Abstract
Research over the past century indicates that the daily timing of physical activity impacts both immediate performance and long-term training efficacy. Recently, several molecular connections between circadian clocks and exercise physiology have been identified. Circadian clocks are protein-based oscillators that enable anticipation of daily environmental cycles. Cell-autonomous clocks exist in almost all cells of the body and their timing is set by a variety of internal and external signals, including hormones and dietary intake. Improved understanding of the relationship between molecular clocks and exercise will benefit professional athletes and public health guidelines for the general population. Here, we discuss the role of circadian clocks in exercise, exploring time-of-day effects and proposed molecular and physiological mechanisms.
Keywords: Exercise, Circadian, Chronotype, Metabolism, Nuclear Receptors
It’s all in the timing
An important safeguard against the development of metabolic disease and age-related physical decline is the maintenance of lean body mass, which can be achieved in part through regular exercise. Along with exercise type, volume and frequency, the daily timing of physical activity impacts its efficacy. Exercise performance is significantly influenced by our internal biological clock, known as the circadian system. The circadian clock represents a series of biological rhythms that oscillate over a 24-hour period, enabling an organism to anticipate daily changes in its environment and adapt its physiology and behavior accordingly. While the primary circadian pacemaker is located in the suprachiasmatic nucleus (SCN), autonomous, self-sustaining oscillators (Box 1) also exist in almost all cells of the body [1]. The timing of these peripheral clocks is set by both neuroendocrine signals from the master clock and by local zeitgebers, such as feeding-fasting cycles or locomotor activity [2]. Improved understanding of the relationship between chronobiology and exercise physiology will benefit professional sports organizations aimed at enhancing athletic performance, as well as aid public health authorities in designing population-wide exercise recommendations. Here, we discuss the role of circadian clocks in exercise, exploring time-of-day effects, proposed physiological mechanisms and potential inputs from the molecular clock, with a focus on recent developments in these areas. This focus does not directly include the consequences for metabolic health and disease, which have recently been evaluated elsewhere [3]. In addition, given inevitable editorial constraints, we regrettably cannot include all key studies in this field, thus we refer to other relevant reviews where appropriate.
Box 1: The molecular circadian clock.
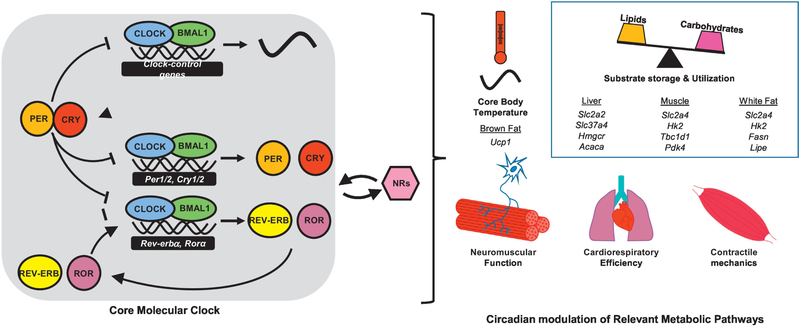
The core cellular machinery of the mammalian circadian system consists of interconnected transcription-translation feedback loops (TTFL). The primary loop is composed of a positive arm with two activator proteins (CLOCK and BMAL1) and a negative arm with two repressor proteins (CRY and PER). CLOCK (Circadian locomotor output control kaput) and BMAL1 (Brain muscle ARNT-like 1) form the heterodimeric transcriptional complex CLOCK:BMAL1 and initiate transcription of the cryptochrome (Cry1, Cry2) and period (Per1, Per2, Per3) genes, as well as a host of other clock-controlled genes. CRYs and PERs heterodimerize to form the repressive complex, subsequently translocating to the nucleus to repress further CLOCK:BMAL1 activity [93]. Proteasomal degradation of CRYs and PERs relieves the repression of CLOCK:BMAL1, allowing the transcriptional cycle to repeat, all of which occurs over approximately 24 hours. A secondary TTFL consists of CLOCK:BMAL1 driving the expression of the nuclear hormone receptors (NRs) REV-ERBα/β and RORα/γ, which in turn repress or activate BMAL1 transcription, respectively [94, 95] (Figure 1, Key Figure).
