Abstract
Children with sickle cell anemia (SCA) have elevated cerebral blood velocity relative to healthy peers. The primary aim of this study was to evaluate the association between cerebral blood velocity, measured by transcranial Doppler (TCD) ultrasound, age, and gender with cognitive function in children with SCA in Nigeria. Eighty-three children (Mage = 9.10, SD = 1.90 years; 55% female) with SCA in Nigeria completed cognitive assessments and a TCD ultrasound. The association between TCD velocity and measures of perceptual reasoning (Raven’s Progressive Matrices), working memory (WISC-IV Digit Span), and executive planning (Tower of London, TOL) were assessed. Results showed that elevated TCD velocity significantly predicted lower scores on TOL Time Violations and Total Problem-Solving Time when controlling for BMI, hemoglobin level, and parent education, suggesting that TCD velocity is related to the efficiency of executive function. Further, age was negatively related to children’s performance on the Ravens Matrices and TOL Total Correct, and boys showed greater deficits on the TOL Total Correct relative to girls. Moderation analyses for gender showed that there was a conditional negative association between TCD velocity and Digit Span for boys, but not for girls. Findings suggest that children with SCA in Nigeria with elevated TCD velocity are at risk for deficits in efficiency of executive planning, and boys with elevated TCD velocity are particularly at increased risk for deficits in auditory working memory. Implications of this study are important for interventions to reduce cerebral blood velocity and the use of TCD in this population.
Keywords: TCD, cerebral blood flow velocity, sickle cell anemia, cognitive function, age, gender
Sickle cell disease (SCD) is one of the most common genetic disorders worldwide, characterized by the abnormal HbS gene (World Health Organization, 1994). Sickle cell anemia (SCA), the most severe form of SCD, is characterized by high rates of ischemic strokes and silent infarcts, with a mortality rate of 20–30% in this group (DeBaun & Kirkham, 2016; Ohene-Frempong et al., 1998, Powars et al., 1978). An important biological risk for strokes in this population is elevated cerebral blood velocity (> 200 cm/sec) measured by transcranial Doppler (TCD) ultrasound (Adams et al., 1992; Jordan & DeBaun, 2017). Due to chronic anemia, children with SCA have decreased blood oxygen content and a global increase in cerebral blood flow relative to healthy peers, which functions as a compensatory mechanism. Elevated TCD velocity then occurs in some patients as a result from arterial stenosis that restricts blood delivery to the brain (Prohovnik, Hurlet-Jensen, Adams, De Vivo, & Pavlakis, 2009).
Elevated TCD velocity is temporally associated with the occurance of stroke (Adams et al., 1992), which can have subsequent effects on cognitive function (Kawadler et al., 2016). Children with SCA routinely score lower on tests of cognitive functioning compared to their peers in multiple domains including overall intelligence, executive function, and processing speed; and these deficits increase with age (e.g., Kawadler et al., 2016; King et al., 2014a; Schatz et al., 2002). Although the occurrence of infarcts increases the risk for and degree of cognitive impairment (Armstrong et al., 1996; King et al., 2014b), deficits are still present in those without a history of stroke or silent infarct. For example, three meta-analyses found that children with SCD without infarcts have deficits in Full Scale IQ and other domains of intelligence compared to healthy siblings, matched controls, or the normative mean (Kawadler et al., 2016; King et al., 2014a; Schatz et al., 2002). Biological characteristics such as elevated TCD velocity and chronic anemia are related to cognitive impairments in this population (Bakker et al., 2014; Hogan et al., 2005; Sanchez et al., 2010; Steen et al., 2003; Strouse et al., 2006).
A more comprehensive analysis of how both child age and gender influence the relation between TCD velocity and cognitive function is needed (Bakker et al., 2014). Although studies consistently show that deficits in cognitive function increase with age (Schatz et al., 2002), no study to our knowledge has examined how age might moderate the relation between TCD velocity and cognitive function. Potentially chronic anemia and deoxygenation of the brain may lead to deficits in cognition relative to age-matched peers to increase over time, and TCD velocity might be more important within one age group relative to another. With regards to gender, boys with SCA are more likely to repeat grade levels (King et al., 2014b), and a study on infant cognition found that boys performed lower on the Bayley Scales of Infant Development Mental Index (Glass et al., 2013). Further, Groen et al. (2012) found that the association between TCD velocity and visuospatial memory was stronger in boys compared to girls in a sample of typically developing children and adolescents. No study to date has assessed this relation in children with SCA and, while hypothesized mechanisms of why boys are more affected by disease characteristics and TCD velocity are not clearly stated in the literature, these relations are also worth investigating in a cross-cultural sample.
Nigeria is an important setting to examine the association between TCD velocity and cognitive problems associated with SCA, as this country has the largest burden of SCD worldwide with nearly 150,000 children born annually with SCA (WHO, 2006). Further, Nigerian health care settings lack the resources for routine imaging of the brain, such as MRI or CT scan, that are part of standard care in high-income settings. The majority of studies on cognitive functioning in children with SCA have been conducted in children living in high-income settings who were receiving treatment. Only one study to date (Oluwole, Noll, Winger, Akinyanju, & Novelli, 2016) examined cognitive function in Nigerian children with SCA. Oluwole et al. (2016) described lower IQ scores and slower processing speed relative to healthy controls, but there were no significant deficits in working memory. This study also found that indicators of child nutritional status (i.e., BMI, height-for-age, weight-for-age) and maternal education were significant correlates of cognitive function in this population. Nevertheless, associations with hemodynamic characteristics (i.e., hemoglobin level and peripheral O2 saturation) were not significant, and TCD velocity was not assessed as a potential correlate. Although studies have described statistically significant associations between TCD velocity and cognitive function in children with SCA (Bakker et al., 2014), only one published study has examined this association in children with sub-Saharan Africa, where other co-morbidities, including malnutrition and severe anemia are more prevalent when compared to high-income settings. In a study conducted in Cameroon, Ruffiex et al. (2013) found that TCD velocity was related to memory using the California Verbal Learning Test; however, the influence of age and gender were not assessed.
