Abstract
Introduction:
Rasagiline is an MAO-B inhibitor with possible neuroprotective effects in patients with amyotrophic lateral sclerosis (ALS).
Methods:
We performed a randomized, double-blind, placebo-controlled trial of 80 ALS participants with enrichment of the placebo group with historical controls (n=177) at 10 centers in the United States. Participants were randomized in a 3:1 ratio to 2 mg rasagiline daily or placebo. The primary outcome was the average slope of decline of the ALS Functional Rating Scale-Revised (ALSFRS-R). Secondary measures included slow vital capacity, survival, mitochondrial and molecular biomarkers, and adverse event reporting.
Results:
There was no difference in the average 12 months ALSFRS-R slope between rasagiline and the mixed placebo and historical control cohorts. Rasagiline did not show signs of drug-target engagement in urine and blood biomarkers. Rasagiline was well tolerated with no serious adverse events.
Discussion:
Rasagiline did not alter disease progression compared to controls over 12 months of treatment.
Clinicaltrials.gov identifier: NCT01786603.
Keywords: Motor Neuron Disease, Randomized Controlled Clinical Trials, Amyotrophic Lateral Sclerosis, MAO-B Inhibitor, Rasagiline, Biomarker
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease with a median survival of approximately 2 years.1 Despite dozens of trials over the last 20 years, only 2 drugs have received Food and Drug Administration approval, riluzole and edaravone, which have modest benefits on survival or disease progression.2–4 There is an urgent need for new therapies. More recent attempts to improve the yield of clinical trials have centered on developing biomarkers to show early drug-target engagement or use of large data sets from prior clinical trials to improve trial efficiency.5–7
Mitochondrial dysfunction leads to downstream ALS pathological features, including oxidative stress and protein misfolding and aggregation.8–10 Many genetic mutations in familial ALS affect enzymes with key roles in mitochondrial function.11 Rasagiline is a monoamine oxidase type B (MAO-B) inhibitor approved for symptomatic treatment of Parkinson’s disease, with a well-defined safety profile. In vitro studies in neuronal cell cultures and in vivo studies in animal models of central neuronal damage have suggested a neuroprotective role for rasagiline mediated by antioxidative or anti-apoptotic actions.12–16 Arguments supporting the use of rasagiline in ALS include: autopsy studies suggesting MAO-B is upregulated in ALS tissues;13,17,18 increased survival in an ALS animal model;19 and a retrospective case series in humans suggesting slowing of functional loss.20 Our prior open-label study of rasagiline in ALS while not showing improved function versus historical controls, showed evidence for drug-target engagement in mitochondrial biomarkers.21
Here we tested whether rasagiline slowed disease progression and engaged mitochondrial biomarkers over 12-months of treatment.
METHODS
Trial Design:
We performed a 12-month randomized, double-blind, placebo-controlled study enriched with historical placebo controls at 10 sites in the United States between November 2012 and August 2017. The trial was approved by Institutional Review Boards at each site. Written informed consent was obtained from all participants, in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. This study was registered at clinicaltrials.gov (NCT01786603). A data safety monitoring board met quarterly by phone to review adverse events and serious adverse events.
Participants were 21 to 80 years of age; had laboratory-supported probable, probable, or definite ALS by El-Escorial criteria; slow or forced vital capacity ≥ 75% predicted; and onset of symptoms within 2 years of enrollment.22 Women of childbearing potential had to be using an effective means of birth control. Participants were excluded if they required tracheostomy or non-invasive ventilation; had a diaphragmatic pacemaker; received an experimental drug within the last 30 days; were taking medications contra-indicated for use with rasagiline (sympathomimetic agents, analgesics with serotoninergic properties, fluoxetine or fluvoxamine, or taking larger doses of antidepressants with serotoninergic properties); had a diagnosis of other neurodegenerative disease; were currently pregnant or breastfeeding; had a history of recent substance abuse, or demonstrated non-compliance with prior clinical study; or had an unstable medical illness.
The trial was conducted by The Western ALS Study Group at 10 academic centers in the United States (Appendix). Data coordinating was through the Muscle Study Group at the University of Rochester Medical Center.
Rasagiline was provided by TEVA Pharmaceuticals. Rasagiline and placebo were over-encapsulated by the University of Iowa’s Research Pharmacy, using identical gelatin capsules.
