Abstract
Background
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are effective for weight loss in adolescents with severe obesity. However, little is known about adverse gastrointestinal symptoms (GIS) following these operations in adolescents. The objective was to examine GIS over 5 years after surgery and differences by surgery type.
Methods
We prospectively studied 228 adolescents (161 RYGB, 67 VSG) undergoing bariatric surgery. Gastrointestinal symptoms were assessed before surgery, at 6-months, and yearly to 5 years after surgery. Symptom severity was dichotomized for analysis. Analysis of post-surgery symptoms involved linear models adjusting for baseline symptoms, BMI, early post-operative complication, sex, and race.
Results
Participants at surgery were 17±1.6 years with preoperative BMI 53±9.4 kg/m2. From 6 months to 5 years, gastroesophageal reflux symptoms (GERS), nausea, bloating, and diarrhea increased. Crude prevalence rates of GERS increased from 4% (1% RYGB, 11% VSG) at 6-months post-surgery to 14% (10% RYGB, 26% VSG) at 5-years. In adjusted analyses, the VSG group experienced 4-fold (4.85 95% CI 2.63, 8.91, p<0.0001) greater odds of GERS compared to RYGB.
Conclusions
Adolescents who underwent VSG experienced greater risk of GERS compared to those undergoing RYGB. Adolescents undergoing VSG should be counseled preoperatively about GERS and objectively monitored postoperatively for gastroesophageal reflux when indicated.
Keywords: Bariatric surgery, Obesity, Gastrointestinal symptoms, Adolescent
INTRODUCTION
Severe obesity represents a rising problem in the adolescent population[1]. For adolescents with severe obesity and comorbid illnesses, metabolic/bariatric surgery (MBS) is increasingly seen as an option for treatment[2, 3]. Anatomical alteration of the gastrointestinal tract may result in adverse gastrointestinal symptoms (GIS). While the literature describing weight loss and other medical outcomes is increasingly available to assist families and physicians to make informed decisions about these operations[4–7], to date, there are few details available regarding patient-reported GIS outcomes[8, 9]. Patients considering MBS often discuss the pros and cons of procedure types with their surgeon and with other patients who have undergone weight loss surgery to get their perspective on which operation is most desirable. GIS often influence these subjective discussions of the outcome of surgery and, anecdotally, patients who are particularly bothered by postoperative GIS can be very influential. Thus, an objective analysis and reporting of the evolution of GIS over the long-term would be valuable for patients and families considering the different MBS approaches.
The aims of this study were to evaluate GIS from 6 months to 5 years following MBS performed in adolescents, and to determine whether there was a difference in symptomatology between participants who underwent gastric bypass versus sleeve gastrectomy.
METHODS
Study Design and Subjects
Methodology and design of the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study (NCT00465829) have been well described previously[10]. Consecutive adolescents (<19 years of age and Tanner Stage 4 or greater) with BMI ≥ 35kg/m2 who met eligibility guidelines for MBS were offered enrollment into Teen-LABS at one of five U.S. centers (February 28, 2007- December 30, 2011). The study protocol, data and safety monitoring plans, and assent and/or consent forms were approved by all institutional review boards and an independent data and safety monitoring board convened by the NIH. Participants who underwent adjustable gastric band (AGB) insertion (n=14) were excluded from the current analysis.
Data Collection
Data were collected at baseline (≤30 days prior to surgery), 6 months, 1 year, 2 years, 3 years, 4 years, and 5 years post-operatively. The majority of follow-up research visits and associated data collection occurred at Teen-LABS centers (89%), while the remaining visits were conducted by trained field examiners at participants’ homes or via telephone and/or electronic communication. Data were available for 184/228 (81%) patients at 5-year follow up, 137/161 (85%) in the RYGB group and 50/67 (75%) in the VSG group (Appendix A).
Data points and Definitions
Demographic data:
Age (years) at the time of surgery, race (white or non-white), sex (male or female), and household income were provided for each study participant.
Weight Loss:
Weight and height were measured and BMI calculated at each pre- and postoperative visit. Percent change in BMI from baseline was calculated for each visit.