Exercise performance and training efficacy across the day
Several studies in human subjects have demonstrated significantly enhanced performance in evening exercise of various types compared to the same exercise performed in the morning [4–7]. Many of these reports have come from dynamic exercise such as cycling, running and swimming, which incorporate both aerobic and anaerobic elements. In compound resistance exercises, this phenomenon is somewhat less robust and is only observed at sub-maximal loads [8–10]. In highly technical sports, evidence suggests that there could be a trade-off between timing of peak cognitive physical abilities, such that measures of accuracy (i.e. in dart-throwing or soccer goal aim) may be greater earlier in the day compared with the typical evening peak in meausres of more purely physical attributes like strength, speed, and endurance [11, 12]. Although it can be difficult to distinguish between the many complex and intertwined factors that influence exercise performance, morning-evening differences in swim performance are at least partly attributable to the body’s internal circadian clock [13].
Some studies incorporated a period of targeted training to establish whether the timing of habitual practice aligns with peak performance and can influence diurnal variation. Indeed, such training time-specific adaptations occur in children and young adults with measures of aerobic and anaerobic capacity, and muscular force [5]. These data suggest that those involved in competitive events would benefit from aligning daily training schedules with the timing of future competitions. If competition time is unknown or spans multiple days and times, morning training may be the best option: evening performance will improve (albeit to a lesser degree) and diurnal differences will be mitigated. In the case of professional sports involving travel across time zones, jet lag causes misalignment of internal clocks and can negatively affect athletic performance. The impairment appears to depend on the direction of air travel, the number of time zones crossed and the specific sport in question [14, 15].
Morning larks versus night owls
It is well established that people vary widely in the timing of their natural behavioral alignment to daily light-dark cycles. Colloquially we refer to someone who wakes up early as being “a morning person” or a “lark”, and to people whose natural inclination is to stay up late and sleep in as “night owls.” Several methods of quantifying an individual’s propensity for early or late alignment with environmental light-dark cycles have been developed, including the Horne-Ostberg Questionnaire [16] and the Munich ChronoType Questionnaire [17]. When analysis of chronotype is included in the study design, the data suggest that overall performance and other related measures are greatest at times that align with the subject’s chronotype [18–20], and that this effect may be further influenced by habitual training time [21, 22]. Thus, exercise capacity and chronotype may exhibit reciprocal feedback (Box 2). As opposed to the clear diurnality of humans, most experimental strains of mice are nocturnal. Additionally, mice display numerous short episodes of sleep and activity during the 24 hour cycle, thus making it difficult to reliably determine chronotype differences and stratify in the context of exercise capacity [23].
Box 2: From genotype, to chronotype, to phenotype.
In more recent years, the elucidation of molecular clock gene polymorphisms as a genetic basis of chronotype has become a consideration for sports performance. A length polymorphism in the human Per3 gene (along with the gene’s endogenous circadian expression) has been associated with altered morning-evening preferences in the general population; the longer allele being linked to morningness and the shorter allele with eveningness [96]. These behavioral correlates are further supported by the preponderance of the shorter form allele in patients with delayed sleep phase syndrome (DSPS) [96]; a condition also now linked to Cry1 coding variants [97]. These relevant differences in Per3 allele length are driven by the variable number of tandem repeats (VNTR). Analysis of this polymorphism in endurance athletes has revealed a greater prevalence of the longer Per3(5) allele relative to control subjects, with the VNTR being predictive of self-reported chronotype distribution [98] and time-of-day differences in exercise performance [21, 99]. It is unclear whether this correlation reflects a direct effect of chronotype on performace or two independent effects of the Per3(5) variant allele. Analysis of male and female elite athletes from a variety of sports reveals an overrepresentation of morning-types in sports generally taking place in the morning, such as marathon running, cycling, or triathlon [100, 101]. These findings suggest that those with strong morning preference may be more drawn to sporting activities that take place during the early morning. Alternatively, those with chronotypes that radically oppose the performance schedules of that sport may simply not progress to the very elite level, resulting in a selection bias.