In the current study, we assessed cognitive function in children with SCA in Kano, Nigeria as part of the baseline assessment in the Stroke Prevention in Nigeria (SPIN) Trial (NCT01801423). The goal of the SPIN trial is to determine the acceptability and safety of hydroxyurea for primary stroke prevention in children with SCA in Nigeria. Initially, hydroxyurea was used to prevent pain and acute chest syndrome (Charache et al., 1995; Ferster et al., 2011; Halsey & Roberts, 2003), but studies show that reduced TCD velocity is a secondary benefit (Ghafuri et al. 2017).
The purpose of this study was to identify biological and demographic predictors of cognitive function (i.e., TCD velocity, age, and gender) in children enrolled in the trial prior to the administration of hydroxyurea. Analyses were also conducted to examine the potential moderating role of child age and gender as suggested by Bakker et al. (2014) in a comprehensive review of TCD velocity and cognitive function. We tested the hypotheses that (1) elevated TCD velocity, older age, and male gender would be significantly related to deficits in cognitive function; (2) TCD velocity and child age would predict deficits even after controlling for nutritional status, hemoglobin level, and parent education in this population to account for potential signifcant relations shown in Oluwole et al. (2016); and (3) child age and gender would significantly moderate the relation between TCD velocity and cognitive function.
Method
Participants
Children were recruited to participate as part of a larger clinical trial, the Primary Stroke Prevention in Nigerian Children with Sickle Cell Disease (SPIN) Trial (NCT01801423; Galadanci et al., 2015). Eligibility requirements for SPIN included: (a) confirmed diagnosis of sickle cell anemia (HbSS or HbSβ0 thalassemia), (b) child age of 5–12 years at study entry, (c) informed consent from a parent or legal guardian and assent from children, and (d) successful completion of screening procedures, including TCD velocity. Children were included in the SPIN trial even if they did not complete the cognitive assessment; however, only participants with complete neurocognitive data were included in the present analyses. Children were screened for overt stroke using the pediatric stroke outcome measure at baseline, and no children were identified as having an overt stroke. The primary determinant of presence of stroke was a neurological deficit that persisted for >24 hours and a clinical picture that was consistent with stroke.
Eighty-three children and adolescents with SCA completed a cognitive evaluation as part of their involvement in the study and were included in the current analyses. Participants were between the ages of 6 and 13 years old (M = 9.10, SD = 1.90) and 55% female (n = 46) at the time of the assessment. Eighty-two children (98.8%) were diagnosed with the HbSS subtype and the remaining participant was diagnosed with HbSB0 thalassemia. All participants had no history of regular red blood cell transfusion.
Seventy-two children (86.7%) identified with the Hausa-Fulani ethnic group, 4 children (4.8%) identified as Yoruba, 1 (1.2%) as Igbo, and 6 (7.2%) as other. Children were also from a range of family environments with regards to parent educational status (8.4% reported no formal education, 12.0% with senior secondary degree, 20.5% with Odinary National Diploma or equivalent, 41% with a Higher National Diploma or Bachelors degree, and 16.9% with a master’s degree) and yearly family income using the current currency conversion rate is 1 US dollar ≈ 361 Nigerian Naira (1.2% earned the equivalent of $1,108 to $1,385; 2.4% earned $1,386 to $1,662; 8.4% earned $1,663 to $1,939; 14.5% earned $1,940 to $2,216; 8.4% earned $2,217 to $2,493; 14.5% earned $2,494 to $2,770; and 41.0% earned $2,771 or above). The vast majority (97.6%) of primary caregivers reported that they are married. Although 83.1% of parents reported that the child was fluent in English, 98.8% reported that English was not the participant’s first language.
Procedure
The study protocol was reviewed and approved by the Institutional Review Boards at Vanderbilt University and the Ethics committee of Aminu Kano Teaching Hospital (AKTH). Participants were recruited from the pediatric SCD clinic housed within the pediatric department of AKTH, located in the second largest metropolis in Nigeria. Participants completed a standardized psychological evaluation at baseline to assess for cognitive function. Cognitive testing was conducted by an experienced pediatric psychiatrist and a research associate who were familiar with the language and culture, after undergoing an extensive 5-day training in test administration under the supervision of a licensed clinical psychologist at the coordinating site in the U.S. All testing took place in an examination room at the hospital. Given the challenges of assessing cognitive development in children in low-income settings, including in sub-Saharan Africa (Wicherts et al., 2010), careful consideration was taken to select measures that had been applied successfully in previous studies with children from sub-Saharan Africa (e.g., Bakare et al., 2009; Wicherts et al., 2010). Measures were selected that included only minimal language demand from participants. Verbal instructions from the examiner were translated to Hausa, which was spoken by all of the participants, as it is the most widely spoken language of Kano, Nigeria.