Outcomes and Measures:
Baseline characteristics included sex, age, and self-reported race/ethnicity. The ALS Functional Rating Scale – Revised (ALSFRS-R), the slow vital capacity, the global ALS quality of life question, and adverse events were assessed at baseline and monthly for 2 months, then every other month for a total of 12 months. If a subject was unable to attend a study visit, the ALSFRS-R was performed by phone. Evaluators at each site were trained at study start up in administration of the ALSFRS-R and slow vital capacity. Blood and urine for biomarkers were collected at baseline, months 6 and 12, except for transactive response DNA binding protein 43 kDa (TDP43) which was collected at baseline and month 12.
The primary outcome for the study was the difference in the average monthly slope of decline over 12 months of the ALSFRS-R between treated and untreated patients (plus historical controls).23 Changes in the ALSFRS-R predict survival, and a change of greater than 20% in the slope of decline is considered to be clinically meaningful.24
Secondary outcomes:
The slow vital capacity is widely used in ALS clinical trials, and for most individuals is virtually identical to the forced vital capacity.25 The baseline vital capacity and decline in vital capacity are both predictors of survival.26,27
The single item ALS quality of life (ALSQOL) asks the participant to rank their global quality of life now taking into account physical, emotional, social, spiritual, and financial aspects of living, and is ranked from 0=very bad to 10 =excellent.28
For survival analysis we defined mortality as death or use of invasive mechanical ventilation.
Exploratory outcomes:
In a prior study, we showed that using commercially available assays of mitochondrial membrane stability or apoptosis, administration of rasagiline appeared to increase mitochondrial membrane potential, decrease oxidative stress, and increase the ratio of anti-apoptotic to pro-apoptotic proteins.21 Several mitochondrial assays were completed using lymphocytes including two measure of lymphocyte mitochondrial membrane potential – JC-1 (MitoProbe JC-1 Assay Kit for Flow Cytometry; Invitrogen),29 and Mitotracker Red CMXRos (Vybrant Apoptotic Assay Kit #11; Molecular Probes);30 and a measure of apoptosis, dye-conjugated Annexin V stain in which the amount of exposed phosphatidylserine was approximated by calculating the percentage of Annexin-positive cells (Supplemental Methods Table e2 for a list of all biomarkers reported in this study).31 Bcl-2 and Bax messenger RNA ratios were determined from whole blood providing information about a cell or tissue’s relative state of apoptosis activity;32 and a measure of free radical stress was completed from plasma, the Oxygen Radical Antioxidant Capacity assay (ORAC; OxiSelect ORAC Activity Assay, Cell Biolabs).33 The primary biomarker analysis was performed on 6-month change, due to early termination affecting the 12-month time point.
In urine, levels of 15-F2t-isoprostane (IsoP), a lipid peroxidation product, reflect oxidative stress. We used a validated, commercially available assay that members of our team showed were increased in a prior ALS study (8-isoPGF2a ELISA Kit, Detroit R&D).34
Frontotemporal lobar degeneration and ALS are both marked by nuclear exclusion and cytoplasmic deposition of TDP43, which is considered a pathological hallmark of both disorders.35,36 Plasma TDP-43 as measured by ELISA has been proposed as a possible biomarker for Alzheimer’s disease and frontotemporal dementia (FTD), with elevations in individuals with Alzheimer’s disease and FTD but not healthy controls, and a phosphorylated TDP43 antibody has been suggested in FTD to correspond to brain pathology on autopsy.37,38 This raises the possibility that phosphorylated TDP-43 may serve as a surrogate marker for progression or of disease state in ALS, and may be measurable in peripheral tissues, such as platelets that represent cytosolic TDP-43 population.39,40 As an exploratory biomarker, platelet TDP-43 was measured from ALS patient samples. Platelet cytosolic samples were analyzed by a high throughput capillary electrophoresis based SimpleWestern system (Proteinsimple, CA, USA). The samples were analyzed with anti TDP-43 (total) and phosphorylated TDP-43 (p(S409/410) antibodies (ProteinTech, USA) that cross-linked to the capillaries. Fluorescent labeled proteins were eluted and intensities were measured. Predictive phosphorylation value (PPV) was calculated by p(S409/410) TDP43 arbitrary units [a.u.] / Total TDP-43 a.u.36,39
Randomization and blinding:
Participants were randomly assigned to rasagiline or placebo in a 3:1 ratio, stratified by riluzole use and bulbar onset (yes/no). Randomization was performed centrally utilizing a computer-generated block structure (4 groups based on stratification, in blocks of 4). Study investigators, evaluators and participants were blinded to treatment assignment.