Early post-operative complications:
Complications within 30-days post-surgery were classified as major, minor or none. Major complications were re-operation due to bowel obstruction, gastrointestinal leak or sepsis, post-operative transfusion, anticoagulant use for DVT or PE, unplanned splenectomy or suicidal ideation. Minor complications were gastrointestinal leak (minimal), postoperative bleeding, atelectasis/pneumonia, urinary tract events, bowel injury, solid organ injury, mesenteric bleeding, over-sedation, hypertension, TPN after discharge, gastrojejunal anastomotic stricture, wound infection, ileus, obstruction, abdominal pain/dehydration, or acute pancreatitis.
Variables considered for sensitivity analyses:
Use of medication for acid reflux is defined as reported use of an “acid reflux” medication or an “antacid” at the study visit. Smoking is defined as cigarette smoking reported within the 3 months prior to the study visit. A study visit is defined as complicated by pregnancy if the study subject is pregnant, within 6 months of delivery or reports currently nursing at the study visit. All these variables were time dependent.
Gastrointestinal Symptoms:
Participants completed a 15-item GIS rating scale[11] before surgery and at all post-operative study visits for 5 years (Appendix B). Of those 15 symptoms, the items pertaining to 1) heartburn and 2) acid reflux were combined into one symptom defined as gastroesophageal reflux symptoms (GERS). Potential responses for each symptom ranged from none through very severe. Based on symptom severity distribution, we considered symptoms reported as “moderate”, “moderate/severe”, “severe” and “very severe” as symptom present, and symptoms reported as “none”, “minor” and “mild” as symptom absent.
Statistical Methods
Descriptive statistics are reported as mean ± standard deviation (SD) for continuous variables or frequency and percentage for categorical variables. Prevalence was defined as presence of the symptom at the time of the visit. Incidence was defined as presence of the symptom at the time of the visit but not at baseline. Remission was defined as not reporting the GIS at the time of the visit but reporting of GIS at baseline.
Adjusted odds ratios (aOR) were determined using general linear models with logit link, to examine the prevalence of GIS at 6 months and yearly at 1 year through 5 years post-surgery. Models were created for each symptom of interest. Each model had the same covariates selected a priori (Appendix C). Covariates included in the analyses were: presence of any early postoperative complication (yes/no), baseline body mass index (BMI), sex, race, and baseline presence of the GIS under analysis. Multicollinearity was assessed by examination of the association of the covariates. Percent change in BMI from baseline was included as a time-dependent covariate, and years since surgery and surgery type were the independent variables of interest. A visit by surgery type interaction was used to examine the difference in GIS rates 6 months to 5 years post-surgery between RYGB and VSG. If the interaction was not significant, the main effect of surgery type was examined to test the overall difference between RYGB and VSG. Sensitivity analyses were done for GERS, adjusting for anti-reflux medication and cigarette use as covariates and by excluding women who were pregnant or nursing at the time of the study visit, as these were identified, a priori, as possibly affecting presence of GERS. SAS®, version 9.4 (SAS Institute, Cary, NC) was used for analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
Participant Characteristics at Baseline
The mean age of participants at surgery was 17 ± 2 years and 75% of the total cohort was female. There were 161 participants who underwent RYGB and 67 who underwent VSG. Mean overall preoperative BMI was 53 kg/m2 with a slightly lower BMI (50 kg/m2) in the VSG group compared to the RYGB group (54 kg/m2), p=0.04. The race distribution in both groups was similar with the majority of participants’ race reported as white. Participants undergoing RYGB tended to be in the higher brackets of household income compared to the VSG group, p=0.06. At baseline, the most prevalent symptoms included GERS (11%), abdominal pain (10%), and nausea (10%) (Table 1).