Measures
Hemodynamic characteristics.
TCD velocity in the proximal portion of middle cerebral artery was measured using a non-imaging Sonara/Tek Portable TCD System at the time of the assessment. Continuous variables of the highest velocity were used in the current analyses; however, TCD < 170 cm/sec was categorized as normal, TCD = 170–199 cm/sec as conditional, and TCD ≥ 200 cm/sec as abnormal, as in the SWiTCH and STOP trials (Adams et al., 1998; Helton et al., 2014) when probing interactions. Hemoglobin level was obtained from complete blood count results at the time of the assessment.
Cognitive function.
Children completed a brief cognitive assessment battery to measure intellectual functioning across several domains. First, children were administered the Raven’s Standard Progressive Matrices (Raven, 1998), a nonverbal test measuring abstract reasoning and regarded as an estimate of fluid intelligence in children ages 6 to 16. The Raven’s has been previously used in research with children in sub-Saharan Africa (Wicherts et al., 2010). Items consist of visual geometric designs with a missing piece. Children are given six to eight choices to pick from to fill in the missing piece. Reliability for this measure is .96 in U.S. samples.
Children also completed the Tower of London – Drexel University: 2nd Edition (TOLDX; Culbertson & Zillmer, 2001) The TOL is a validated measure of higher-order problem solving, specifically executive planning abilities, in children ages 7 to 15. The test taker is instructed to rearrange beads on a peg board to match the configuration presented by the examiner. For the TOL timed scales, higher time yields lower standard scores. Four scores from this measure were used in the current analyses: Total Correct, Rule Violations, Time Violations, and Total Problem-Solving Time (i.e., Total Time). Both Time Violations and Total Time were used as they are independent measures of efficiency of executive planning. Initiation Time and Execution Time are components of Total Time, and they were not used to reduce the number of analyses. Reliability across scores fall between .42 and .81.
Children were administered the Digit Span subtest of the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003). The WISC-IV is a widely used measure of intelligence in children 6 to 16 years of age. The Digit Span subtest requires children to recall a sequence of orally presented numbers in the same order and reverse order. Digit Span is a measure of verbal short-term and working memory, and the ability to register, maintain, and manipulate information in conscious awareness. U.S. standardized norms were used in the absence of normative data from children in Nigeria for each measure of cognitive function. Validity and reliability estimates for this test have been shown to be .74 to .89 and .88 to .97, respectively, in national U.S. samples; however, this measure has been used in previous research in sub-saharan Africa (e.g., Oluwole et al., 2016; Ruffieux et al., 2013).
Nutritional status.
Children’s height and weight at the time of the assessment were used to calculate body mass index (BMI), which was used as a proxy for child nutritional status.
Parent Education.
Parents were asked to report the head of household’s highest educational degree. Ordinal variables were used in the analyses.
Statistical Analyses
Statistical analyses were conducted using SPSS (24th edition). We conducted descriptive statistics and bivariate correlations among study variables. Hierarchical multivariate linear regression analyses with covariates (BMI, hemoglobin, and parent education) in Step 1, and primary study variables (continuous TCD velocity, age, and gender) added in Step 2. Finally, we tested age and gender as possible moderators of the association between TCD velocity and scores on measures of cognitive function in this. Interactions between TCD velocity with gender and TCD velocity with child age were assessed. Age was centered by subtracting the sample mean from each individual, and the centered variable, continuous TCD velocity variable, the product interaction term, and additional covariates were included in the analyses. Gender was dummy coded to designate boys (0) and girls (1), and this variable was included along with TCD velocity, the product interaction term, and covariates in the analysis. Post hoc probing was conducted for significant interactions to determine whether simple slopes differed significantly from zero, and predicted associations were plotted separately at high and low values (i.e., ± 1 SD for age; boys (0) and girls (1) for gender) of the moderator (Aiken & West, 1991; Holmbeck, 2002). Further, TCD velocity was dummy coded for the recommended categorical cut-offs outlined in the SWiTCH and STOP trials (Adams et al., 1998; Helton et al., 2014) for normal (−1), conditional (0), and abnormal TCD velocity (1) when plotting interactions found with the continuous variable. To correct for possible Type I error in the bivariate correlation analyses, supplemental analyses were conducted using the False Discovery Rate (Benjamini & Hochberg, 2000) .
Results
Descriptive statistics
Descriptive statistics for the measures of study variables are described in Table 1. On average, children scored at the 15.21 (SD = 14.76) percentile rank on the Raven’s Standard Progressive Matrices, which corresponds to a standard IQ score of 85. Participants obtained a mean scaled score of 9.84 (SD = 3.98) on the Digit Span subtest of the WISC-IV. Finally, the mean scores across five of the TOL tests (i.e., Rule Violations, Time Violations, Execution Time, and Problem-Solving Time) were below age-based U.S. norms, whereas Initiation Time was above the normative mean. Descriptive statistics for hemodynamic and health characteristics are also described in Table 1. Participants had a mean hemoglobin level of 7.72 (SD = .91) at the time of the assessment. Mean TCD velocity was 138.0 cm/sec (SD = 29.28), 84.3% of participants were in the normal range, 8.4% were in the conditional range, and 7.2% were in the abnormal range. Finally, the mean BMI was 13.74 (SD = 2.27, range = 9.60 to 22.50), which is lower than healthy children in Nigeria and the expected BMI for normal weight in children world wide (range = 18.5 to 24.9; Anyiam, Ogala, & Onuor, 2008; WHO, 2000).