Sample size:
In order to increase efficiency, we enriched our placebo cohort with historical placebo controls from clinical trials of minocycline.41,42 For the primary outcome only we included the subset of subjects within the historical placebo database with disease durations less than 2 years and baseline FVC greater than or equal to 75%. This cohort had a mean decline in the ALSFRS-R of 1 unit per month. With 80 subjects, 20 placebo (augmented with n=177 placebo participants from a previous randomized controlled trial of minocycline) and 60 treated, we estimated 80% power (at two-sided 5% level of significance) to detect a treatment-response where there is a 30% or greater reduction in the ALSFRS-R slope of decline. The power calculations were based on 1000 simulations where the slopes for a random subset of 60 placebo patients from our placebo database were reduced by 30% and then compared to the remaining placebos using the same linear mixed effects (LME) model as was used in the current trial.43
Statistical analysis:
Descriptive statistics (mean and standard deviation, or median, minimum and maximum) were used to describe the study population and adverse event frequencies. Balanced randomization was tested using a 3 group ANOVA. For the primary outcome (ALSFRS-R slope of decline) we fit an LME model to the data from each patient. The model for each fit is given by the equation: Yij = (B0 + b0i) + (B1 + B2*dose(i) + b1i)*tij + eij, where Yij is the ALSFRS-R score for the ith patient at time tij and dose(i) is zero for placebo and equal to 1 for rasagiline; B0, B1 and B2 are fixed effects for initial value, slope and slope change due to drug, respectively; b0i, b1i and eij are random effects for initial value, slope and within patient variation. All participant data was used, regardless of length of follow-up. The model is self-weighting so that patients with longer follow-up have greater influence on parameter estimates than patients with shorter follow-up.43 Additional terms were added to the model to adjust for effects of cofactors that differ significantly between the treated, controls, and historical controls (riluzole use, symptom duration). The test for efficacy is based on testing H0: B2=0, where B2 is the parameter for the change in the slope of ALSFRS-R over time due to rasagiline. The same LME model was used for secondary outcomes (vital capacity and quality of life). For the ALSFRS-R each data set was fit separately: first only data from the 80 subjects in the trial was used; next the 80 subjects were augmented with data from historical placebo controls. Death from any cause or tracheostomy were considered survival endpoints. Kaplan-Meier curves were created to compare survival, and a two-sided log-rank test was used to test equality of survival time.42 The blood and urine mitochondrial biomarker assays were analyzed by paired Student’s T-test. The effect of rasagiline on these measures was tested using a LME previously described. For quality assurance, we used plots of individual measurements vs time to detect outlier values for ALSFRS-R, VC, QoL, and biomarkers. Any measurement that was beyond 3 standard deviations (SD) of the best fitted value (from the LME model) was flagged and rechecked for accuracy by the participant’s institution. All statistical testing was two-sided, and p<0.05 was considered significant. Statistical analysis was performed using Stata (version 12, College Station, TX).
RESULTS
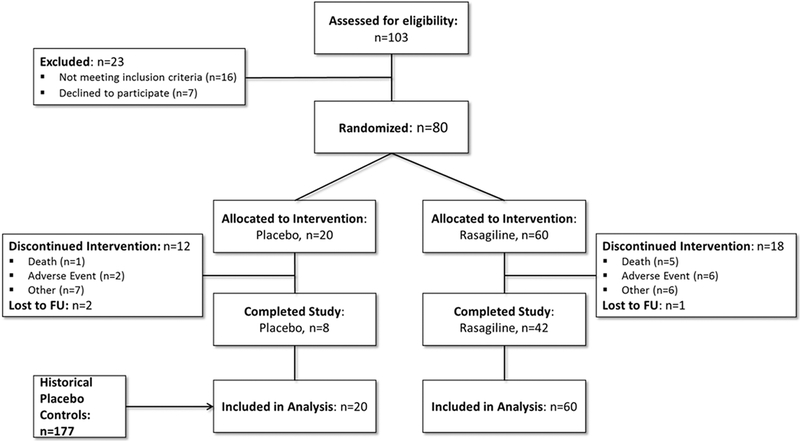
One hundred and three individuals were screened for eligibility, and 80 were randomized, 60 to rasagiline, and 20 to placebo (Figure 1). Reasons for screen failure included failure to meet inclusion criteria and individuals who declined to participate. Thirty individuals discontinued the study (60% from placebo, compared to 30% rasagiline), most commonly due to adverse events. There was 1 death in the placebo group (5%) and 8 deaths in the rasagiline group (13.3%, not statistically significant). No subject met mortality criteria due to need for invasive mechanical ventilation. All 80 participants were included in the intention to treat analysis.
Figure 1. Study Flow Diagram.
