Abstract
Background
Total knee arthroplasty (TKA) is associated with a risk of thromboembolism requiring routine thromboprophylaxis, but there is debate about the risk with unicondylar knee arthroplasty (UKA) as it is believed to be a less invasive, more minor procedure with regard to surgical trauma. Because of this gap in knowledge, we sought to investigate the relative risk of thromboembolism with UKA compared to TKA and one-staged bilateral TKA (BTKA) by measuring the increase in circulating biochemical markers of coagulation at various time-points during the procedures. At the same time, we wanted to assess the relative degree of surgical trauma during the procedures by measuring IL-6, a marker of metabolic injury.
Methods
We prospectively studied a total of 75 patients: 25 patients undergoing UKA, unilateral TKA and BTKA respectively. All patients had neuraxial anesthesia, surgery performed with tourniquet and received no heparin or tranexamic acid (TXA). Radial artery blood samples were taken at four periods during surgery and assayed for circulating markers of thrombin generation: prothrombin fragment 1+2 (F1+2) and thrombin-antithrombin complexes (TAT); and a marker of metabolic injury, interleukin-6 (IL6), using ELISA assays.
Results
The circulating marker of thrombin generation, TAT, increased during all time-points (p<0.001) but was not significantly different between surgical treatment groups. F1+2 also rose significantly during surgery, with no significant difference between UKA and TKA. There was, however, a significant difference in F1+2 between BTKA and UKA or TKA (p<0.02). IL6 rose minimally with UKA but rose significantly with TKA and BTKA (p<0.001).
Conclusion
Based on this data of circulating biochemical markers, patients undergoing UKA are at similar risk of thromboembolism with respect to TKA despite a lower index of metabolic injury. Therefore, we believe that UKA patients should receive thromboprophylaxis comparable to TKA patients. BTKA had the greatest increase in F1+2, a circulating marker of thrombogenesis; and IL6, a marker of metabolic injury.
Keywords: total knee arthroplasty, unicompartmental knee arthroplasty, deep vein thrombosis, risk, metabolic injury, coagulation
Introduction
Unicondylar knee arthroplasty (UKA) is being used more frequently for knee arthritis [1–3], as more attention is being turned toward dissatisfaction following total knee arthroplasty (TKA), and UKA is proposed as a possible solution [2–4]. The operation is of smaller magnitude than TKA based upon the amount of bone preparation and blood loss, presumably triggering less metabolic injury. However, tourniquet times are similar for UKA and TKA, which has raised the debate as to whether the two procedures carry the same thromboembolic risk [5, 6]. A number of studies have been published demonstrating the risk of deep vein thrombosis (DVT)/pulmonary embolism (PE) to be lower with UKA [6, 7] whereas others have noted a similar risk of PE or death from PE [5, 8]. As such, there is a wide variation in thromboprophylaxis after UKA. Some authors utilize chemoprophylaxis similar to TKA [4–6], others use only mechanical thromboprophylaxis [9], and still others believe that thromboprophylaxis is not necessary after UKA [10].
The “gold” standard to compare comparative risk of DVT/PE between procedures is to perform contrast venography in patients 5-7 days following surgery [11, 12]. This is impractical to do in modern times, as these patients typically leave the hospital on postoperative day 1 or 2 and no such studies have been published. The second option is to prospectively follow-up large cohorts of patients for risk of clinical DVT, PE and lethal PE following UKA and compare to historical TKA controls. A third option is to study markers of thrombin generation during surgery to determine the degree of activation of thrombosis during surgery compared to TKA. This has been utilized in multiple studies in joint arthroplasty providing information on the timing of thrombogenesis and relative risks between procedures [13–17]. Circulating markers of thrombogenesis are byproducts of the activated coagulation cascade, and therefore should be elevated when clot formation is occurring. The final step in the coagulation cascade is the conversion of fibrinogen into fibrin clot. Prothrombin F1+2 (F1+2) is produced when prothrombin is cleaved to form thrombin, which then acts upon fibrinogen. Thrombin anti-thrombin complexes (TAT) are formed after thrombin acts on fibrinogen to form fibrin clot, and is then inactivated.