Table 1.
Descriptive statistics of study variables
| M (SD) | n (%) | |
|---|---|---|
| Cognitive Function | ||
| Raven’s Matricesa | 15.21 (14.76) | |
| Digit Spanb | 9.84 (3.98) | |
| TOL Correctc | 96.22 (14.16) | |
| TOL Rule Violationsc | 73.32 (15.03) | |
| TOL Time Violationsc | 80.59 (14.69) | |
| TOL Total Timec | 78.44 (15.44) | |
| Hemodynamic Characteristics | ||
| Hemoglobin | 7.72 (.91) | |
| TCD Velocity d (continuous) | 138.0 (29.28) | |
| Normal | 70 (84.3) | |
| Conditional | 7 (8.4) | |
| Abnormal | 6 (7.2) | |
| Age | 9.10 (1.89) | |
| Gender | ||
| Male | 37 (44.6) | |
| Female | 46 (55.4) | |
| BMI | 13.74 (2.27) | |
| Parent Education | ||
| None | 7 (8.4) | |
| Senior Secondary | 10 (12.0) | |
| OND or equivalent | 17 (20.5) | |
| HND, Bachelors | 34 (41.0) | |
| Master’s Degree | 14 (16.9) |
Percentile rank from a standardized distribution (M = 50).
Scaled scores from a standardized distribution (M = 10, SD = 3).
Standard scores from a standardized distribution (M = 100, SD = 15).
TCD velocities measured cm/sec.
N = 83
Bivariate analyses
Bivariate pearson corrrelations amongst the primary predictor variables showed that TCD velocity was not related to either age, r = −.21, p = .062, or gender, r = .16, p = .142, and gender was not related to child age, r = −.17, p = .117. Correlations were also used to assess whether elevated TCD velocity, older age, and male gender are related to deficits in cognitive function (hypothesis 1) are shown in Table 2. Partially supportive of Hypothesis 1, TCD velocity was significantly negatively related to scores on TOL Time Violations and TOL Total Time, meaning that children with higher TCD velocity had more time violations and took longer to complete the tasks. The relation between TCD velocity and the ToL time scores, however, were no longer significant after controlling for False Discovery Rate. Further, TCD velocity was not related to scores on the Raven’s Matrices or Digit Span. Child age was significantly related to Raven’s Matrices and TOL Total Correct, such that deficits increased with age. The relations between age with Raven’s Matrices and ToL Correct both remained significant after controlling for False Discovery Rate. Child gender was positively related to TOL Total Correct, such that girls performed signficiantly better than boys. The relation between the gender and ToL Correct remained significant after controlling for False Discovery Rate. BMI, hemoglobin, and parent education were not significantly related to any measure of cognitive function.
Table 2.
Bivariate Correlations of Cognitive Function with Hemodynamic, Health, and Demographic Characteristics
| Ravan’s Matrices | Digit Span | TOL |
||||
|---|---|---|---|---|---|---|
| Total Correct | Rule Violation | Time Violations | Total Time | |||
| BMI | −.09 | −.04 | −.19 | .01 | −.03 | −.02 |
| Hemoglobin | .04 | .06 | −.03 | .14 | −.13 | −.00 |
| Parent Education | −.04 | .12 | .00 | .08 | −.10 | −.04 |
| TCD Velocity | −.07 | .06 | .16 | −.10 | −.25* | −.26* |
| Age | −.40***a | −.17 | −.29**a | −.13 | .10 | −.06 |
| Gender (girls) | −.12 | .20 | .29**a | −.02 | .03 | −.01 |
p < .05
p <.01
p < .001
Correlations remain significant after controlling for False Discovery Rate.
Main effects in regression analyses
Hierarchical linear regressions were conducted to determine if TCD velocity and child age were unique predictors of deficits in cognitive function (hypothesis 2). Results in Table 3 show that BMI, hemoglobin, and parent education did not predict any score across the cognitive assessments. In step 2, Elevated TCD velocity significantly predicted lower scores on TOL Time Violations and Total Time when controlling for additional covariates, providing evidence that higher TCD velocity is uniquely associated with deficits in efficiency of executive planning in this sample. Age significantly predicted scores on the Raven’s Matrices and TOL Total Correct scores in both models. Older children had significantly lower scores on these measures compared to younger children. Finally, child gender significantly predicted Raven’s Matrices, Digit Span, and TOL Total Correct in both models, such that girls performed better compared to boys.
Table 3.