Table 1:
Patient Demographic, Anthropomorphic Characteristics and Gastrointestinal Symptoms at Baseline
| Variable | Overall (n=228) |
RYGB (n=161) |
VSG (n=67) |
p-value RYGB vs Sleeve |
|---|---|---|---|---|
| Age at surgery (years) | 16.5 (1.6) | 16.6 (1.6) | 16.4 (1.7) | 0.33 |
| Sex (Female) | 171 (75) | 126 (78) | 45 (67) | 0.08 |
| Race: | 0.23 | |||
| Hispanic | 16 (7) | 15 (9) | 1 (2) | 0.04 |
| Household income: | 0.06 | |||
| Weight (kg) | 148.8 (31.2) | 150.9 (30.3) | 143.6 (33.0) | 0.04 |
| Height (cm) | 167.9 (9.1) | 167.5 (8.5) | 168.7 (10.5) | 0.18 |
| BMI (kg/m2) | 52.6 (9.4) | 53.7 (9.6) | 50.1 (8.3) | 0.004 |
| Gastrointestinal symptoms | ||||
| Abdominal Pain | 22 (10) | 15 (9) | 7 (10) | 0.79 |
| Bloating | 18 (8) | 12 (7) | 6 (9) | 0.70 |
| Constipation | 6 (3) | 5 (3) | 1 (1) | 0.67 |
| Diarrhea | 18 (8) | 13 (8) | 5 (7) | 0.88 |
| Belching | 8 (4) | 7 (4) | 1 (1) | 0.44 |
| GERS* | 25 (11) | 19 (12) | 6 (9) | 0.53 |
| Nausea | 23 (10) | 17 (11) | 6 (9) | 0.70 |
| Early post-operative complications | 54 (24) | 42 (26) | 12 (17.9) | 0.19 |
Data presented as mean (standard deviation) or n (%)
RYGB is Roux-en-Y gastric bypass and VSG is vertical sleeve gastrectomy
GERS – Gastroesophageal reflux symptoms(defined as the presence of heartburn or acid reflux)
Gastrointestinal Symptoms Post-Surgery
Between 6 months and 5 years post-surgery, the odds of reporting GERS, nausea, bloating, and diarrhea modestly increased when adjusted for baseline GIS, early post-operative complications, surgery type, baseline BMI, change in BMI, sex, and race. There were no statistically significant changes in abdominal pain, belching, or constipation (Table 2).
Table 2:
6-month to 5-year Crude Prevalence Rates and Adjusted Odds Ratio (aOR) for change (per year) in presence of Moderate to Very Severe Gastrointestinal Symptoms 6-months to 5-years Post Operatively and for type of surgery
| Prevalence | aOR (95%CI) | Change in prevalence | |||
|---|---|---|---|---|---|
| 6 months | 5 years | Change per year | Surgery (reference RYGB) | 6m to 5 years p-value | |
| Abdominal Pain | 8% | 13% | 1.10 (0.99, 1.23) | 1.15 (0.63, 2.11) | 0.09 |
| Bloating | 5% | 13% | 1.28 (1.13, 1.43) | 0.92 (0.51, 1.68) | 0.0008 |
| Constipation | 6% | 10% | 1.14 (0.98, 1.32) | 1.18 (0.56, 2.52) | 0.15 |
| Diarrhea | 2% | 6% | 1.28 (1.06, 1.54) | 1.25 (0.53, 2.94) | 0.03 |
| Belching | 9% | 7% | 0.95 (0.83, 1.09) | 1.84 (0.92, 3.69) | 0.56 |
| GERS* | 4% | 14% | 1.31 (1.15, 1.49) | 4.85 (2.63, 8.91) | 0.0001 |
| Nausea | 10% | 15% | 1.13 (1.01, 1.26) | 1.57 (0.91, 2.73) | 0.051 |
Adjusted odds ratios (aOR), 95% confidence intervals and p-values presented for change per year and surgery type were adjusted for baseline BMI, percent change in BMI, early post-operative complications, sex, race, and baseline presence of the specific gastrointestinal symptom. This analysis focused on postoperative symptoms from 6 months to 5 years, thus the aOR were calculated based on data from 6 months to 5 years after surgery.
GERS – Gastroesophageal reflux symptoms (defined as the presence of heartburn or acid reflux)
For GERS, crude overall prevalence changed from 4% at 6 months to 14% at 5 years with a 31% increase in the odds of having moderate to very severe GERS per year (aOR 1.31 (95% CI 1.15, 1.49); p<0.0001). Adjusting for reflux medication use resulted in an aOR of 1.43 (95% CI 1.25, 1.63), adjusting for cigarette use resulted in an aOR of 1.30 (95% CI 1.13, 1.48), and excluding visits complicated by pregnancy resulted in an aOR of 1.31 (95%CI 1.14, 1.50). Adjusting for medication use and cigarette use and excluding visits complicated by pregnancy resulted in an aOR of 1.38 (95% CI 1.19, 1.60).