Proposed physiological mechanisms underlying diurnal variation
Body temperature
One of the circadian mechanisms put forward to explain improved evening performance in humans is the well-established rhythmic daily oscillation (~0.4–1.0°C) in core body temperature (CBT) [24]. The pattern of anaerobic power output across the day closely mirrors that for body temperature [13, 25, 26]; moreover, pre-warming or pre-cooling of the limbs can alter diurnal differences in muscle strength [27, 28]. Studies of isolated rodent skeletal muscles have suggested that elevation in CBT (and subsequently muscle temperature) alleviates muscle stiffness and improves thermodynamic muscular efficiency [29], which could contribute to improved evening performance in humans.
Substrate utilization
Diurnal differences in muscle function could also arise from changes in substrate utilization and cellular metabolism. Although circadian rhythms in glycogen accumulation have been well documented in rodents [30], the extent to which tissue glycogen content cycles diurnally in humans is less clear [31, 32], possibly due to differences in overall muscle mass and fiber type composition, as well as lifestyle factors such as diet and sleep amount [33].
Both glycogenolysis and gluconeogenesis during intense/prolonged exercise are mediated, in part, by sympathoadrenal responses. Long-term training adaptations can include enhanced epinephrine release to hard exercise [34], increased gluconeogenesis and greater lactate clearance [35, 36]. However, While there is a clear circadian rhythm in the production and circulation of hormones such as cortisol and catecholamines [37], the peaks and nadirs of these hormones do not align reliably with the diurnal variation in human exercise capacity. Mouse studies have revealed that molecular clock components can influence the response to cortisol [38] and otherwise influence substrate selection [39] in peripheral organs, which may also contribute to daytime-dependent differences in glucose, lipid, and glycogen storage and/or utilization. Diurnal variation in muscle fatigue has been evaluated elsewhere [40] and falls outside the scope of this review.
Cardiovascular effects
Sustained physical activity is highly dependent on the delivery of oxygen and nutrients to, and the removal of waste products from, the working musculature. Thus, diurnal variation in exercise capacity could be linked to alterations in blood flow. Endothelium-dependent and -independent vasodilation in the forearm vary significantly across the day, related to the circadian oscillation of endothelin-1 (ET-1), a major endogenous vasoconstrictor [41, 42]. However, the time of peak perfusion may differ between vascular beds [43, 44], thus standard experimental measures from the brachial artery may not be representative of diurnal changes in muscles heavily engaged in athletics, such as the legs and trunk.
Neuromuscular recruitment and central governance
Time-of-day differences in exercise performance would be influenced by both peripheral (e.g. cardiovascular, muscular) and neurological mechanisms. Studies of different muscle groups in humans have demonstrated that while contractile force increases over the course of the day, there is no difference in electromyographic parameters of muscle excitation, suggesting minimal diurnal changes in neural drive [45, 46]. Other relevant aspects of central command include cognitive processing and reaction times to complex tasks, which may be subject to circadian regulation, but are beyond the scope of this review.