One hundred-three were assessed for eligibility and 80 were randomized in a 3:1 ratio. Deaths were similar between groups. The placebo group was enriched with historical placebo controls from completed minocycline clinical trials.41,42
Participants were mostly male, middle aged, with limb-onset (Table 1). Compared to historical placebo controls, study participants were more likely to be taking riluzole, and had longer symptom duration.
Table 1.
Baseline Characteristics.
| Item | Rasagiline Treated | Rasagiline Controls | Historical Controls41,42 | p-value* |
|---|---|---|---|---|
| Number participants | 60 | 20 | 177 | |
| Number female (%) | 20 (33%) | 7 (35%) | 65 (37%) | 0.89 |
| Number bulbar onset (%) | 10 (17%) | 3 (15%) | 44 (25%) | 0.29 |
| Number taking riluzole (%) | 49 (82%) | 16 (80%) | 121 (68%) | <.001 |
| Mean age in years (SD/range) | 58.4 (10.2 / 28–75) | 57.5 (8.5 / 40–73) | 57.3 (11.0 / 30–80) | 0.22 |
| Mean symptom duration years (SD/range) | 1.23 (0.49 / 0.43–2.04) | 1.37 (0.46 / 0.52–2.05) | 1.06 (0.46 / 0.23–2.0) | 0.004 |
| Mean baseline % predicted vital capacity (SD/range) | 92.5 (12.3 / 73–122) | 94.4 (12.5 / 75–124) | 94.9 (15.3 / 75–178) | 0.52 |
| Mean baseline ALSFRS-R (SD/range) | 38.2 (6.1 / 17–48) | 35.9 (7.5 / 14–45) | 38.7 (4.8 / 23–47) | 0.08 |
P-value for 3 way ANOVA comparing treated, controls, and historical controls.
Outcomes and Measures:
Rasagiline did not slow the rate of disease progression compared to placebo as measured by ALSFRS-R, slow vital capacity, or ALSQOL (Table 2). The 20% difference in the ALSFRS-R slope of decline between rasagiline and placebo was further attenuated on augmenting the placebo group with historical controls, which reinforced the initial impression of no clinical benefit on disease progression (Table 3, 4).
Table 2.
Outcome Measures: rasagiline n=60, placebo n=20
| Outcome | Placebo | Rasagiline | Difference Ras - Plac | % Difference | p-Value |
|---|---|---|---|---|---|
| ALSFRS-R | −1.25 (0.22 / −1.70,−0.81) | −1.01 (0.13 / −1.27,−0.75) | 0.24 (0.26 / −0.27,0.76) | 20 | 0.35 |
| FVC % | −2.48 (0.54 / −3.54,−1.43) | −2.24 (0.31 / −2.86,−1.64) | 0.24 (0.62 / −0.98,1.45) | 11 | 0.65 |
| ALSQoL | 0.12 (0.03 / −0.18,−0.05) | 0.09 (0.02 / −0.13,−0.06) | 0.03 (0.04 / −0.05,0.09) | 21 | 0.47 |
SD = standard deviation; CI = confidence interval; Ras = rasagiline; Plac = placebo;
Table 3.
Analysis of slope by treatment group from the MLE model
| Rasagiline Treated | Rasagiline Controls | Historical Controls | |
|---|---|---|---|
| N subjects | 60 | 20 | 177 |
| Ave. ALSFRS-R Slope | −1.00 | −1.26 | −1.12 |
| 95% Confidence Interval | (−1.22,−0.77) | (−1.64,−0.87) | (−1.25,−0.99) |
Table 4.
Test for treatment effect from LME model
| Tests – difference in slope between groups | Change | 95% CI | p-value |
|---|---|---|---|
| Rasagiline v Placebo | 0.26 | (−0.19,+0.71) | 0.26 |
| Rasagilne v Historical Controls | 0.12 | (−0.14,+0.38) | 0.36 |
| Placebo v Historical Controls | −0.14 | (−0.55,+0.27) | 0.50 |
| Rasagiline v (Placebo + Historical Controls) | |||
| Unadjusted | 0.13 | (−0.12,+0.39) | 0.30 |
| Adjusted for Symptom Duration | 0.09 | (−0.16,+0.34) | 0.16 |
| Adjusted for Riluzole Use and Symptom Duration | 0.10 | (−0.15,+0.35) | 0.43 |
V = versus; CI = confidence interval
An adjusted analysis for baseline differences between study participants and historical placebo controls (riluzole use and symptom duration) did not affect the estimates of treatment effects of rasagiline on ALSFRS-R slope of decline (Table 4).