The prevalence of nausea increased from 10% at 6 months to 15% at 5 years with a 13% increase in the odds of having moderate to very severe nausea per year (aOR 1.13 (95% CI 1.01, 1.26); p=0.04). The prevalence of bloating increased from 5% at 6 months to 13% at 5 years after surgery with a 28% increase in the adjusted odds of having moderate to very severe bloating per year (aOR 1.28 (95% CI 1.13, 1.43); p<0.0001). The prevalence of diarrhea increased from 2% at 6 months to 6% at the 5-year visit. After adjustment, the odds of having moderate to severe diarrhea during the post-operative period significantly increased by 28% per year (aOR 1.28 (95% CI 1.06, 1.54); p=0.01). There was no statistically significant effect of early post-operative complications on GIS.
Gastrointestinal Symptoms by Surgery Type
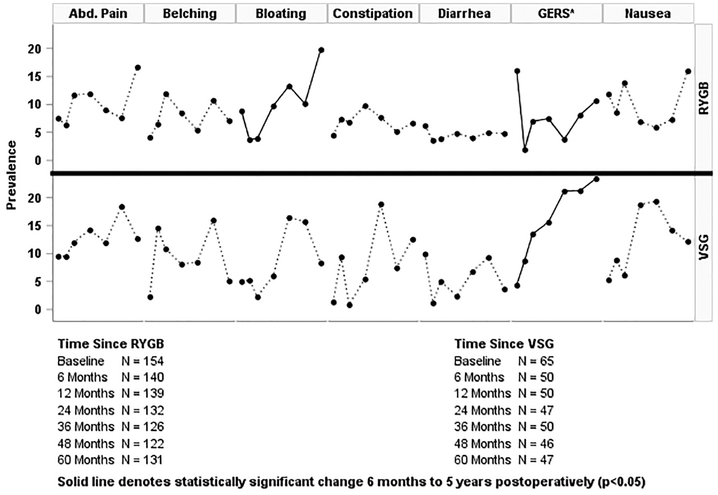
Examination of the difference between RYGB and VSG for GIS only revealed a statistically significant overall difference for GERS, p=0.0006 (Table 2). In adjusted analyses, VSG had more than 4-fold (aOR 4.85 95% CI 2.63–8.91) greater odds of moderate to very severe GERS compared to RYGB post-surgery. The crude prevalence of GERS among VSG patients ranged from 11% at 6 months to 26% at 5 years, whereas the crude prevalence in RYGB patients ranged from 1% to 10% (Figure 1).
Figure 1:
Prevalence of Moderate to Very Severe Gastrointestinal Symptoms at Baseline and from 6 months to 5 years after Gastric Bypass and Sleeve Gastrectomy
*GERS - Gastroesophaqeal reflux (defined as the presence of heartburn or acid reflux)
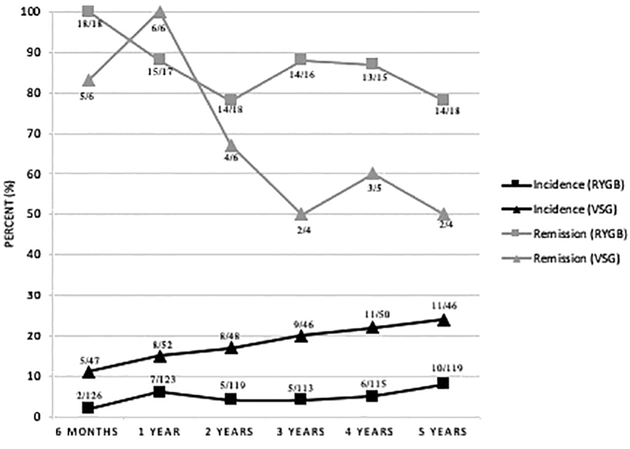
In addition, the incidence of GERS post-surgery was significantly higher in the VSG group (11–24%) compared to the RYGB group (2–8%), p<0.0001. Remission from baseline GERS at 5 years was more common in the RYGB group (remission rate of 78%, 14 of 18 participants) than the VSG group (50%, 2 of 4 participants) (Figure 2). Use of anti-reflux medications initially increased post operatively but use decreased over time. At 5 years post-surgery, 10% of VSG patients used anti-reflux medications compared to 14% in the RYGB group.
