Abstract
The dismal prognosis of patients with disseminated Ewing sarcoma necessitates the development of novel treatment strategies. Pazopanib is an oral multi-targeted tyrosine kinase inhibitor that is active against advanced soft tissue sarcoma. However, the clinical activity and feasibility of pazopanib for treating Ewing sarcoma remain poorly understood. Moreover, clinical information on the use of tandem high-dose chemotherapy for Ewing sarcoma is limited. A 14-year-old boy with Ewing sarcoma was transferred to our hospital for treatment. Magnetic resonance imaging, computed tomography scans, and bone scintigraphy revealed multiple lesions in the pubis, ilium, ischium, femur, rib, cranial bone, thoracic vertebrae, sacrum, obturator muscle, adductor magnus muscle, testicular cord, and lungs. Bone scintigraphy after intensive chemotherapies confirmed that multiple abnormal accumulations were still present in the cranial bone and pubis. Subsequently, the patient received tandem high-dose chemotherapy including topotecan, and radiotherapy. Abnormal accumulations have disappeared in bone scintigraphy. Subsequently, pazopanib maintenance therapy was initiated. Despite the presence of innumerable lesions at diagnosis, the patient has been in near-complete remission for the past 1 year with pazopanib administration. This confirms that adding pazopanib maintenance therapy after tandem high-dose chemotherapy is a therapeutic option for cases with disseminated Ewing sarcoma.
Keywords: Disseminated Ewing sarcoma, Pazopanib, Tandem high-dose chemotherapy
Introduction
The prognosis of patients with disseminated Ewing sarcoma is dismal with a survival rate in the range of 0–30% despite the administration of intensive cytotoxic chemotherapy including high-dose chemotherapy [1–5]. Accordingly, novel treatment strategies are urgently needed for treating these patients. Pazopanib is an oral multi-targeted tyrosine kinase inhibitor that has been demonstrated to prolong progression-free survival of patients with advanced soft tissue sarcoma [6]. However, the clinical activity and feasibility of pazopanib for treating patients with Ewing sarcoma remains poorly understood. Moreover, the clinical experience of using tandem high-dose chemotherapy for Ewing sarcoma is limited. Here, we describe the case of a patient with disseminated Ewing sarcoma who underwent pazopanib maintenance therapy after tandem high-dose chemotherapy including topotecan. Despite having innumerable lesions at diagnosis, the patient remains in near-complete remission for the past 1 year.