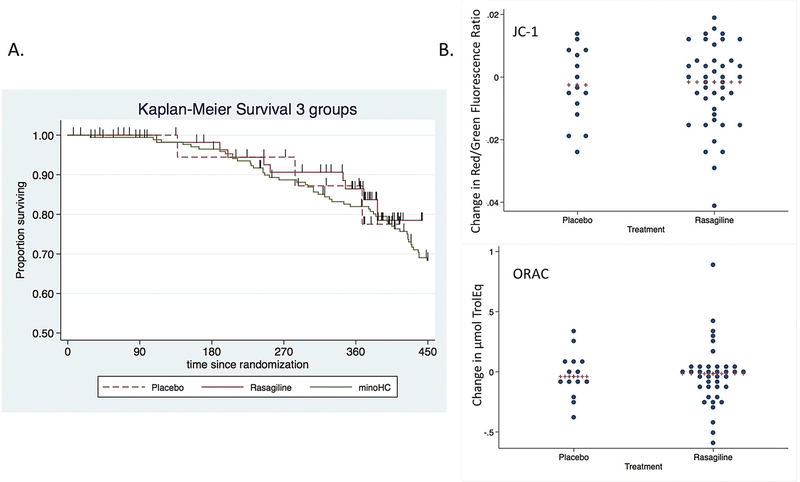
Rasagiline provided no benefit on survival compared to placebo participants or to historical placebo controls (Figure 2A). We did not see any consistent trend in blood biomarker change, either between baseline and 6 months follow up or between rasagiline and placebo (Figure 2B, Supplemental table e3). We saw no rasagiline-related difference in urine IsoP or platelet TDP43 predicted phosphorylation value; however, both rasagiline and placebo groups decreased in predicted TDP43 phosphorylation value over 1 year.
Figure 2. Survival curves and mitochondrial biomarkers.
A) There was no difference in survival between the rasagiline, placebo, and historical controls (Log-rank test Chi Squared=0.41, p=0.81). Vertical hash lines indicate withdrawals (dropouts). B) We found no treatment-related difference in the 6 months change in blood biomarkers (JC-1 above, ORAC below) which showed drug-target engagement in our prior study.
Harms:
There were no treatment-related severe adverse events. The most frequent adverse events were gastrointestinal disturbances, followed by musculoskeletal, both more frequent in placebo group (Supplemental able e4).
DISCUSSION
Rasagiline did not slow the rate of functional decline in this study and showed no evidence for biomarker engagement. We experienced an unanticipated high rate of discontinuations from the study. However, when evaluating the reasons for discontinuation there is no clear explanation (e.g. more adverse events in one group). Comparing this study to the minocycline randomized controlled trials from which the historical controls were obtained; we saw an overall increased study-wide discontinuation rate (37.5% versus 8%). The statistical analysis counts all available data, creating slopes of decline for each participant. To some degree this adjusts for the problem of missing data, assuming the relative rates of discontinuation were equal across the study timeline and treatment groups. In addition, many decisions went into the study design which are important for discussion: the use of historical controls to increase the study power; the choice to power the study for a 30% difference in ALSFRS-R slope of decline; and use of peripheral mitochondrial and other molecular biomarkers to show drug-target engagement.
This study utilized a screening study design which enriched our placebo group with historical placebo controls to increase power.7 This decision was based on the observation that on aggregate the rates of decline in the ALSFRS-R have been fairly constant across studies with similar inclusion criteria.7 WALS has an extensive historical placebo control data base to pull from, and more recently large data sets like The Pooled Resource Open-Access ALS Clinical Trials database (PRO-ACT) have become available.44 The decision to use a screening study design is based on an assumption that if negative, the likelihood of any meaningful benefit is statistically quite small.45
Several assumptions underlie the use of historical controls. The first assumption is that matching for basic inclusion and exclusion criteria will yield similar groups. Here we found differences in the frequency of riluzole use and time from symptom onset, which required adjustment in an ancillary analysis. A second assumption is that given similar inclusion and exclusion criteria rates of functional decline on the ALSFRS-R will be consistent. Indeed there were no significant differences in rates of functional decline between our placebo group and historical controls. That said, while on the aggregate the slope in decline in the ALSFRS-R appears to be fairly constant, which would support this study design, more recent modeling with PRO-ACT have shown the group with symptom duration < 2 years and FVC > 75% is actually quite diverse, and splits into rapid and slow progressers.46 The strongest driver for this is time since symptom onset – and while there was a difference in time from symptom onset between study participants and historical controls, the adjusted analysis confirmed our primary analysis. While it is likely true that a study such as this rules out a large benefit for rasagiline, more sophisticated strategies for historical control matching, a run-in period to stratify into fast and slow progressers, and trial simulation could increase the power of such screening studies.47 Finally the recent approval of edaravone may limit the ability to use such historical data sets, as the current standard of care will include edaravone, which may affect disease progression.2
While differences in the slope of progression >20% are generally considered to be clinically meaningful, the ALSFRS-R documents the decline of our basic activities of daily living, and as such, much smaller changes projected over time would likely be meaningful to ALS patients.24 Here we chose 30% as we felt the role of such a screening trial should be to select for drugs with potential robust effects: the desired effect of 30% reduction in slope is roughly equivalent to a 4–6 month life extension (the effect measured for taking riluzole).