Figure 2:
Incidence and Remission of Moderate to Very Severe Gastroesophageal Reflux Symptoms Over Time by Surgery Type
DISCUSSION
This study is one of the first to characterize changes in gastrointestinal symptoms after bariatric surgery in adolescents. It demonstrates a significant increase in prevalence of moderate to severe GERS, nausea, bloating, and diarrhea over the 5 years after MBS in a large multicenter adolescent cohort. Furthermore, the odds of developing new moderate to severe GERS were significantly greater for VSG compared to RYGB. As little is known about gastrointestinal symptoms after RYGB and VSG in adolescents, these findings may inform preoperative discussions between the physician and patient regarding post-operative expectations.
Previous studies of MBS in adolescents using the Teen-LABS cohort have highlighted outcomes related to weight loss, comorbidities resolution, and improvement in quality of life with RYGB and VSG[5, 6, 12]. However, no prior studies have evaluated GIS after these procedures in adolescents. The adult literature contains data pertaining to some of the GIS that may occur after MBS with a focus on gastroesophageal reflux and changes in bowel habits. Several adult retrospective reviews found a greater prevalence of gastroesophageal reflux disease after sleeve gastrectomy[13–15]. The data regarding changes in bowel habits are more heterogeneous. Some studies highlight an increased risk of constipation after RYGB with a possible link to decreased dietary fiber intake[16]. The majority of studies, however, report findings similar to ours, showing diarrhea is the more common bowel disturbance after MBS. This is related to a variety of factors, including the finding that those with severe obesity have higher baseline levels of diarrhea than individuals who do not have obesity [17]. Postoperatively, dietary type and composition, altered gastrointestinal motility, changes in microbiota, nutrient malabsorption or maldigestion, as well as length of the common channel in RYGB may variably affect the prevalence of diarrhea after surgery [18–20].
The increase in the sensation of nausea over time may be related to the increased sensation of bloating, or may even be related to the increasing perception of reflux. It is interesting to note that there was no significant change in belching, which could be expected to be associated with reflux and bloating. An unexpected finding was the lack of association between GIS and early post-operative complications as it could be hypothesized that certain gastrointestinal complications of MBS would lead to an increased sensation of symptoms. Similar to adult studies, the current analysis demonstrates a greater increase in the prevalence of GERS after VSG than after RYGB[21]. The proposed mechanisms for greater GERS after VSG include decreased gastric compliance, increased intraluminal pressure, and shape of the sleeve[22].
In addition to informing the patient’s preoperative understanding of changes in gastrointestinal symptoms after surgery, this study highlights that changes in follow up for these patients may be necessary. As the prevalence of subjective reflux and heartburn increases significantly, especially after VSG, objective testing and treatment for esophageal inflammation and injury may be warranted. Furthermore, longitudinal monitoring for Barrett’s esophagus are indicated in those with GERD. The true prevalence of erosive esophagitis and Barrett’s esophagus after VSG is unclear, but a few recent studies have reported prevalence up to 15%[23, 24]. As of yet, there are no data regarding Barrett’s esophagus in adolescents, and it is unclear how adolescents should be managed in this regard. Further research to gather objective longitudinal data on GERD and its consequences after VSG are needed.
Limitations of this study include a small sample size, specifically in the VSG group. In addition, the evaluated symptoms are subjective in nature predisposing the data to recall bias. We did not adjust for testing multiple symptoms due to the exploratory nature of this study. Lastly, the observational study design introduces heterogeneity into the data, but this was addressed by adjusting for potential confounders in logistic regression analyses. Despite these limitations, the prospective design along with the uniformity of data collection, length of follow up, geographically distinct sites, standardized definitions (specifically the GIS rating scale), and high subject retention make our findings an important contribution to the literature.
In conclusion, adolescents undergoing MBS experience an increasing risk over time of GERS, nausea, bloating, and diarrhea postoperatively. Furthermore, the risk of GERS in this cohort is more pronounced following the VSG procedure. Patients should be counseled regarding these post-operative risks when contemplating their choice of MBS procedure and those undergoing VSG may require additional evaluation and treatment for GERD and its long-term consequences.