Methods
We obtained informed consent from the patient’s guardians regarding the off-label use of topotecan, melphalan, and cyclophosphamide (TMC) conditioning high-dose chemotherapy and pazopanib maintenance therapy for Ewing sarcoma, both of which were approved by the Institutional Review Board of the Kobe Children’s Hospital.
Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Case report
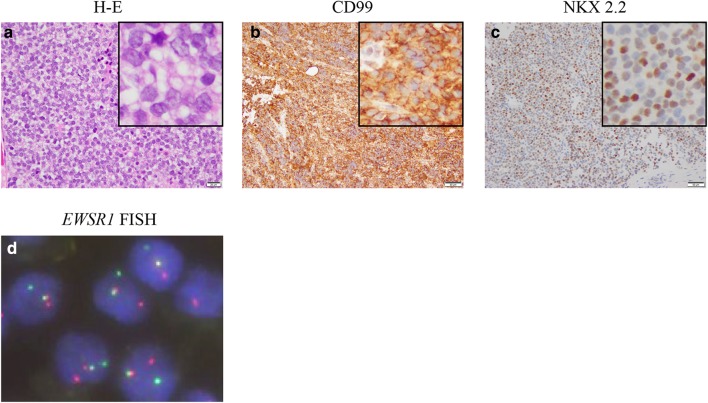
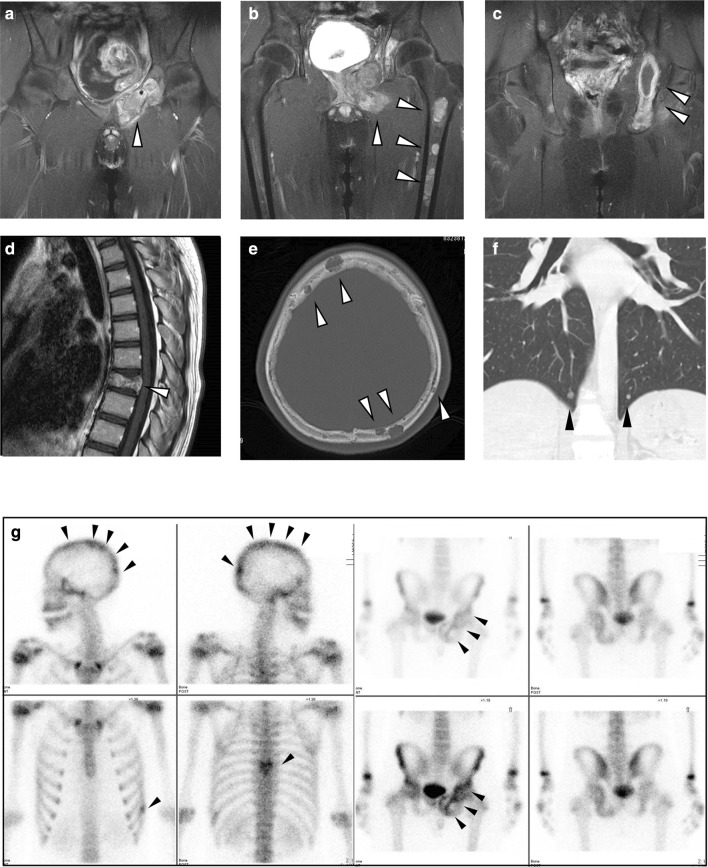
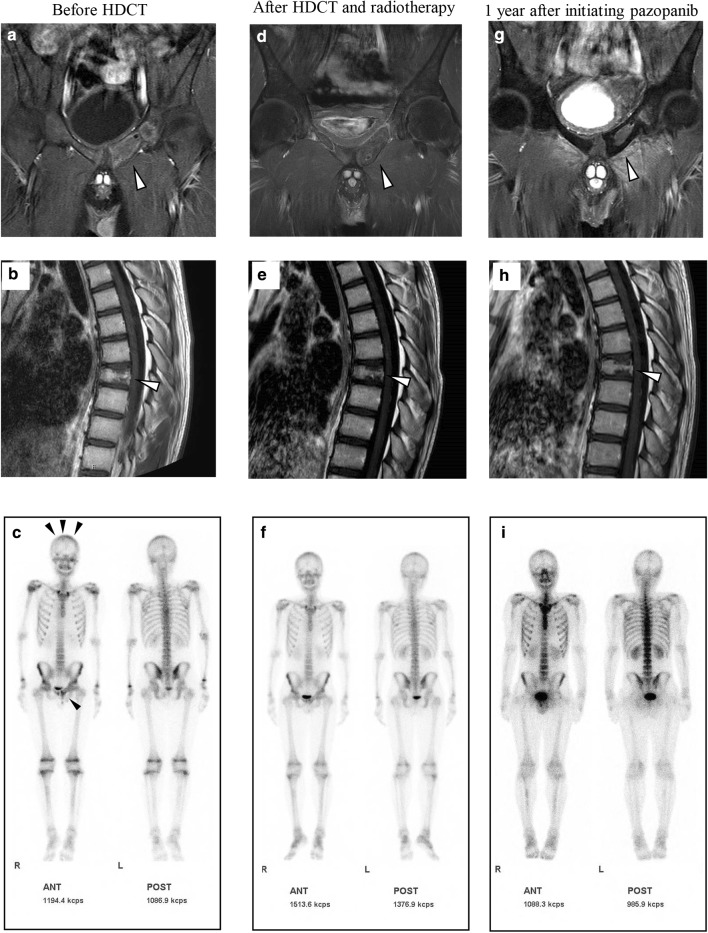
A previously healthy 14-year-old boy visited his family physician clinic complaining of pain in the left hip. A computed tomography (CT) scan revealed a tumor in the pelvis. He was referred to the local hospital and underwent a biopsy of the left pubis. A pathological examination revealed a small round cell tumor, which was immunohistochemically positive for CD99 and NKX2-2 (Fig. 1a–c). FISH indicated rearrangement of EWSR1 (Fig. 1d). Based on these findings, he was diagnosed with Ewing sarcoma and transferred to Kobe Children’s Hospital (Kobe, Japan) for treatment. Magnetic resonance imaging (MRI) and CT scans revealed innumerable lesions in the pubis, ilium, ischium, femur, rib, cranial bone, thoracic vertebra, sacrum, obturator muscle, adductor magnus muscle, testicular cord, and lungs (Fig. 2a–f). Bone scintigraphy showed multiple abnormal accumulations in the pelvis, cranial bone, thoracic vertebrae, and left rib (Fig. 2g), although the bone marrow was not involved. He received six courses of vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) at 3-week-intervals, according to the European Ewing Tumor Working Initiative of National Groups (Euro-EWING 99) trial [1, 7], without major side effects. Bone scintigraphy after six cycles of VIDE showed that multiple abnormal accumulations were still present, albeit slightly reduced in size and number. Subsequently, he received two courses of vincristine, actinomycin-D, and ifosfamide (VAI). Surgical indication was not an option because of persistent disseminated lesions. Alternatively, two courses (3-week interval) of salvage chemotherapy consisting of temozolomide (100 mg/m2/day; day 1–5) and irinotecan (20 