Mitochondrial pathology appears to be a consistent finding in ALS – in both autopsy tissues and in animal models.21,48 Many heritable ALS mutations are in genes which affect mitochondrial function. Oxidative changes are also a common downstream event. The assays we used here and in our prior study have the advantage of being commercially available. The decision to use peripheral markers, e.g. from lymphocytes, platelets or urine, would only indirectly reflect activity in the central nervous system. In our prior study we used a combination of established biomarkers for oxidative stress (i.e. ORAC), but also more exploratory assays (annexin, mitotracker, JC-1, and Bcl/Bax) that are used in cell culture experiments in basic science laboratories; our intention was to treat participant blood samples as if they are a “cell culture.” Indeed in our prior study we saw consistent shifts in lymphocyte biomarker values consistent with a potential beneficial effect of rasagiline, on the mitochondrial membrane potential, oxidative stress, or apoptosis markers. Here we saw higher variability than anticipated based on results from our prior study and did not see consistent trends either in the rasagiline group comparing baseline to 6 months, or between rasagiline and placebo. The possibilities for the difference in biomarker findings between the studies include: higher than anticipated variability; differences in wait-time before processing for samples from different sites; need for better strategies for handling outlier data; and the possibility the original observation was erroneous.
Platelet TDP43 is an exploratory biomarker, and while there were shifts in both treated and placebo towards lower values it is not clear what this means for the overall utility of platelet TDP43 as a biomarker. While it is possible this would reflect a reduction in the phosphorylated TDP43 population, it is also possible this reflects an increase in total TDP43.
Limitations to our current study include the small sample size – to definitively answer the question of a small benefit for rasagiline would require a much larger study. In addition, the participants recruited for this study may or may not reflect ALS patients in the general population. Additional limitations include incomplete matching of baseline characteristics between our study participants and historical controls. Finally, only 50 participants completed the study, and so incomplete data could have affected estimates if some unknown factor contributed to individuals exiting the study, and this was different between groups.
In this study rasagiline was well tolerated but we were unable to detect a 30% improvement in the rate of functional decline. The recently completed randomized controlled study of rasagiline in Germany also did not meet its primary endpoint of a benefit on survival; however a post-hoc analysis was able to discern approximately 40% slowing of functional loss in the half of participants who were most rapidly progressing.49 This is in line with the experience of edaravone, which also used a strategy to select for the more rapidly progressing participants. This means in the future for drugs which may provide a protective benefit an inclusion strategy which selects for normal-to rapid progressers may reduce the overall variability and improve the power to detect a small benefit on functional decline compared to standard practice. Such an approach could also be used with studies that utilize historical controls. The same selection using rates of progression could be applied to historical placebo data sets, assuming such data sets including edaravone become available. The main limitation may be to trial generalizability, because such approaches raise the question of whether such benefits in more rapidly progressing participants early in the disease course will persist or accrue to patients with longer disease courses or much slower rates of functional loss.
Supplementary Material
Study Funding:
This study was funded by Food and Drug Administration Orphan Products Division RO1 FD003739. Additional funding was provided in part by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (# UL1TR000001). Dr. Statland’s work on this project was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (# KL2TR000119). Columbia’s Biomarkers Laboratory is supported by ES009089. Biomarker studies performed by the KU Alzheimer’s Disease Center Mitochondrial Genomics and Metabolism Core was supported by P30 AG035982.
Dr. Statland’s work on this project was supported by an NCATS grant awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119.
Dr. Barohn’s work on the project was supported by Food and Drug Administration Orphan Products Division RO1 FD003739.
Dr. Swerdlow’s work on the project was supported by Food and Drug Administration Orphan Products Division RO1 FD003739, and by P30 AG035982.