Summary of Linear Regression Analyses Predicting Measures of Cognitive Functioning
| Ravan’s Matrices |
Digit Span |
TOL |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correct |
Rule Violation |
Time Violations |
Total Time |
||||||||||||||||||||
| Predictors: | B | β | R2 | B | β | R2 | B | β | R2 | B | β | R2 | B | β | R2 | B | β | R2 | |||||
| Step 1: | .03 | .04 | .04 | .04 | .04 | .00 | |||||||||||||||||
| BMI | −1.05 | −.15 | −.18 | −.10 | −.32 | −.20 | .01 | .00 | −.09 | −.01 | −.09 | −.01 | |||||||||||
| Hemoglobin | .57 | .03 | .48 | .11 | −.30 | −.02 | 2.79 | .17 | −3.11 | −.19 | −.55 | −.03 | |||||||||||
| Parent Education | −.68 | −.08 | .31 | .13 | −.21 | −.02 | .99 | .11 | −.90 | −.10 | −.13 | −.01 | |||||||||||
| Step 2 | .30 | .12 | .19 | .07 | .11 | .08 | |||||||||||||||||
| BMI | −.09 | −.01 | −.02 | −.01 | −.38 | −.06 | .39 | .06 | −.35 | −.05 | .05 | .05 | |||||||||||
| Hemoglobin | −.38 | −.02 | .32 | .07 | −1.10 | −.07 | 2.30 | .14 | −3.80 | −.23 | −1.61 | −.76 | |||||||||||
| Parent Education | −1.28 | −.14 | .37 | .15 | .03 | .00 | .83 | .09 | −.73 | −.08 | −.16 | −.14 | |||||||||||
| TCD Velocity | −.07 | −.15 | .01 | .06 | .05 | .09 | −.04 | −.08 | −.13 | −.25* | −.15 | −2.26* | |||||||||||
| Child Age | −4.20 | −.53*** | −.28 | −.13 | −1.89 | −.25* | −1.56 | −.19 | .48 | .06 | −1.12 | −1.02 | |||||||||||
| Child Gender | −7.75 | −.26* | 1.90 | .24* | 7.49 | .36* | −1.83 | −.06 | 2.54 | .09 | .07 | .02 | |||||||||||
p < .05
p <.01
p < .001
Moderation analyses
Moderation analyses were used to test if child age and gender significantly moderated the relation between TCD velocity and cognitive function (hypothesis 3). Results of an interaction in a linear regression analysis with gender and TCD velocity, R2 = .22, F(7, 67) = 2.68, p = .017, showed that child gender significantly moderated the association of TCD velocity with scores on Digit Span, gender* TCD velocity: β = .58, p = .005, when controlling for all covariates in the model. An analyses of simple slopes (shown in Figure 1) showed that there was a significant conditional effect for boys, B = −3.71, p = .005, but not for girls, B = 1.32, p = .141. This interaction term did not significantly predict any other neurocognitive score, and there were no significant interactions between TCD velocity and age across all outcome measures.
Figure 1.
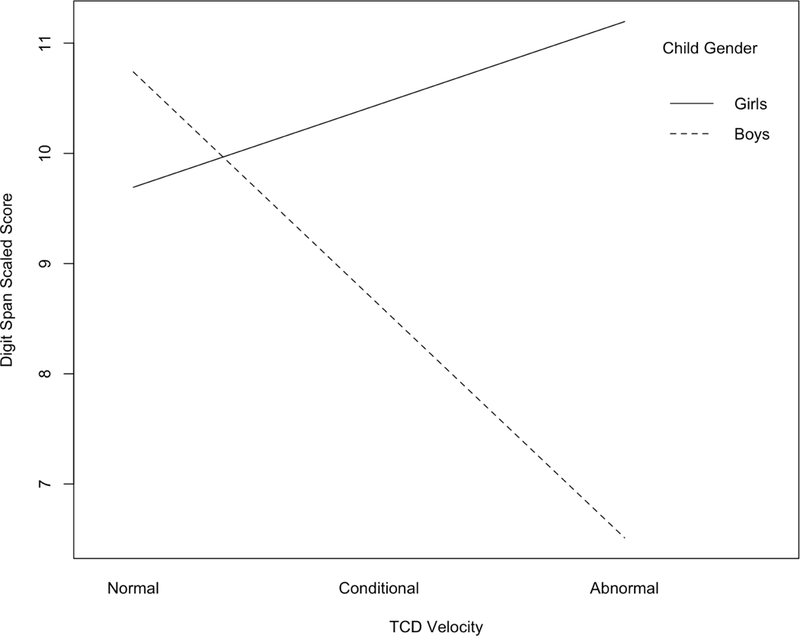
Interaction of TCD velocity and child gender as predictors of Digit Span scaled score. Child gender moderated the association of TCD velocity with WISC-IV Digit Span, such that there was a significant conditional effect for boys (B = −3.71, p = .005) but not for girls (B = 1.32, p = .141).
Discussion
The current study provides new and valuable information on cognitive function in children with SCA living in a low-resource setting, namely Africa where the majority of children with SCD are born. In the tests of the primary hypotheses of the study, we found that poorer performance on cognitive tests was associated with higher TCD velocity, older age, and male gender.
On average, children scored below the normative mean on the Raven’s Progressive Matrices as an index of perceptual reasoning. Scores from this sample, however, were consistent with those reported in a systematic review of 40 samples with test performance on the Raven’s Progressive Matrices in sub-Saharan Africans that did not specify the proportion of participants with or without SCD (Wicherts et al., 2010). Findings also showed that children with SCA in Nigeria did not differ from the normative U.S. mean on the working memory task measured by the WISC-IV Digit Span, which is consistent with the one previous study using this measure in children with SCA in Nigeria (e.g., Oluwole et al., 2016). Finally, the mean scores across the TOL (i.e., Total Correct, Rule Violations, Time Violations, and Total Time) were significantly below age-based norms. These findings are similar to those reported in a study of children with SCD in Germany which found that SCD patients took significantly more time in responding and solved significantly fewer problems on the TOL than healthy peers (Burkhardt et al., 2016).