Molecular clock-exercise reciprocal regulation
The central circadian pacemaker is located in the suprachiasmatic nucleus (SCN) at the base of the anterior hypothalamus, and its timing is set primarily by light cues. Molecular clocks in peripheral tissues oscillate in a cell-autonomous fashion. Moreover, elegant studies in mice have demonstrated that these decentralized clocks can be dissociated from SCN-generated rhythms and entrained by other environmental cues, including the timing of food availability [47]. Since acute exercise and long-term training adaptations are whole-body challenges, the application of chronobiology to athletic performance and sports medicine must consider these multi-level circadian concepts. Namely, exercise may be governed centrally by generalized behavioral rhythms and entrained by photoperiod (e.g. sleep-wake cycles and travel across time-zones). It may also be influenced by tissue-specific clocks in organs such as skeletal muscle and the liver (including any cross-talk), and act itself as a physiological cue to entrain the circadian system. Rodent studies revealed that habitual, voluntary running-wheel activity at specific times of day is sufficient to synchronize behavioral rhythms in mice and regulate expression of core clock genes in the SCN [48]. Phase-shifting effects of exercise have also been observed in humans [49–51], suggesting that exercise could be a valuable tool to mitigate the effects of transmeridian travel (i.e. “jet-lag”) on sleep timing and quality.
Muscular rhythms
Hundreds of transcripts, representing approximately four percent of all expressed genes, undergo circadian oscillations of expression in mouse skeletal muscle [52], many of them being involved in processes relevant to exercise capacity. Genes encoding components of fatty acid (FA) transport and β-oxidation peak in the middle of the inactive phase, whereas lipogenic genes peak late into the active phase, likely in response to feeding. Transcripts involved in glucose uptake and glycogen storage are most highly expressed in the active phase [53], consistent with previously documented rhythms in skeletal muscle glycogen [30]. However, most studies have focused on large muscle groups of mixed fiber types (e.g. gastrocnemius or quadriceps). Humans and rodents have many different types of muscles, each of which influences exercise in different ways. The vast diversity of muscle subtypes was recently elegantly demonstrated by transcriptional profiling of a wide array of muscle groups in rodents [54]. When several mouse muscle groups have been analyzed for rhythmic transcript expression in parallel (e.g. plantaris, soleus, tibialis anteriorus), the group of transcripts that undergo circadian oscillation is specific to each muscle group [55, 56].
Biopsy samples have revealed that human skeletal muscle transcription is also regulated in a time-of-day dependent manner, resulting in rhythmic expression of transcripts involved in substrate storage and utilitzation, as well as the basal production of various myokines [57, 58]. Given that many of these pathways are known to be important for exercise capacity and training adaptations, these findings imply that the timing of exercise may be optimized to coincide with a rhythmic metabolic milieu. Supporting this idea, mitochondrial oxidative capacity of human muscle fibers and whole-body energy expenditure exhibit synchronous circadian rhythms that peak in the evening, consistent with peaks in exercise performance [59].
CLOCK:BMAL1 control
In addition to observed rhythmicity, the direct influence of the intrinsic circadian clock on muscle function has been highlighted through models employing genetic manipulation of core molecular components. Several studies have now examined the impact of deleting BMAL1 specifically in mouse skeletal muscles, enabling robust interpretation of tissue-autonomous clock disruption in the context of normal sleep-wake cycles, and feeding and activity rhythms. Deletion of BMAL1 selectively in skeletal muscles impairs insulin sensitivity and causes a switch from carbohydrate to lipid oxidation. These changes are accompanied by decreased muscle force generation, despite only modest changes in fiber type distribution [53, 60, 61]. Analysis of global RNA expression in mice lacking BMAL1 specifically in muscles revealed sweeping changes in metabolic transcriptional programs. These changes likely reflect a loss of both direct CLOCK:BMAL1 target gene regulation and impaired hypoxia-responsive transcription, since BMAL1−/− myotubes express much less HIF1α than control samples [62]. The interaction of the circadian clock with HIF1α has strong implications for intense exercise, when there is an increased reliance on glycolytic metabolism and a potential drop in the partial pressure of oxygen in the muscle. Indeed, wildtype mice exercised to exhaustion have variable induction of transcripts regulated by HIF1α in skeletal muscle depending on the time of day; the lowest transcriptional response being in the middle of the inactive phase [62]. Further supporting a role for cell-autonomous muscle clocks in driving oscillations of gene expression and metabolic functions, disruption of CLOCK in cultured human myotubes leads to upregulation of genes encoding lipid transport and downregulation of genes involved in exercise- and insulin-stimulated glucose uptake, along with altered myokine production [57, 58] and potential changes to hypertrophic signaling (Box 3).