In this study, we prospectively measure changes in the circulating markers of thrombin generation, F1+2 and TAT, during UKA as well as unilateral TKA and one-staged BTKA, to determine the relative thrombogenic risk of each procedure. In addition, we measured interleukin-6 (IL-6) for each procedure, which has been established previously as a measure metabolic injury [18]. Assessment of the metabolic injury associated with the different knee arthroplasty procedures has not been done previously.
Methods
Following IRB approval and patient consent, we recruited 25 patients undergoing unicondylar knee arthroplasty, 25 undergoing unilateral total knee arthroplasty and 25 undergoing one-staged bilateral total knee arthroplasty. All patients had avascular necrosis or osteoarthritis. Exclusion criteria included inflammatory arthritis or patients on preoperative anticoagulation, as we were uncertain how this would affect circulating markers of thrombogenesis or metabolic injury. Patients were operated upon by different fellowship trained knee surgeons. All operations were performed with tourniquet inflated prior to incision and deflated prior to wound closure. All patients received either spinal or epidural anesthesia.
All patients had radial artery catheter (20 gauge) inserted before surgery. A total of 4 blood samples were drawn: (1) following the induction of the epidural or spinal anesthesia, (2) 20 minutes following surgical incision with the tourniquet inflated (3) immediately following deflation of the tourniquet and (4) at the end of wound closure. With the bilaterals, the second sample was drawn 20 minutes after incision on the first knee and the third sample was drawn immediately following deflation of the tourniquet on the second knee.
Blood samples were withdrawn from the radial artery catheter and placed into citrated Vacutainer® tubes, immediately placed on ice, centrifuged at 2000g for 10 minutes at 4 degrees centigrade. Plasma was stored in −70 degrees centigrade until it was ready for assay. Samples were subsequently assayed for prothrombin F1+2, TAT and IL-6 using ELISA Assays (Enzygnost® TAT and Enzygnost® F1 + F2, Siemens Healthcare Diagnostics Inc. Duluth, GA and Human IL-6 Quantikine®, R&D Systems, Minneapolis, MN) by lab technicians blinded to the type of surgery. Given the three cohorts with four time-points, 900 assays for these circulating markers were performed.
Blood was not transfused during surgery. No patients received intraoperative heparin or tranexamic acid. The only fluid infused was lactated Ringer’s. Patients were sedated with a combination of intravenous propofol, midazolam with or without fentanyl. 47 had spinal/CSE and 28 had epidural anesthesia.
Surgery
Unicondylar knee arthroplasty was performed on either the medial or lateral femoro-tibial compartment, through a mini mid-vastus arthrotomy. The tibial cut was made using an extramedullary alignment guide. An intramedullary rod was used to align the femoral cutting guide. Components were cemented for fixation and the tourniquet deflated prior to incision closure. Drainage tubes were used at the discretion of the operating surgeon.
Total knee arthroplasty was performed via a medial parapatellar arthrotomy. Extramedullary tibial guide and intramedullary femoral guides were utilized. The patella was always resurfaced, and components were cemented. The tourniquet was deflated prior to incision closure, and drainage tubes used at the discretion of the surgeon.
One-stage bilateral total knee arthroplasty was performed just as above, beginning with the more symptomatic knee. After the tourniquet was deflated, the hemodynamic status of the patient was evaluated by the anesthesiologist, and if stable, the procedure was initiated on the second knee. The surgeon performed the second TKR while another team completed the closure on the first TKR, hence the bilateral TKR were performed sequentially.
Statistics
Our sample size was based upon changes in markers of coagulation during TKA from a prior study [15]. To detect a 25% difference between TKA and UKA, assuming an alpha of 0.05 and a beta of 0.2, 20 patients in each group would be required. We added 5 patients to each group in case of laboratory error.
Descriptive statistics are presented as medians; first and third quartiles for continuous variables and as frequencies and percentages for categorical variables. The generalized estimating equations (GEE) approach was used to assess the effect of procedure (UKA, TKA, and BTKA) and time (1, 2, 3 and 4), on IL-6. Predictors of IL-6 elevation were assessed in the same fashion. To assess the relationship between tourniquet time (TT) and change in IL-6 level, differences between times 2 and 3 were chosen. A Spearman’s Correlation Coefficient was done to examine the relationship between TT and change in IL-6 level between time 2 and 3. All analyses were performed with SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA); a p-value less than 0.05 was considered statistically significant.