The primary finding of the current study was that elevated TCD velocity was significantly related to lower performance in efficiency of executive planning when controlling for biological, nutritional, and social characteristics. Specifically, higher TCD velocity was associated with slower problem-solving time and more time violations on the TOL. These results suggest that elevated TCD velocity may have a specific effect on brain regions in the prefrontal cortex that are responsible for these skills. While the association of TCD velocity and cognitive function has been reported in children with SCA (Bakker et al., 2014), this study is the first to show that TCD velocity is significantly related to the efficiency of executive planning in children with SCA. Our results, however, should be viewed cautiously because the bivariate association between TCD velocity with cognitive function were no longer significant after controlling for False Discovery Rate. Nevertheless, the importance of these findings are discussed here given the relatively understudied area of research and the difficulty of conducting research in sub-Saharan Africa, where the majority of the children with SCA are born (WHO, 2006). Most importantly, findings from regression analyses showed that TCD velocity predicted the ToL Time scores even after controlling for BMI and hemodynamic and health characteristics.
Further, previous studies examining the relation between elevated TCD velocity and cognitive function have found similar associations in children with SCA who have received transfusion therapies, which has been shown to lower TCD velocity. Kral and Brown (2004) found that deficits in executive function measured by parent and teacher reports on the Behavioral Rating Inventory of Executive Function (BRIEF) were higher in children with elevated TCD velocity. Findings from the current study are particularly relevant to this population because the participants did not have a history of red blood cell transfusion. Our results, provide more evidence on the inverse relationship between between TCD velocity and executive function.
The increased demand for blood flow in the brain during cognitive tasks is one potential mechanism through which TCD velocity affects cognitive function (Sasoh et al., 2003). Resting TCD velocity in children with SCA is significantly higher than their healthy peers (Bakker et al., 2014). As a consequence, the additional need for blood flow during mental tasks that involve problem-solving, working memory, and executive planning in the prefrontal cortex may go unmet because TCD velocity in children with SCA may already be at its peak during resting states.
Results also showed that age and gender were significant predictors of cognitive function. Age was negatively related to the scores on the Raven’s Matrices and TOL Total Correct score, but it was not associated with working memory on the WISC-IV. The negative association with age is consistent with previous research that has found declines in cognitive performance with age in children with SCD (Schatz et al., 2002). Another study of Nigerian children with SCD, Oluwole et al. (2016) also found a negative correlation between age and the WMI from the WISC-IV which was not found here. Child gender was a significant predictor of cognitive performance on the working memory task and TOL, such that girls performed significantly better on the total number of puzzles they solved correctly. Findings of gender and cognitive function in children with SCA have been mixed. Some previous studies have found that girls obtain significantly higher scores on measures of cognitive function and achievement (e.g., Glass et al., 2013; King et al., 2014a, b), while others have not (e.g., Cichowitz et al., 2014; Oluwole et al., 2016).
Gender also moderated of the association between TCD velocity and WISC-IV Digit Span, such that there was a significant association for boys, but not for girls after accounting for additional covariates. As mentioned above, Groen et al. (2012) demonstrated an association between TCD velocity and visuospatial memory was stronger in boys compared to girls. We found a similar effect here in the conditional effect of TCD velocity on digit span for boys. Mechanisms of why boys with SCA show greater impairment on cognitive function tasks in this population have not been clearly defined; however, findings from the field of neuroscience provide evidence that brain maturation might occur at different rates for boys relative to girls (De Bellis et al., 2001). Although research on various domains of cognitive functioning favor boys in some instances, findings from imaging studies suggest that brain regions associated working memory might develop later for boys relative to girls (Bell, Willson, Wilman, Dave & Silverstone, 2006; Christakou et al., 2009; De Bellis et al., 2001; Goldstein et al., 2005). Studies assessing the effect of gender on the relation between cardiovascular fitness with working memory are greater for boys relative to girls (Drollette et al., 2016). Further, studies show that girls also have more developed social skills (Mashburn et al., 2008; Rimm-Kaufman, Curby, Grimm, Nathanson, & Brock, 2009), which might provide better scaffolding for learning skills related to working memory. Future research is warrented in further investigating the role of gender in the effects of SCA on child cognition.
Findings from the current study highlight the potential universality of the association between TCD velocity and cognitive function, as it was conducted in a sample in a low-income setting. Bakker et al. (2014) conducted a meta-analysis on TCD velocity findings in association to cognitive functioning in children with SCD and other populations. Studies included in this meta-analysis were primarily conducted in the United States and Europe, with the exception of Ruffieux et al. (2013), which was conducted in Cameroon. Ruffieux et al., however, found that TCD velocity was significantly related to measures of memory in Cameroonian children with SCD, as opposed to efficiency of executive planning shown here. Overall, results from Ruffiex et al. and the current study conducted in Nigeria emphasize the importance of using TCD as a potential cost-effective mechanism of screening for potential cognitive deficits in children with SCA living in a low-resource medical environment without access to the gold standard measurements of brain structure and functioning obtained from MRI.
The current study had several strengths, including the use of multiple methods, using TCD measurements of cerebral blood velocity, standardized assessments of cognition, and demographic information in a sample of Nigerian children with SCA. Nevertheless, there are weaknesses that must be addressed in future research. First, the study did not incorporate the use of healthy control group to determine how these relationships may differ across children with and without SCA in Nigera. Although these findings cannot suggest that TCD velocity is uniquely related to cognitive function in children with SCA in Nigeria, our results highlight that TCD velocity is an important predictor for children with SCA. Further, findings presented here rely on cross-sectional analyses of measurements, which limits an interpretation for the directionality of the associations. Future research should incorporate a control group of children without SCA within a prospective design. Finally, although the cognitive measures were selected due to their minimal language demand from participants, the assessments were not specifically developed for non-English speakers. These limitations notwithstanding, this study provides evidence that TCD velocity is related to the efficiency of executive planning in children with SCA in Nigeria, and it is also related to working memory for boys. Further, boys appear to have greater deficits in perceptual reasoning and planning relative to girls, and age is negatively associated with these measures as well. These findings are important for understanding risk for cogntiive deficits in this population.
