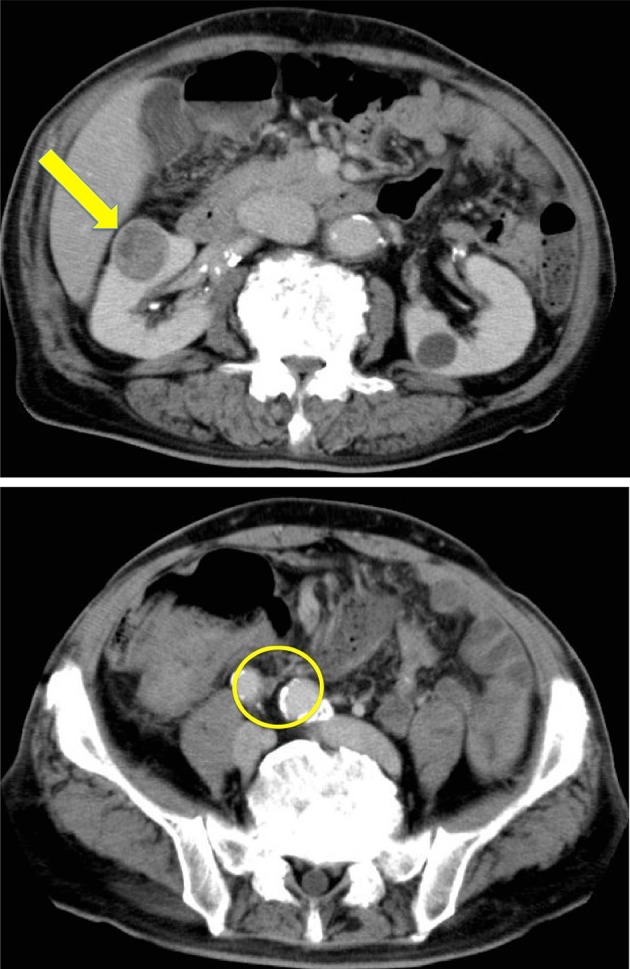
Abdominal CT scan showed the presence of a 2.5 cm × 3.0 cm solid mass at the hilum of the right kidney (arrow). The signs of malignancy were not observed at the right ureter (circle)
Fig. 2.
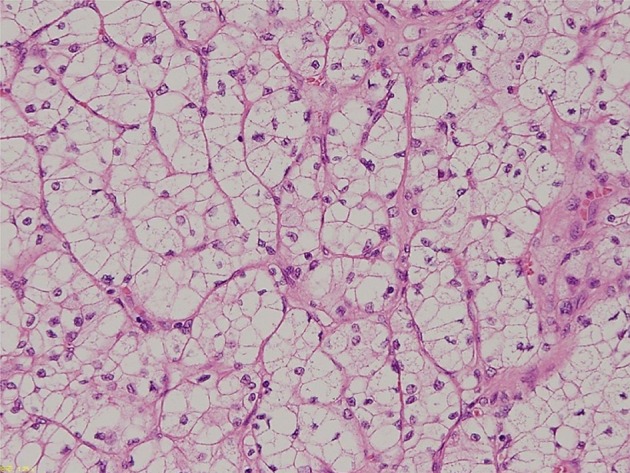
Pathological specimen of the renal cell carcinoma showing clear cell type, stage I (hematoxylin&eosin staining, × 400)
Fig. 3.
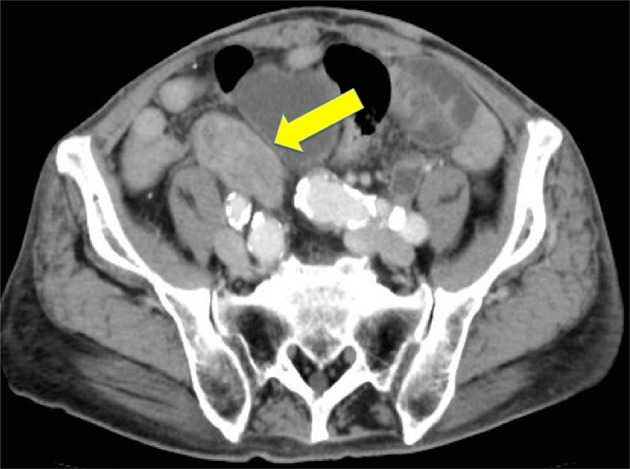
Abdominal CT scan showed the presence of a 5.0 cm × 7.0 cm solid mass at the site of the iliac artery (arrow)
Fig. 4.