Dr. Wilkins’ work on the project was supported by Food and Drug Administration Orphan Products Division RO1 FD003739, and by P30 AG035982.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale – Revised
- ALSQOL
ALS quality of life
- ELISA
enzyme linked immunosorbent assay
- IsoP
isoprostane
- MAO-B
monoamine oxidase B
- MLE
mixed linear effects
- ORAC
oxygen radical antioxidant capacity
- PRO-ACT
Pooled Resource Open-Access ALS Clinical Trials
- RCT
randomized controlled trial
- SVC
slow vital capacity
- TDP43
TAR DNA-binding protein 43
Footnotes
Disclosures: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
All other authors no disclosures to report.
REFERENCES
- 1.Goyal NA, Mozaffar T. Experimental trials in amyotrophic lateral sclerosis: a review of recently completed, ongoing and planned trials using existing and novel drugs. Expert opinion on investigational drugs 2014;23(11):1541–1551. [DOI] [PubMed] [Google Scholar]
- 2.Writing G, Edaravone ALSSG. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet Neurology 2017;16(7):505–512. [DOI] [PubMed] [Google Scholar]
- 3.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 1994;330(9):585–591. [DOI] [PubMed] [Google Scholar]
- 4.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996;347(9013):1425–1431. [DOI] [PubMed] [Google Scholar]
- 5.Gordon PH, Cheung YK, Levin B, Andrews H, Doorish C, Macarthur RB, et al. A novel, efficient, randomized selection trial comparing combinations of drug therapy for ALS. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 2008;9(4):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson KA, Cudkowicz ME, Berry JD. Clinical Trial Designs in Amyotrophic Lateral Sclerosis: Does One Design Fit All? Neurotherapeutics 2015;12(2):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RG, Moore DH, Forshew DA, Katz JS, Barohn RJ, Valan M, et al. Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design. Neurology 2011;77(10):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cozzolino M, Rossi S, Mirra A, Carri MT. Mitochondrial dynamism and the pathogenesis of Amyotrophic Lateral Sclerosis. Frontiers in cellular neuroscience 2015;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lezi E, Swerdlow RH. Mitochondria in neurodegeneration. Adv Exp Med Biol 2012;942:269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palomo GM, Manfredi G. Exploring new pathways of neurodegeneration in ALS: the role of mitochondria quality control. Brain Res 2015;1607:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weishaupt JH, Hyman T, Dikic I. Common Molecular Pathways in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Trends Mol Med 2016;22(9):769–783. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Raya S, Blaugrund E, Trembovler V, Shilderman-Bloch E, Shohami E, Lazarovici P. Rasagiline, a monoamine oxidase-B inhibitor, protects NGF-differentiated PC12 cells against oxygen-glucose deprivation. J Neurosci Res 1999;58(3):456–463. [PubMed] [Google Scholar]
- 13.Chen JJ, Swope DM. Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease. J Clin Pharmacol 2005;45(8):878–894. [DOI] [PubMed] [Google Scholar]
- 14.Finberg JP, Takeshima T, Johnston JM, Commissiong JW. Increased survival of dopaminergic neurons by rasagiline, a monoamine oxidase B inhibitor. Neuroreport 1998;9(4):703–707. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama W, Akao Y, Youdim MB, Davis BA, Naoi M. Transfection-enforced Bcl-2 overexpression and an anti-Parkinson drug, rasagiline, prevent nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase induced by an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol. J Neurochem 2001;78(4):727–735. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama W, Youdim MB, Naoi M. Antiapoptotic properties of rasagiline, N-propargylamine-1(R)-aminoindan, and its optical (S)-isomer, TV1022. Ann N Y Acad Sci 2001;939:320–329. [DOI] [PubMed] [Google Scholar]
- 17.Jossan SS, Ekblom J, Aquilonius SM, Oreland L. Monoamine oxidase-B in motor cortex and spinal cord in amyotrophic lateral sclerosis studied by quantitative autoradiography. J Neural Transm Suppl 1994;41:243–248. [DOI] [PubMed] [Google Scholar]
- 18.Youdim MB, Gross A, Finberg JP. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. British journal of pharmacology 2001;132(2):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waibel S, Reuter A, Malessa S, Blaugrund E, Ludolph AC. Rasagiline alone and in combination with riluzole prolongs survival in an ALS mouse model. Journal of neurology 2004;251(9):1080–1084. [DOI] [PubMed] [Google Scholar]
- 20.Drory V Evalutation of treatment with rasagiline in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2007;Suppl 1:145. [Google Scholar]
- 21.Macchi Z, Wang Y, Moore D, Katz J, Saperstein D, Walk D, et al. A multi-center screening trial of rasagiline in patients with amyotrophic lateral sclerosis: Possible mitochondrial biomarker target engagement. Amyotroph Lateral Scler Frontotemporal Degener 2015;16(5–6):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1(5):293–299. [DOI] [PubMed] [Google Scholar]