Acknowledgments
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (R01NS094041) and a training grant from the National Institute of Mental Health (T32-MH18921).
References
- Adams RJ, McKie VC, Brambilla D, Carl E, Gallagher D, Nichols FT, …, Waclawiw MA (1998). Stroke prevention trial in sickle cell anemia. Controlled Clinical Trials, 19(1), 110–129. [DOI] [PubMed] [Google Scholar]
- Adams R, McKie V, Nichlos F, Carl E, Zhang DL, McKie K, …, Hess D (1992). The use of transcranial ultrasonogrphy to predict stroke in sickle cell disease. New England Journal of Medicine, 326(9), 605–610. [DOI] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions Newbury Park, CA: Sage. [Google Scholar]
- Anyiam JO, Ogala WN, & Onuora CU (2008). Body mass index of healthy Nigerian children. Nigerian Journal of Medicine, 17(4), 407–413. [DOI] [PubMed] [Google Scholar]
- Armstrong FD, Thompson RJ, Wang W, Zimmerman R, Pegelow CH, Miller S, … Vass K (1996). Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics, 97(6), 864–870. [PubMed] [Google Scholar]
- Bakker M, Hofmann J, Churches OF, Badcock NA, Kohler M, & Keage HAD (2014). Cerebrovascular function and cognitiion in childhood: A systematic review of transcranial doppler studies. BMC Neurology, 14(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Ubochi VN, Okoroikpa IN, Aguocha CM, & Ebigbo PO (2009). Agreement between clinicians’ and care givers’ assessment of intelligence in Nigerian children with intellectual disability: ‘Ratio IQ’ as a viable option in the absence of standardized “deviance IQ’ tests in sub-Saharan Africa. Behavioral and Brain Functions, 5(39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, & Silverstone PH (2006). Males and females differ in brain activation during cognitive tasks. NeuroImage, 30, 529–538. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (2000). On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics, 25, 60–83. [Google Scholar]
- Burkhardt L, Lobitz S, Koustenis E, Rueckriegel SM, & Driever PH (2016). Cognitive and fine motor deficits in a pediatric sickle cell disease cohort of mixed ethnic origin. Annals of Hematology, 96(2), 199–213. [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, & Bonds DR (1995). Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. The New England Journal of Medicine, 332(20), 1317–1322. [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M et al. (2009). Sex-dependent age modulation of fronto-striatal and temporo-parietal activation during cognitive control. NeuroImage, 48 (1), 223–236. [DOI] [PubMed] [Google Scholar]
- Cichowitz C, Carroll CP, Strouse JJ, Haywood C, & Lanzkron S (2014). Screening for neurocognitive dysfunction in an adult population with sickle cell disease. Blood, 124(21), 2717–2717. [Google Scholar]
- Culbertson WC, & Zillmer E (2001). Tower of London-Drexel University (TOLDX) Multi-Health Systems. [Google Scholar]
- DeBaun MR, & Kirkham F (2016). Central nervous system complications and management in sickle cell disease. Blood, 127(7), 829–838. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, …, & Boring AM (2001). Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex, 11(6), 552–557. [DOI] [PubMed] [Google Scholar]
- Ferster A, Tahriri P, Vermylen C, et al. (2001). Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood, 97(11), 3628–3632. [DOI] [PubMed] [Google Scholar]
- Galadanci NA, Abdullahi SU, Tabari MA, Abubakar S, Belonwu R, …, DeBaun MR (2015). Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): Challenges of conducting a feasibility trial. Pediatric Blood and Cancer, 62(3), 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafuri DL, Chaturvedi S, Rodeghier M, Stimpson SJ, McClain B, Byrd J, & DeBaun MR (2017). Secondary benefit of maintaining normal transcranial Doppler velocities when using hydroxyurea for prevention of severe sickle cell anemia. Pediatric Blood and Cancer, 64(7), e26401–e26405. [DOI] [PubMed] [Google Scholar]
- Glass P, Brennan T, Wang J, Lutchman-Jones L, Hsu L, Bass CM, … Gordeuk V (2013). Neurdevelopmental deficits among infants and toddlers with sickle cell disease. Journal of Developmental and Behavioral Pediatrics, 34(6), 299–405. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC et al. (2005). Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology, 19, 509–519. [DOI] [PubMed] [Google Scholar]
- Groen MA, Whitehouse AJO, Badcock NA, & Bishop DVM (2012). Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain and Behavior, 2(3), 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey C, & Roberts IA (2003). The role of hydroxyurea in sickle cell disease. British Journal of Haematology, 120(2), 177–186. [DOI] [PubMed] [Google Scholar]
- Helton KJ, Adams RJ, Kesler KL, Lockhart A, Aygun B, Driscoll C, …, Ware RE (2014) Magnetic resonance imaging/angiography and transcranial Doppler velocities in sickle cell anemia: results from the SWiTCH trial. Blood, 124(6), 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AM, Kirkham FJ, Prengler M, Telfer P, Lane R, Vargha-Khadem F, & de Haan M (2006). An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anemia. British Journal of Haematology, 132(1), 99–107. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27, 87–96. [DOI] [PubMed] [Google Scholar]