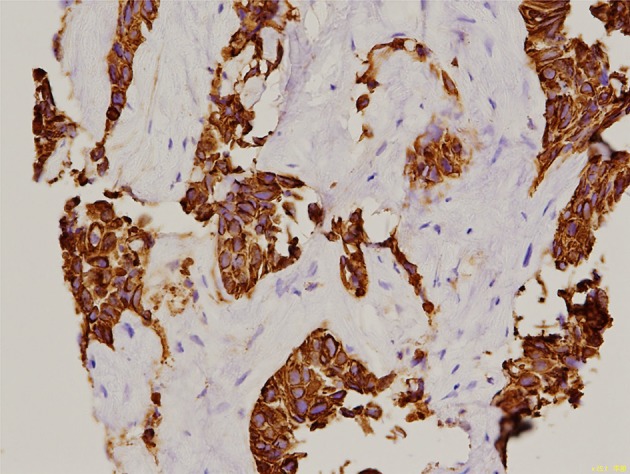
Pathological specimen of the tumor biopsy showing lymphatic follicles (CK7 antigen, × 400)
Fig. 5.
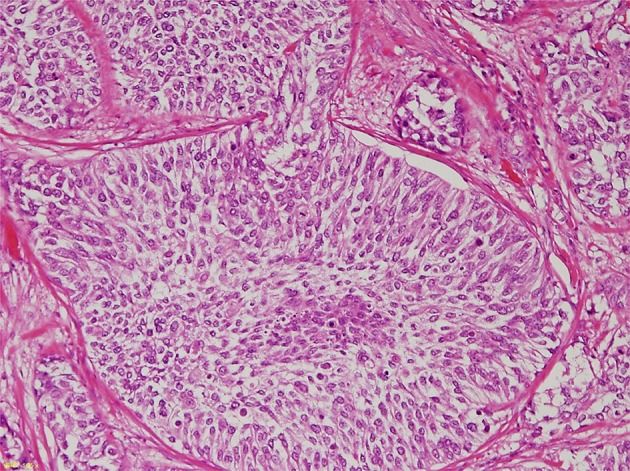
Microscopic examination of the right ureteral stump revealing high-grade urothelial carcinoma with focal interstitial infiltrates (hematoxylin&eosin staining, × 400)
Discussion
The occurrence of primary carcinoma of the ureteral stump following a radical nephrectomy is very rare. To our knowledge, only nine cases were reported [1–9]. Kim et al. reported that 8 of 318 patients who underwent a simple nephrectomy for benign renal disease or renal organ donation presented with a ureteral stump carcinoma; the incidence rate of the disease was 2.51% [10]. Generally, the reported incident rate of primary ureter cancer is 0.01–0.08% [11]. The incident rate of primary ureter carcinoma of the ureteral stump appears to be lower than the occurrence of primary carcinoma of the ureteral stump following a radical nephrectomy.
Table 1 summarizes the backgrounds of the reported cases (including the present patient’s case) of RCC progressing to ureteral stump carcinoma. All of the patients were elderly, with a mean age of 67.2 years. Only one of the ten patients was female. The laterality of renal cancer was right in six cases, left in four. The time interval to the appearance of the primary carcinoma of the ureteral stump following a radical nephrectomy ranged from 1 to 23 years. The first symptom in almost all of the patients was asymptomatic macroscopic hematuria. Thus, the detection of the ureteral stump may be very difficult because of the late appearance of macrohematuria that progression would be easier.
Table 1.