Acknowledgments
Clinical Trial Registration: ClinicalTrials.gov, Identifier: NCT00474318, https://clinicaltrials.gov/ct2/show/NCT00474318?term=Teen-LABS&rank=1
APPENDIX
A: Study Participant Flow Diagram
B: GSRS: Gastrointestinal Symptom Rating Scale
C: Table 2a: Adjusted Odds Ratio (aOR) for models used to examine Moderate to Very Severe Gastrointestinal Symptoms 6-months to 5-years Post Operatively
Discussion
Change in Gastrointestinal Symptoms over the First 5 Years After Bariatric Surgery in a Multicenter Cohort of Adolescents
Presenter: Lindel C. Dewberry MD
DR. BRAD WARNER-ST. LOUIS, MO: Congratulations. That’s a great study. You know, I just have a little issue with the statistic of the binary separation of yes/no. But in that no was, I think, mild to moderate symptoms--
DR. DEWBERRY: [Interposing] In that no was no to minor symptoms.
DR. WARNER: Minor, okay. What’s the incidence of minor symptoms then with the two? And is there any disparity there, because that still can be a problem.
DR. DEWBERRY: Thank you very much for your question. I don’t remember the exact prevalence of the minor symptoms, but I do remember it was lower. And the reason we chose that specific cutoff is because there weren’t that many symptoms in that group. And we really wanted to look at the severe symptoms, although I agree with you that mild symptoms can also affect patients to just see how severe symptoms are after surgery. And for analysis purposes, again, it was better for our model to - -into binary outcome. But that is a valid question. Thank you.
DR. KUOJEN TSAO, HOUSTON, TX: Can you tell me a little bit about the experience of doing the two operations--one’s been around a little bit longer in our field compared to the other--and the ability to manage those patients postoperatively. You may have greater experience in the Roux-en-Y group. Is there any difference in the timing of how long the patients have been around?
DR. DEWBERRY: We didn’t look at that specifically. But maybe I’m misunderstanding your question.
DR. TSAO: I guess I’m asking, is there a greater experience with one operation than the other operation in their ability to manage symptoms for one group versus the other group?
DR. DEWBERRY: So I think in the data--the most significant finding is definitely the higher incidence and prevalence of reflux after sleeve. So I think a lot of times, patients who have reflux at baseline, it’s recommended they undergo bypass. So I think that is something to consider. I don’t think that’s a reason that we should switch to bypass necessarily. I think these patients just need to be monitored more closely.
DR. TSAO: Okay. Any other questions?
DR. SALEEM ISLAM, GAINESVILLE, FL: I could ask one. So what are you recommending then at this point? The sleeve is clearly a much easier operation. Most of the adult folks have gone to it, despite the known incidence of reflux. So you’ve shown that reflux is there, which the adult people have shown as well. So are you guys doing something different now?
DR. DEWBERRY: So again, we’re not advocating switching to bypass. And we agree that our data mirrors the adult data in terms of reflux. I think what’s needed is that this may be another good project for the Teen-LABS group, and those who perform adolescent bariatric surgeries to come up with guidelines for these patients after sleeve in terms of monitoring, because again, I don’t think everyone needs an EGD, and not everyone needs to be placed on anti-reflux medication. But I think that is definitely something to work on in the future.
DR. TSAO: Thank you.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lindel C. Dewberry, Department of Surgery, University of Colorado, Aurora, CO.
Jane C Khoury, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Shelley Ehrlich, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Todd M. Jenkins, Department of Surgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Andrew J. Beamish, Research Department, Royal College of Surgeons of England, London, UK.
Heidi J. Kalkwarf, Department of Pediatrics, Digestive Health Center, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Stavra A. Xanthakos, Department of Pediatric Gastroenterology, Hepatology and Nutrition, Cincinnati, Children’s Hospital Medical Center, Cincinnati, OH.
Thomas Inge, Department of Pediatric Surgery, Children’s Hospital Colorado, Aurora, CO.