Results
Demographics in the three groups are shown in Table 1. The progression of F1+2, TAT and IL-6 throughout the patient’s episode of care are shown in figures 1–3 in all 3 groups. F1+2 and TAT increased significantly over time in all 3 groups. There was no significant difference in TAT levels between the 3 cohorts at any time-point (Figure 2). There was no significant difference in the F1+2 level between UKA and TKA at any time-point (Figure 1). However, there was a significant difference in F1+2 between UKA (mean 525 pmol/L) or TKA (mean 563 pmol/L) and BTKA (mean 668 pmol/L) at time-point 4 (wound closure) (p<0.02) (Figure 1). IL-6 increased minimally with UKA from time-point 1 to 4 (mean value 2.79 pg/mL to 3.05 pg/mL) but was significantly higher in TKA (mean value 4.06 pg/mL to 6.83 pg/mL p<0.001) and BTKA (mean value 4.96 pg/mL to 12.21 pg/mL, p<0.0003) at time-point 4. IL-6 levels were significantly different between UKA and TKA (p<0.001) and between UKA and BTKA (p<0.0003), but not between TKA and BTKA at time-point 4 (Figure 3).
Table 1.
Patient demographics.
| UKA | TKA | BTKA | p Value | |
|---|---|---|---|---|
| Age (years) | 63.76 ± 8.69 | 66.28 ± 8.96 | 65.2 ± 6.87 | NS |
| Gender (M / F) | 16 / 9 | 8 / 17 | 10 / 15 | NS |
| Height (cm) | 171.8 ± 8.89 | 167.13 ± 13.12 | 165.79 ± 10.99 | NS |
| Weight (kg) | 85.72 ± 17.42 | 81.22 ± 19.48 | 86.04 ± 13.68 | NS |
| BMI (kg/m2) | 28.9 ± 4.548 | 29.278 ± 7.354 | 31.4 ± 4.686 | NS |
| ASA | NS | |||
| 1 | 2 | 0 | 1 | |
| 2 | 20 | 17 | 22 | |
| 3 | 3 | 8 | 2 | |
| Tourniquet Time (min) | 52.2 ± 17.47 | 46.4 ± 8.42 | 94.84 ± 24.64 | <0.0001 |
| Intraoperative Fluid (mL) | 1386 ± 429.03 | 1500 ± 278.39 | 2668 ± 623.64 | <0.0001 |
Values are mean ± SD
Figure 1.
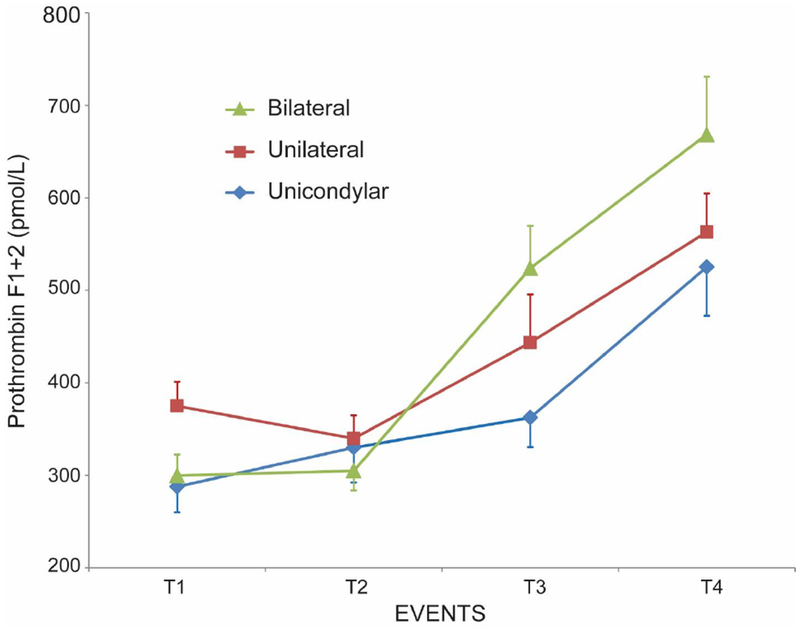
Changes in prothrombin F1+2 (mean ± SEM) at 4 time-points during surgery: (1) After neuraxial anesthesia, (2) 20 minutes following surgical incision with the tourniquet inflated [With the bilaterals, the second sample was drawn 20 minutes after incision on the first knee] (3) following tourniquet deflation [With the bilaterals, the third sample was drawn immediately following deflation of the tourniquet on the second knee] and (4) at the end of wound closure.