Summary of reported cases of renal cell carcinoma (RCC) progressing to ureteral stump carcinoma
| No. | Age | Gender | Laterality | Complaint | Interval | Histology | Treatment | Authors | Year |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | R | Macrohematuria | 25 mos | UC | Total excision, partial cystectomy | Grey et al. [1] | 1987 |
| 2 | 49 | M | L | Macrohematuria | 12 yrs | UC | Total excision, partial cystectomy | Gojiet al [2] | 1993 |
| 3 | 49 | M | R | Macrohematuria | 23 yrs | UC | Total excision, partial cystectomy | Michel et al. [3] | 1993 |
| 4 | 88 | M | L | Macrohematuria | 6 yrs | UC | Total excision, partial cystectomy | Mitsui et al. [4] | 1998 |
| 5 | 68 | M | R | Macrohematuria | 1 yrs | UC | Total excision, partial cystectomy | Nagatsuma et al. [5] | 1999 |
| 6 | 64 | M | R | No | 6 yrs | UC | Total excision, partial cystectomy | Suzuki et al. [6] | 1999 |
| 7 | 66 | M | L | No | 16 yrs | UC | Neoadjuvant chemotherapyTotal resection, partial cystectomy | Ikeda et al. [7] | 2008 |
| 8 | 76 | M | R | Macrohematuria | 30 mos | UC | Total excision, partial cystectomy | Tsai et al. [8] | 2013 |
| 9 | 61 | F | L | Macrohematuria | 3 yrs | UC | Total excision, partial cystectomy | Shihua et al. [9] | 2016 |
| 10 | 76 | M | R | No | 3 yrs | UC | Total excision only | Present case | 2017 |
M male, F female, R right, L left, K kidney
Cytology may be useless to detect ureter cancer because of a lack of the secretion of urine at the affected side. Endoscopy played an important role, but cannot always provide definite answers; the combination of endoscopy with CT or MRI might be useful in diagnosing cases [12]. Early diagnosis by CT/MRI or selective retrograde ureterography is reported to be possible and could be used for surveillance. In any case, it is important to clarify the pathological aspect by obtaining tissue during surgery or by a needle biopsy.
Although an infectious/inflammatory background was identified in almost all of the 10 cases in Table 1, a significant correlation could not be established when the parameters were examined separately. The development of a ureteral stump might be a cause of primary carcinoma due to the existence of hyperplasia and metaplasia resulting from chronic irritation due to an infection or urinary calculi [6]. The poor secretion of mucosa from the residual ureter might be related to the occurrence of infection due to damage of the feeding vessel to ureter by surgery.
Histologically, all 10 cases (including the present case) were urothelial carcinoma (UC). UC appears to be the prevalent histological type in the majority of cases. Some reports showed that the incidence of squamous cell variety in an intact ureter is between 4% and 12%, while the incidence in the ureteric stump has been reported to be as high as 50% [13]. The present patient’s case was also an UC with an invasion of plasma cell. Within 10 years post-surgery, almost all cases including the present case, were transitional cell carcinoma, whereas at > 10 years post-surgery, almost all of the reported cases were reported to be squamous cell carcinoma (SCC) [14]. Some reports provided evidence of an increased incidence of the squamous cell variety in ureteric stump neoplasms in a comparison with the features of primary malignant ureteric tumors [13, 14]. The prognostic implication of these findings remains unclear.
All 10 of the reported cases were treated by an operation such as a complete ureterectomy with the removal of the bladder cuff, previously resected endoscopically. We should consider the treatment of choice, similarly to UC of the lower ureter. In our patient’s case, we were unable to trace the ureter to the bladder because of the tight adhesion. Therefore, only a total excision except partial cystectomy was done. Three patients (including the present patient) received adjuvant chemotherapy. An important finding was that metastases at diagnosis or at follow-up were significantly correlated with cancer-specific mortality [13]. Tumor stage and grade failed to correlate in the overall review. Future prospective and multicenter studies may help identify subgroups of patients with a higher possible risk of recurrence, who might require long-term follow-up.