- Jordan LC, & DeBaun MR (2017). Cerebral hemodynamic assessment and neuroimaging across the lifespan in sickle cell disease. Journal of Cerebral Blood Flow and Metabolism, e-publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawadler JM, Clayden JD, Clark CA, & Kirkham FJ (2016). Intelligence quotient in paediatric sickle cell disease: a systematic review and meta-analysis. Developmental Medicine and Child Neurology, 58(7), 672–680. [DOI] [PubMed] [Google Scholar]
- King AA, Rodeghier MJ, Panepinto JA, Strouse JJ, Casella JF, Quinn CT, … Debaun MR (2014a). Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. American Journal of Hematology, 89(10), E188–E192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, Mckinstry RC, … Debaun MR (2014b). Parent education and biologic factors influence on cognition in sickle cell anemia. American Journal of Hematology, 89(2), 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral MC, & Brown RT (2004). Transcranial doppler ultrasonography and executive dysfunction in children with sickle cell disease. Journal of Pediatric Psychology, 29(3), 185–195. [DOI] [PubMed] [Google Scholar]
- Lagunju IA, Brown BJ, & Sodeinde OO (2013). Chronic blood transfusion for primary and secondary stroke prevention in Nigerian children with sickle cell disease: A 5-Year appraisal. Pediatric Blood and Cancer, 60, 1940–1945. [DOI] [PubMed] [Google Scholar]
- Mashburn A, Pianta R, Barbarin O, Bryant D, Hamre B, Downer J, … Howes C (2008). Measures of Classroom Quality in Prekindergarten and Children’s Development of Academic, Language, and Social Skills. Child Development, 79(3), 732–749. [DOI] [PubMed] [Google Scholar]
- Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, …, & Gill FM (1998). Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood, 91(1), 288–294. [PubMed] [Google Scholar]
- Oluwole OB, Noll RB, Winger DG, Akinyanju O, & Novelli EM (2016). Cognitive functioning in children from Nigeria with sickle cell anemia. Pediatric Blood & Cancer, 63(11), 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H. (1994). Guidelines for the Control of Haemoglobin Disorders, ed. Sardinia, Italy, WHO. [Google Scholar]
- Organization, W.H. (2000). Obesity: Preventing and Managing the Global Epidemic, ed. Geneva, Switzerland, WHO. [PubMed] [Google Scholar]
- Organization, W. H. (2006). Sickle cell anaemia. Fifty-Ninth World Health Assembly, A59/9(April), 1–5. [Google Scholar]
- Powars D, Wilson B, Imbus C, Pegelow C, & Allen J (1978). The natural history of stroke in sickle cell disease. The American Journal of Medicine, 65(3), 461–71. [DOI] [PubMed] [Google Scholar]
- Prohovnik I, Hurley-Jensen A, Adams R, De Vivo D, & Pavlakis SG (2009). Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. Jounal of Cerebral Blood Flow and Metabolism, 29, 803–810. [DOI] [PubMed] [Google Scholar]
- Raven JC (1998). Raven’s progressive matrices Oxford: Oxford Psychologists Press. [Google Scholar]
- Rimm-Kaufman SE, Curby TW, Grimm KJ, Nathanson L, & Brock LL (2009). The contribution of children’s self-regulation and classroom quality to children’s adaptive behaviors in the kindergarten classroom. Developmental Psychology, 45(4), 958–972. [DOI] [PubMed] [Google Scholar]
- Ruffieux N, Njamnshi AK, Wonkam A, Hauert CA, Chanal J, …, Sztajzel R (2013). Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychology, 19(2), 143–160. [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Schatz J, & Roberts CW (2010). Cerebral blood flow velocity and language functioning in pediatric sickle cell disease. Journal of the International Neuropsychological Society, 16(2), 326–34. [DOI] [PubMed] [Google Scholar]
- Sasoh M, Ogasawara K, Kuroda K, Okuguchi T, Terasaki K, …, & Ogawa A (2003). Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surgical Neurology, 59, 455–463. [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, & Kramer JH (2002). Cognitive functioning in children with sickle cell disease: a meta-analysis. Journal of Pediatric Psychology, 27(8), 739–748. [DOI] [PubMed] [Google Scholar]
- Steen RG, Miles MA, Helton KJ, Strawn S, Wang W, Xiong X, & Mulhern RK (2003). Cognitive impairment in children with hemoglobin SS sickle cell disease: Relationship to MR Imaging findings and hematocrit. Americal Journal of Neuroradioly, 24(3), 382–389. [PMC free article] [PubMed] [Google Scholar]
- Stroobant N, & Vingerhoets G (2000). Transcranial Doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: A review. Neuropsychology Review, 10(4), 213–231. [DOI] [PubMed] [Google Scholar]
- Strouse JJ, Cox CS, Melhem ER, Lu H, Kraut MA, Razumovsky A, … Casella JF (2006). Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA), 108(1), 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children--Fourth edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wicherts JM, Dolan CV, Carlson JS, & van der Maas HLJ (2010). Raven’s test performance of sub-Saharan Africans: Average performance, psychometric properties, and the Flynn Effect. Learning and Individual Differences, 20(3), 135–151. [Google Scholar]