Figure 3.
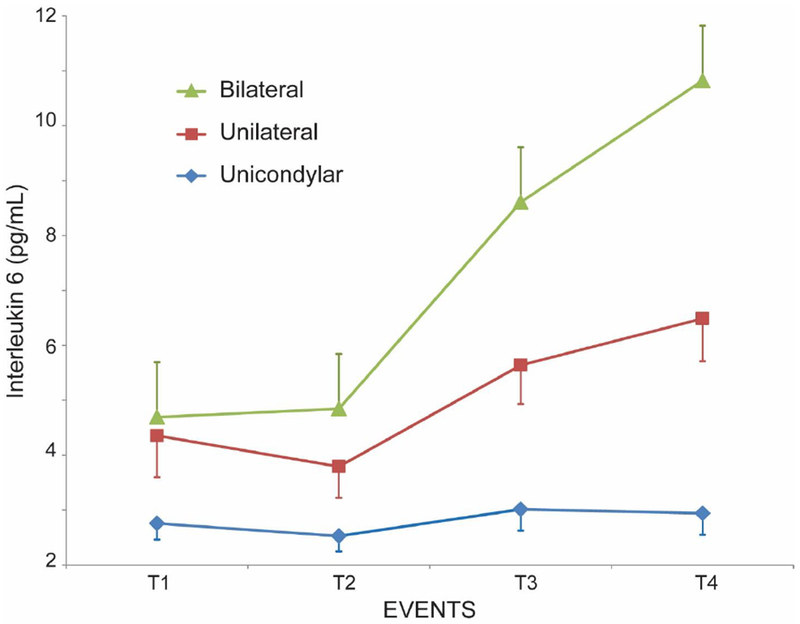
Changes in interleukin-6 (mean ± SEM) at 4 time-points during surgery: (1) After neuraxial anesthesia, (2) 20 minutes following surgical incision with the tourniquet inflated [With the bilaterals, the second sample was drawn 20 minutes after incision on the first knee] (3) following tourniquet deflation [With the bilaterals, the third sample was drawn immediately following deflation of the tourniquet on the second knee] and (4) at the end of wound closure.
Figure 2.
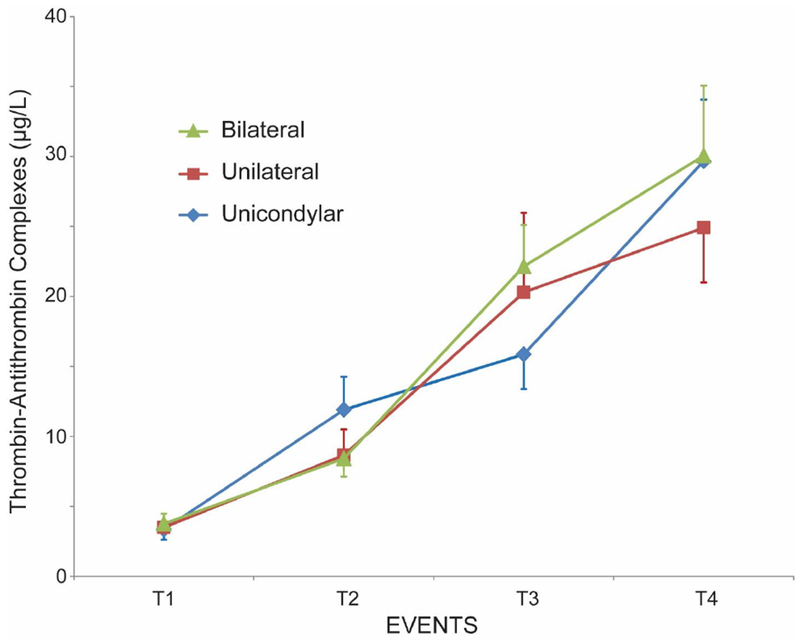
Changes in thrombin-antithrombin complexes (mean ± SEM) at 4 time-points during surgery: (1) After neuraxial anesthesia, (2) 20 minutes following surgical incision with the tourniquet inflated [With the bilaterals, the second sample was drawn 20 minutes after incision on the first knee] (3) following tourniquet deflation [With the bilaterals, the third sample was drawn immediately following deflation of the tourniquet on the second knee] and (4) at the end of wound closure.