mg/m2/day; day 1–5 and day 8–12) (TMZ/CPT-11) were administered [8]. MRI after chemotherapy (6 cycles of VIDE, 2 cycles of VAI, and 2 cycles of TMZ/CPT-11) revealed multiple lesions still present in the pubis, ilium, ischium, femur, rib, cranial bone, thoracic vertebra, and sacrum (Fig. 3a, b). Bone scintigraphy confirmed that multiple abnormal accumulations were still present in the cranial bone and pubis, although in a reduced size and number (Fig. 3c). The patient received the initial high-dose chemotherapy with autologous stem cell rescue. The conditioning regimen consisted of topotecan (3.5 mg/m2/day × 5 days), melphalan (70 mg/m2/day × 2 days), and cyclophosphamide (1000 mg/m2/day × 3 days). No major adverse event other than hematological toxicity was observed (white blood cell count decreased, grade 4; lymphocyte count decreased, grade 4; neutrophil count decreased, grade 4; platelet count decreased, grade 4; febrile neutropenia, grade 3; anemia, grade 2; nausea, grade2). Two months after the first high-dose chemotherapy, he underwent the second high-dose chemotherapy; no major side effects other than hematological toxicity were observed. The conditioning regimen consisted of busulfan (12.8 mg/kg) and melphalan (180 mg/m2). Subsequently, he received intensity-modulated radiation therapy (IMRT) to the pelvis and cranial bone at doses of 50.4 and 30.6 Gy, respectively. The dose of radiotherapy to the cranial bone was limited because of anticipated neurotoxicity following high-dose chemotherapy that included busulfan.
Fig. 1.
Histological and fluorescence in situ hybridization (FISH) findings of the tumor at diagnosis. Boxed images in the right upper corner are the magnification of each image. Hematoxylin–eosin (H–E) stain showed small round tumor cells (a). Immunohistochemical analysis demonstrated positivity for CD99 and NKX 2.2 (b, c). FISH demonstrated EWSR1 split signal pattern with a pair of fused yellow signals and split red (5′ EWSR1) and green (3′ EWSR1) signals (d)
Fig. 2.
Representative images of contrast-enhanced T1-weighted magnetic resonance imaging (a–d), computed tomography (e, f), and bone scintigraphy (g) at diagnosis. Disseminated tumors in the pelvis (a–c, and g), left femur (b), thoracic vertebrae (d and g), cranial bone (e and g), and bilateral lungs (f) are shown. Arrowheads indicate lesions (a–f) and abnormal accumulations (g)
Fig. 3.