REFERENCES
- [1].Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012; 307: 483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kelleher DC, Merrill CT, Cottrell LT, Nadler EP, Burd RS. Recent national trends in the use of adolescent inpatient bariatric surgery: 2000 through 2009. JAMA Pediatr 2013; 167: 126–32. [DOI] [PubMed] [Google Scholar]
- [3].Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg 2013; 48: 2401–7. [DOI] [PubMed] [Google Scholar]
- [4].Beamish AJ, Olbers T, Kelly AS, Inge TH. Cardiovascular effects of bariatric surgery. Nat Rev Cardiol 2016; 13: 730–43. [DOI] [PubMed] [Google Scholar]
- [5].Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med 2016; 374: 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Inge TH, Courcoulas AP, Xanthakos SA. Weight Loss and Health Status after Bariatric Surgery in Adolescents. N Engl J Med 2016; 374: 1989–90. [DOI] [PubMed] [Google Scholar]
- [7].Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gribsholt SB, Pedersen AM, Svensson E, Thomsen RW, Richelsen B. Prevalence of Self-reported Symptoms After Gastric Bypass Surgery for Obesity. JAMA Surg 2016; 151: 504–11. [DOI] [PubMed] [Google Scholar]
- [9].Hogestol IK, Chahal-Kummen M, Eribe I, Brunborg C, Stubhaug A, Hewitt S et al. Chronic Abdominal Pain and Symptoms 5 Years After Gastric Bypass for Morbid Obesity. Obes Surg 2017; 27: 1438–45. [DOI] [PubMed] [Google Scholar]
- [10].Inge TH, Zeller M, Harmon C, Helmrath M, Bean J, Modi A et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg 2007; 42: 1969–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res 1998; 7: 75–83. [DOI] [PubMed] [Google Scholar]
- [12].Inge TCY, Bazzano L, Xanthakos S,McTigue K, Srterburn D, Williams N, Wellman R, Coleman K, Courcouals A, Desai N, Anau J, Pardee R, Toh S, Janning C, Cook A, Sturtevant J, Horgan C, Zebrink A, Michalsky M. Comparative Effectiveness of Gastric Bypass, Sleeve Gastrectomy, and Adjustable Gastric Banding for Weight Loss among adolescents: The National Patient-Centered Clinical Research Network (PCORnet) Bariatric Study. SOARD 2018. [Google Scholar]
- [13].Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med 2013; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg 2010; 252: 319–24. [DOI] [PubMed] [Google Scholar]
- [15].Prachand VN, Alverdy JC. Gastroesophageal reflux disease and severe obesity: Fundoplication or bariatric surgery? World J Gastroenterol 2010; 16: 3757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Afshar S, Seymour K, Kelly SB, Woodcock S, van Hees VT, Mathers JC. Changes in physical activity after bariatric surgery: using objective and self-reported measures. Surg Obes Relat Dis 2017; 13: 474–83. [DOI] [PubMed] [Google Scholar]
- [17].Borbely YM, Osterwalder A, Kroll D, Nett PC, Inglin RA. Diarrhea after bariatric procedures: Diagnosis and therapy. World J Gastroenterol 2017; 23: 4689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borbely Y, Kroll D, Egermann U, Nett PC. [Aftercare following bariatric surgery]. Ther Umsch 2013; 70: 123–8. [DOI] [PubMed] [Google Scholar]
- [19].Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients--an evidence-based review. Obes Surg 2011; 21: 119–24. [DOI] [PubMed] [Google Scholar]
- [20].Potoczna N, Harfmann S, Steffen R, Briggs R, Bieri N, Horber FF. Bowel habits after bariatric surgery. Obes Surg 2008; 18: 1287–96. [DOI] [PubMed] [Google Scholar]
- [21].Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kroll D, Borbely Y et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 2018; 319: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol 2015; 21: 10348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Felsenreich DM, Kefurt R, Schermann M, Beckerhinn P, Kristo I, Krebs M et al. Reflux, Sleeve Dilation, and Barrett’s Esophagus after Laparoscopic Sleeve Gastrectomy: Long-Term Follow-Up. Obes Surg 2017; 27: 3092–101. [DOI] [PubMed] [Google Scholar]
- [24].Soricelli E, Casella G, Baglio G, Maselli R, Ernesti I, Genco A. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis 2018; 14: 751–6. [DOI] [PubMed] [Google Scholar]