- 23.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of the neurological sciences 1999;169(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- 24.Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 2010;11(1–2):178–180. [DOI] [PubMed] [Google Scholar]
- 25.Pinto S, de Carvalho M. Correlation between Forced Vital Capacity and Slow Vital Capacity for the assessment of respiratory involvement in Amyotrophic Lateral Sclerosis: a prospective study. Amyotroph Lateral Scler Frontotemporal Degener 2017;18(1–2):86–91. [DOI] [PubMed] [Google Scholar]
- 26.Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. Journal of the neurological sciences 2008;275(1–2):69–73. [DOI] [PubMed] [Google Scholar]
- 27.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: Analysis of a clinic population, 1997–2011. Neurology Clinical practice 2013;3(4):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons Z, Felgoise SH, Bremer BA, Walsh SM, Hufford DJ, Bromberg MB, et al. The ALSSQOL: balancing physical and nonphysical factors in assessing quality of life in ALS. Neurology 2006;67(9):1659–1664. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie HD, Welch GR. Determination of high mitochondrial membrane potential in spermatozoa loaded with the mitochondrial probe 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) by using fluorescence-activated flow cytometry. Methods Mol Biol 2008;477:89–97. [DOI] [PubMed] [Google Scholar]
- 30.Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, et al. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem 2011;400(8):2383–2390. [DOI] [PubMed] [Google Scholar]
- 31.Jetzek-Zader M, Gudowius S, Feyen O, Stevens M, Lipfert P, Niehues T. A single intravenous dose of prednisolone induces phosphatidylserine externalization, loss of surface marker expression and a 24-h net increase in human peripheral blood lymphocytes ex vivo. Rheumatol Int 2007;27(7):667–673. [DOI] [PubMed] [Google Scholar]
- 32.de Siqueira EC, Souza FT, Diniz MG, Gomez RS, Gomes CC. Hsp27 (HSPB1) differential expression in normal salivary glands and pleomorphic adenomas and association with an increased Bcl2/Bax ratio. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014. [DOI] [PubMed] [Google Scholar]
- 33.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clinical chemistry 1998;44(6 Pt 1):1309–1315. [PubMed] [Google Scholar]
- 34.Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, et al. Oxidative stress biomarkers in sporadic ALS. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 2008;9(3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314(5796):130–133. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Annals of neurology 2008;64(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta neuropathologica 2008;116(2):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, et al. Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta neuropathologica 2009;118(5):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhite R, Sage G, Bouzid A, Primavera A, Agbas A. Platelet phosphorylated TDP-43: an exploratory study for a peripheral surrogate biomarker development for Alzheimer’s disease. Future Sci OA 2017;3(4):FSO238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi AH, Chaoji V, Maiguel D, Faridi MH, Barth CJ, Salem SM, et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: insights into integrin signaling. PLoS One 2009;4(10):e7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon PH, Moore DH, Gelinas DF, Qualls C, Meister ME, Werner J, et al. Placebo-controlled phase I/II studies of minocycline in amyotrophic lateral sclerosis. Neurology 2004;62(10):1845–1847. [DOI] [PubMed] [Google Scholar]
- 42.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. The Lancet Neurology 2007;6(12):1045–1053. [DOI] [PubMed] [Google Scholar]
- 43.Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 44.Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology 2014;83(19):1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz J, Agosta J, Moore D, Miller R. P09 EXPLORING STOPPING RULES FOR ALS SCREENING TRIALS USING A BAYESIAN MODEL AND HISTORICAL PLACEBO DATABASE. Amyotrophic Lateral Sclerosis 2008;9(Suppl. 1):59–66.17924236 [Google Scholar]
- 46.Gomeni R, Fava M, Pooled Resource Open-Access ALSCTC. Amyotrophic lateral sclerosis disease progression model. Amyotroph Lateral Scler Frontotemporal Degener 2014;15(1–2):119–129. [DOI] [PubMed] [Google Scholar]
- 47.Zach N, Ennist DL, Taylor AA, Alon H, Sherman A, Kueffner R, et al. Being PRO-ACTive: What can a Clinical Trial Database Reveal About ALS? Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2015;12(2):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muyderman H, Chen T. Mitochondrial dysfunction in amyotrophic lateral sclerosis - a valid pharmacological target? British journal of pharmacology 2014;171(8):2191–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludolph AC, Schuster J, Dorst J, Dupuis L, Dreyhaupt J, Weishaupt JH, et al. Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial. The Lancet Neurology 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.