Though there was no significant difference in the tourniquet time between UKA and TKA, there was a correlation between longer tourniquet times and higher levels of IL-6. The relationship was strongest with UKA (r=0.6, p=0.009) and weakest for unilateral TKA (r=0.28, p=0.21). When we examined the effect of procedure and time on IL-6, we found that there was an interaction between procedure and time (p=0.05). However, the most significant predictor of IL-6 was procedure type, with TKA (bilateral, p=0.007; unilateral, p=0.052) as the greatest predictor of IL-6 levels. A multiple regression was then performed to investigate the influence of several factors on IL-6 elevation (Table 2). After adjustment of factors such as body mass index (BMI), American Society Anesthesiologist score (ASA), race, tourniquet time, and procedure, we found a significant interaction between BMI and tourniquet time (p<0.0001); meaning tourniquet time modifies the influence of BMI on IL-6 elevation.
Table 2.
Predictors of IL-6 elevation.
| Covariates | Adjusted Estimate (95% CI) | p value |
|---|---|---|
| Age | −0.06 (−0.14 – 0.03) | 0.202 |
| Gender | 1.01 (−0.75 – 2.78) | 0.259 |
| ASA | 2.3 (0.44 – 4.25) | 0.016 |
| Race | 1.7 (0.62 – 2.83) | 0.002 |
| proc (UKA) | Ref | |
| proc (BTKA) | 3.08 (0.66 – 5.51) | 0.013 |
| proc (TKA) | 0.99 (−1.0 – 3.0) | 0.334 |
| Intraoperative Fluid | −0.002 (−0.003 – 0.0003) | 0.016 |
| BMI*Tourniquet Time | 0.01 (0.01 – 0.02) | <0.0001 |
p<0.05 considered significant
Discussion
In this prospective cohort study of 75 patients, we noted an activation of thrombin generation during UKA performed under tourniquet, as measured by circulating markers of thrombogenesis. This increase was similar to that noted during both TKA and BTKA. This was unexpected, as we had expected to see less activation in the markers of thrombin generation since the UKA is a less invasive operation. However, there was a significant difference in the level of F1+2 between TKA and BTKA at wound closure, which corresponds with a known higher clot forming potential of BTKA in the literature [19, 20].
With regard to metabolic injury, we noted a greater activation of IL-6 with BTKA and TKA than with UKA. UKA resulted in a minimal increase in IL-6, indicating relatively little metabolic injury, whereas both TKA and BTKA showed significant increases. Thus, although UKA appeared to show minimal metabolic injury according to IL-6, the activation of thrombin generation was similar to that of TKA.
Changes in F1+2 and TAT as markers of thrombin generation have been extensively studied as markers of thrombosis during total joint arthroplasty [13–17, 21]. They have been useful in defining the timing of onset of thrombogenesis (e.g., surgery on the femur during total hip arthroplasty) [14, 16] and the relative thromboembolic risks of different procedures e.g., cemented vs. non-cemented hip arthroplasty [14]. Another study showed no difference between uncemented THA via the posterior approach and resurfacing hip arthroplasty [17]. Prior studies have demonstrated an increase in markers of thrombosis during TKA [15, 21] performed with or without tourniquet [13]. In non-orthopaedic literature including neurosurgery and cardiac surgery, F1+2 appears to be more specific to clot formation than TAT [22]. With regard to metabolic injury, circulating levels of IL-6 have been established to correlate with injury severity score (ISS) in trauma patients [18].