In summary, the occurrence of primary carcinoma of the ureteral stump following a radical nephrectomy for renal cell carcinoma is rare. However, if a patient who has undergone a radical nephrectomy experiences hematuria, the possibility of ureteral stump carcinoma should be considered. Urologists need paying attention to the occurrence of primary carcinoma of the ureteral stump following a radical nephrectomy.
Funding
There is no funding source.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article dose not contain any studies with human participants performed by any of the authors.
Informed consent
The patients and/or their families were informed that data from the case would be submitted for publication and provided their consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grey LF, Sorial RF, Levin HJ, et al. Transitional cell carcinoma in ureteral stump after radical nephrectomy for renal cell carcinoma. Urology. 1987;29:209–210. doi: 10.1016/0090-4295(87)90156-7. [DOI] [PubMed] [Google Scholar]
- 2.Gohji K, Ueno K, Higuchi, et al. A case of asynchronous renal cell carcinoma and urothelial cancer of the urinary bladder and left ureter. Hinyokika Kiyo. 1993;39:927–930. [PubMed] [Google Scholar]
- 3.Cher ML, Milchgrub S, Sagalowsky AI, et al. Transitional cell carcinoma of the ureteral stump 23 years after radical nephrectomy for adenocarcinoma. J Urol. 1993;149:106–108. doi: 10.1016/S0022-5347(17)36013-5. [DOI] [PubMed] [Google Scholar]
- 4.Mitsui K, Yamada Y, Taki T, et al. A case of asynchronous renal cell carcinoma, hepatocellular carcinoma and residual ureteral cancer. Hinyokika Kiyo. 1998;39:927–930. [PubMed] [Google Scholar]
- 5.Nagatsuma K, Tachibana M, Miyakawa A, et al. Transitional cell carcinoma of ureteral stump following a nephrectomy for renal cell carcinoma. Int J Urol. 1999;6:627–629. doi: 10.1046/j.1442-2042.1999.00117.x. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Tsuchiya N, Otomo R, et al. Primary tumor of the ureteral stump following a nephrectomy for renal cell carcinoma. Int J Urol. 1999;6:41–43. doi: 10.1046/j.1442-2042.1999.06124.x. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Iwamura M, Reii S, et al. Late relapse and urothelial carcinoma of residual ureter 16 years after radical nephrectomy: a case report. Hinyokika Kiyo. 2008;54:415–417. [PubMed] [Google Scholar]
- 8.Tsai SW, Lin CM, Lin CY, et al. Urothelial carcinoma in a remnant ureter after a radical nephrectomy for renal cell carcinoma: a case report. Urol Sci. 2013;1:65–68. doi: 10.1016/j.urols.2013.04.005. [DOI] [Google Scholar]
- 9.Shihua J, Gang W, Chengfan Y. Primary carcinoma of the ureteral stump following radical nephrectomy for renal cell carcinoma: case report and literature review. Onc Lett. 2016;11:3324–3326. doi: 10.3892/ol.2016.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YJ, Jeon SH, Huh JS, et al. Long-term follow-up of ureteral stump tumors after nephrectomy for benign renal disease. Eur Urol. 2004;46:748–752. doi: 10.1016/j.eururo.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Abehouse BS. Primary benign and malignant tumors of the ureter. Am J Surg. 1956;91:237–271. doi: 10.1016/0002-9610(56)90420-2. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe J, Friedman AC, Seidmon EJ, et al. Diagnosis of ureteral stump transitional cell carcinoma by CT and MRI. Am J Roentgenol. 1987;149:741–742. doi: 10.2214/ajr.149.4.741. [DOI] [PubMed] [Google Scholar]
- 13.Andreas B, Imran S, Geraldine S, et al. Transitional cell carcinoma of the ureteric stump: a systematic review of the literature. Urol Int. 2013;91:170–174. doi: 10.1159/000349884. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y, Yamamoto S, Akamatsu S, et al. Primary transitional carcinoma of the remaining ureter after nephrectomy for pyonephrosis: a case report. Hinyokika Kiyo. 2005;51:101–103. [PubMed] [Google Scholar]