Contrast-enhanced T1-weighted magnetic resonance imaging and bone scintigraphy before the high-dose chemotherapy (HDCT) (a–c), after HDCT and radiotherapy (d–f), and 1 year after initiating pazopanib (g–i). Abnormal accumulations were noted in the cranial bone and pubis before HDCT (c). Abnormal accumulations in bone scintigraphy have diminished after HDCT and radiotherapy (f) and remained undetectable 1 year after initiating pazopanib (i). Lesions in the pubis have reduced in size, with almost negligible contrast enhancement during the treatment (a, d, and g). Remaining contrast enhancement in the thoracic vertebrae before HDCT (b) has diminished after HDCT and radiotherapy (e) and remained undetected 1 year after initiating pazopanib (h). Arrowheads indicate lesions (a, b, d, e, g, and h) and abnormal accumulations (c)
After the radiotherapy, MRI revealed that multiple lesions in the pubis, ilium, and cranial bone were reduced in a size, number, and contrast enhancement (Fig. 3d). Abnormal accumulations diminished in bone scintigraphy as well as the contrast enhancement in the thoracic vertebrae in MRI (Fig. 3e, f). Based on these findings, we concluded that the patient achieved near-complete remission. Subsequently, pazopanib maintenance therapy was initiated at 400 mg/day, which was eventually increased to 800 mg/day. At the last follow-up (1 year after initiating pazopanib), MRI revealed that multiple lesions were reduced in size, showing almost negligible contrast enhancement (Fig. 3g, h). In addition, abnormal accumulations remain undetected in bone scintigraphy (Fig. 3i). In this case, whitening of hair (grade 1), mild exfoliative dermatitis (grade 1), and mildly elevated thyroid stimulating hormone (grade 1) were observed, but did not require intervention. After continuing pazopanib therapy, he has been in near-complete remission for 1 year without the presence of abnormal accumulations (based on bone scintigraphy findings).
Discussion
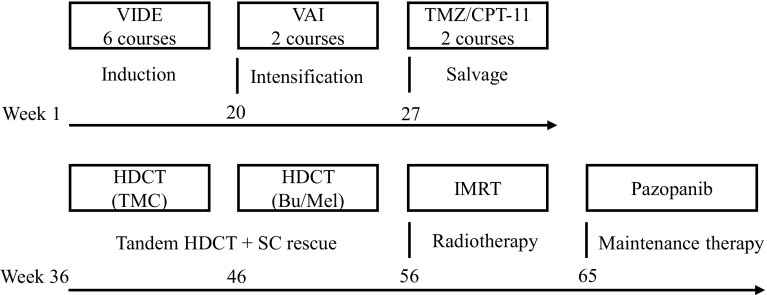
Here, we report on a novel treatment strategy of pazopanib maintenance therapy after tandem high-dose chemotherapy for disseminated Ewing sarcoma (Fig. 4). Despite having innumerable lesions at diagnosis, our patient remains in a state of near-complete remission.
Fig. 4.
Schematic overview of the treatment strategy for disseminated Ewing sarcoma. Bu busulfan, CPT-11 irinotecan, HDCT high-dose chemotherapy, IMRT intensity-modulated radiation therapy, Mel melphalan, SC stem cell, TMC topotecan, melphalan, and cyclophosphamide, TMZ temozolomide, VAI vincristine, actinomycin-D, and ifosfamide, VIDE vincristine, ifosfamide, doxorubicin, and etoposide
Previous clinical trials incorporating tandem high-dose chemotherapy, including high-dose thiotepa, have failed to improve outcomes of high-risk Ewing sarcoma [4, 9]. Based on these findings, our aim was to develop a novel conditioning regimen that would achieve treatment success. High-dose topotecan, melphalan, and cyclophosphamide (TMC) with autologous stem cell support is reported to be a safe and effective regimen for adult patients with ovarian cancer and multiple myeloma [10, 11]. To the best of our knowledge, our case is the first report on the feasibility of tandem high-dose chemotherapy incorporating the TMC regimen for treating Ewing sarcoma. The commonly used busulfan and melphalan conditioning regimen was chosen for the second high-dose chemotherapy [1].