This study reaffirms the clinical studies demonstrating that there is a risk of DVT/PE following UKA. Of the seven studies we were able to identify, all noted evidence of DVT/PE or lethal PE on follow-up of UKA patients [2, 4–9]. In several, the risk of DVT was lower with UKA than TKA [6, 7]. Others reported a very low rate of DVT (0.1%) and no PE in a series of 828 patients (1000 UKA) [4]. However, several studies have noted lethal PE after UKA 0.07% [5] and 0.6% [9] and 0.96% with single stage bilateral UKA [5]. All but one of these series utilized chemoprophylaxis and early mobilization. Only one relied upon mechanical thromboprophylaxis alone [9].
The major limitation of this data is that an increase in markers of thrombosis does not mean patient will develop a DVT. Furthermore, the degree of activation of these markers has never been linked to venographic evidence of DVT [17]. It merely demonstrates an activation of thrombosis has occurred which in this case was independent of the degree of metabolic injury from surgery. We also found in our study, as have other studies, that there are some variations in the levels of F1+2 and TAT between patients. We believe that there are variations in markers for different patients, much like there would be different levels of WBC or different protimes. However, overall, we believe that each individual responds in the same way to a procedure. Statistical analysis using multivariate regression demonstrated that patient factors were not associated with elevation in the levels of circulating markers of thrombogenesis or IL-6.
Our study did, however, indicate a significantly higher level of one circulating marker of thrombogenesis (F1+2) in bilateral TKA, a procedure known to have an elevated risk of clot formation. Based on our findings that the circulating level of thrombogenesis were significantly elevated with all three procedures we examined, and that there was no difference in the levels between UKA and TKA, we believe that patients undergoing UKA should receive postoperative thromboprophylaxis similar to that given for TKA. We do not believe that patients undergoing UKA should receive no prophylaxis, as has been suggested in the past [10]. However, in view of the early mobilization with UKA, a less powerful agent such as aspirin may be most appropriate, unless otherwise medically indicated, as it is as effective as more powerful agents [23] and is less likely to result in wound hematomas or wound infections [24].
Contributor Information
Edwin P. Su, Associate Attending Orthopaedic Surgeon, Hospital for Special Surgery, 535 East 70th Street, New York, NY, 10021
Lauren Mount, Research Coordinator, Hospital for Special Surgery, 535 East 70th Street, New York, NY, 10021
Allina A. Nocon, Director, Clinical Research, Complex Joint Reconstruction Center, Hospital for Special Surgery, 535 East 70th Street, New York, NY, 10021.
Thomas P. Sculco, Attending Orthopedic Surgeon, Surgeon-in-Chief Emeritus, Hospital for Special Surgery, 535 East 70th Street, New York, NY, 10021
George Go, Research Assistant, Hospital for Special Surgery, 535 East 70th Street, New York, NY, 10021.
Nigel Sharrock, Anesthesiologist-in-Chief Emeritus, Attending Anesthesiologist and Senior Scientist, Hospital for Special Surgery, 535 East 70th Street, New York, NY 10021.
References
- 1.Price AJ, Svard U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2011;469: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger RA, Meneghini RM, Jacobs JJ, Sheinkop MB, Della Valle CJ, Rosenberg AG, Galante JO. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87: 999–1006. [DOI] [PubMed] [Google Scholar]
- 3.Riff AJ, Sah AP, Della Valle CJ. Outcomes and complications of unicondylar arthroplasty. Clin Sports Med. 2014;33: 149–160. [DOI] [PubMed] [Google Scholar]
- 4.Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20: 218–220. [DOI] [PubMed] [Google Scholar]
- 5.Asopa V, Cobain W, Martin D, Keene G, Bauze A. Staged venous thromboemolic events prophylaxis with low-molecular-weight heparin followed by aspirin is safe and effective after arthroplasty. ANZ J Surg. 2015;85: 652–657. [DOI] [PubMed] [Google Scholar]
- 6.Willis-Owen CA, Sarraf KM, Martin AE, Martin DK. Are current thrombo-embolic prophylaxis guidelines applicable to unicompartmental knee replacement? J Bone Joint Surg Br. 2011;93: 1617–1620. [DOI] [PubMed] [Google Scholar]
- 7.Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 8.Brown NM, Sheth NP, Davis K, Berend ME, Lombardi AV, Berend KR, Della Valle CJ. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27: 86–90. [DOI] [PubMed] [Google Scholar]
- 9.Chan WC, Musonda P, Cooper AS, Glasgow MM, Donell ST, Walton NP. One-stage versus two-stage bilateral unicompartmental knee replacement: a comparison of immediate post-operative complications. J Bone Joint Surg Br. 2009;91: 1305–1309. [DOI] [PubMed] [Google Scholar]
- 10.Koh IJ, Kim JH, Kim MS, Jang SW, Kim C, In Y. Is Routine Thromboprophylaxis Needed in Korean Patients Undergoing Unicompartmental Knee Arthroplasty? J Korean Med Sci. 2016;31: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassen MR, Fisher W, Mouret P, Agnelli G, George D, Kakkar A, Mismetti P, Turpie AG. Semuloparin for prevention of venous thromboembolism after major orthopedic surgery: results from three randomized clinical trials, SAVE-HIP1, SAVE-HIP2 and SAVE-KNEE. J Thromb Haemost. 2012;10: 822–832. [DOI] [PubMed] [Google Scholar]
- 12.Le Gal G, Righini M. Controversies in the diagnosis of venous thromboembolism. J Thromb Haemost. 2015;13 Suppl 1: S259–265. [DOI] [PubMed] [Google Scholar]
- 13.Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res. 2000;371: 169–177. [DOI] [PubMed] [Google Scholar]
- 14.Sharrock NE, Go G, Harpel PC, Ranawat CS, Sculco TP, Salvati EA. The John Charnley Award. Thrombogenesis during total hip arthroplasty. Clin Orthop Relat Res. 1995;319: 16–27. [PubMed] [Google Scholar]
- 15.Sharrock NE, Go G, Sculco TP, Ranawat CS, Maynard MJ, Harpel PC. Changes in circulatory indices of thrombosis and fibrinolysis during total knee arthroplasty performed under tourniquet. J Arthroplasty. 1995;10: 523–528. [DOI] [PubMed] [Google Scholar]
- 16.Sharrock NE, Go G, Sculco TP, Salvati EA, Westrich GH, Harpel PC. Dose response of intravenous heparin on markers of thrombosis during primary total hip replacement. Anesthesiology. 1999;90: 981–987. [DOI] [PubMed] [Google Scholar]
- 17.Su EP, Chatzoudis N, Sioros V, Go G, Sharrock NE. Markers of thrombin generation during resurfacing and noncemented total hip arthroplasty: a pilot study. Clin Orthop Relat Res. 2011;469: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Bruckner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135: 291–295. [DOI] [PubMed] [Google Scholar]
- 19.Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28: 87–91. [DOI] [PubMed] [Google Scholar]
- 20.Sharrock NE, Haas SB, Hargett MJ, Urquhart B, Insall JN, Scuderi G. Effects of epidural anesthesia on the incidence of deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Am. 1991;73: 502–506. [PubMed] [Google Scholar]
- 21.Sharrock NE, Go G, Williams-Russo P, Haas SB, Harpel PC. Comparison of extradural and general anaesthesia on the fibrinolytic response to total knee arthroplasty. Br J Anaesth. 1997;79: 29–34. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Toyohira H, Kariyazono H, Yamada K, Moriyama Y, Taira A. Relationship between changes in F1+2 and TAT levels and blood coagulation early after prosthetic valve replacement. Thromb Res. 1997;86: 161–171. [DOI] [PubMed] [Google Scholar]
- 23.Sharrock NE, Gonzalez Della Valle A, Go G, Lyman S, Salvati EA. Potent anticoagulants are associated with a higher all-cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res. 2008;466: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang R, Buckley PS, Scott B, Parvizi J, Purtill JJ. Administration of Aspirin as a Prophylaxis Agent Against Venous Thromboembolism Results in Lower Incidence of Periprosthetic Joint Infection. J Arthroplasty. 2015;30: 39–41. [DOI] [PubMed] [Google Scholar]