To date, only four case reports have shown efficacy of pazopanib in treating Ewing sarcoma. However, in three out of four cases, the duration of the effect was limited to several months [12–15]. Furthermore, the patients in these studies did not receive high-dose chemotherapy. To the best of our knowledge, this is the first to report on the feasibility of pazopanib after tandem high-dose chemotherapy. There is a possibility that the near complete remission status induced by HDCT and radiotherapy was maintained regardless of pazopanib treatment. Despite the limitation, given that the estimated survival rate of similar patients (aged > 14 years, > 5 bone metastases, primary tumor volume > 200 ml, and lung metastases) is less than 10% [1], this novel treatment strategy was well tolerated and has potential efficacy.
Our findings indicate that pazopanib maintenance therapy after tandem high-dose chemotherapy is a therapeutic option for disseminated Ewing sarcoma. Based on the outcome of this case, we recommend that large prospective clinical trials are undertaken to assess the efficacy of pazopanib maintenance therapy after tandem high-dose chemotherapy for disseminated Ewing sarcoma.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose. This study was conducted in accordance with the Declaration of Helsinki. Written and oral informed consent for publication was obtained from the patient’s guardians.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ladenstein R, Pötschger U, Le Deley MC, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 3.Paulussen M, Ahrens S, Burdach S, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 1998;9:275–281. doi: 10.1023/A:1008208511815. [DOI] [PubMed] [Google Scholar]
- 4.Loschi S, Dufour C, Oberlin O, et al. Tandem high-dose chemotherapy strategy as first-line treatment of primary disseminated multifocal Ewing sarcomas in children, adolescents and young adults. Bone Marrow Transpl. 2015;50:1083–1088. doi: 10.1038/bmt.2015.118. [DOI] [PubMed] [Google Scholar]
- 5.Thiel U, Wawer A, von Luettichau I, et al. Bone marrow involvement identifies a subgroup of advanced Ewing sarcoma patients with fatal outcome irrespective of therapy in contrast to curable patients with multiple bone metastases but unaffected marrow. Oncotarget. 2016;7:70959–70968. doi: 10.18632/oncotarget.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 7.Juergens C, Weston C, Lewis I, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47:22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 8.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 9.Burke MJ, Walterhouse DO, Jacobsohn DA, et al. Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer. 2007;49:196–198. doi: 10.1002/pbc.21182. [DOI] [PubMed] [Google Scholar]
- 10.Donato ML, Gershenson DM, Wharton JT, et al. High-dose topotecan, melphalan, and cyclophosphamide (TMC) with stem cell support: a new regimen for the treatment of advanced ovarian cancer. Gynecol Oncol. 2001;82:420–426. doi: 10.1006/gyno.2001.6326. [DOI] [PubMed] [Google Scholar]
- 11.Kazmi SM, Saliba RM, Donato M, et al. Phase II trial of high-dose topotecan, melphalan and CY with autologous stem cell support for multiple myeloma. Bone Marrow Transpl. 2011;46:510–515. doi: 10.1038/bmt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Nozawa M, Shimizu N, et al. Pazopanib for recurrent extraosseous Ewing’s sarcoma of the retroperitoneum. Int J Urol. 2014;21:1183–1184. doi: 10.1111/iju.12546. [DOI] [PubMed] [Google Scholar]
- 13.Attia S, Okuno SH, Robinson SI, et al. Clinical activity of pazopanib in metastatic extraosseous Ewing sarcoma. Rare Tumors. 2015;7:5992. doi: 10.4081/rt.2015.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcindor T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol. 2015;54:1063–1064. doi: 10.3109/0284186X.2014.971938. [DOI] [PubMed] [Google Scholar]
- 15.Mori Y, Kinoshita S, Kanamori T, et al. The successful treatment of metastatic extraosseous ewing sarcoma with pazopanib. Intern Med. 2018;57:2753–2757. doi: 10.2169/internalmedicine.9879-17. [DOI] [PMC free article] [PubMed] [Google Scholar]