Box 3: Clock control of anabolic responses.
A critical aspect of training adaptation (particularly for strength-based athletes) and the efficacy of exercise with age is the hypertrophic response within the muscle. While extensive research suggests that athletic performance peaks in the evening, it is unclear whether the muscle-intrinsic hypertrophic response is affected by the time of day at which resistance training occurs [102]. Hypertrophy is correlated with mTOR (mechanistic target of rapamycin) signaling [103], which exhibits circadian regulation in mouse liver [104]. Myogenic genes such as myogenin and myoD are also under circadian control in skeletal muscle [105], and may contribute to resistance exercise-induced muscle hypertrophy [106].
TTFL negative arms
A role for the primary and secondary negative arms of the core clock in muscle function and exercise physiology has also been recognized. Deletion of Per2, but not Per1, appears to increase muscle mass and reduce running endurance capacity of mice [63]. Conversely, mice deficient in CRY1 and CRY2 have improved capacity for sprint exercise, but do not exhibit increased running endurance. Skeletal muscle and liver from these mice exhibited greater glycogen storage, while Cry1−/−;Cry2−/− myotubes showed an upregulation of genes involved in FA utilization. These findings suggest that CRY1/2 help regulate substrate switching [39]. Loss of input from REV-ERB also impacts skeletal muscle morphology and metabolism, as well as exercise ability. Reverbα−/− mice have disrupted fuel partitioning [64], reduced skeletal muscle mitochondrial content, and impaired running endurance [65]. Because all of these studies have been done in animals harboring germline disruption of repressive clock components, further analysis is required to determine the muscle-intrinsic roles of PERs, CRYs, and REVERBs in exercise physiology.
Input from other peripheral clocks
Exercise requires an integrated response across many organ systems, so peak performance likely depends on synchronized function of multiple peripheral clocks. The liver supplies substrates to working muscles at all levels of exercise intensity and circadian rhythms in liver have been extensively studied [66]. The importance of the clock in this process is apparent even at rest, as liver-specific deletion of Bmal1 in mice leads to impaired glucose homeostasis throughout the day [67]. In addition, NAD+ biosynthesis and oxidative metabolism in the liver is under clock control [68, 69], which may influence exercise physiology [70]. Energetic stressors like exercise activate AMPK, which has been shown to phosphorylate and degrade CRY1, thereby altering hepatocyte rhythms [71]. Mitochondria are the powerhouse for energy production in cells and are subject to a surprisingly high amplitude oscillation in proteome composition in mouse liver [72, 73]. Moreover, hepatic BMAL1 seems to be required for directing mitochondrial dynamics in the liver and defend redox balance during different nutrient conditions [74]. Together, these data describe circadian regulation of key metabolic pathways in the liver, many of which converge to appropriately time energetic supply with physiological demands. Circadian regulation of several other tissues important for exercise physiology, including bone [75, 76] and adipose tissue [77–80], may also contribute to daily changes in physical performance.
Role of nuclear receptors
One of the major targets of the molecular clock, and a well-established pathway for regulation of metabolic health and exercise capacity, is the superfamily of nuclear hormone receptors (NRs) [81]. Multiple core clock proteins interact with NRs, thus providing a molecular bridge connecting the circadian clock to endocrine and metabolic outputs [38, 39, 82–84]. REV-ERBα/β and RORα/γ are NRs that participate directly in core clock function. Genetic manipulation of REV-ERBα in mice alters skeletal muscle expression of the NR transcriptional coactivator (Box 4) PGC-1α (Peroxisome proliferator-activated receptor γ coactivator 1-α), leading to changes in mitochondrial biogenesis and running endurance [65].
Box 4: NR coregulators.
The ability of NRs to respond to and coordinate circadian and metabolic signals is regulated by transcriptional coactivators or corepressors. For example, PPARγ coactivators 1 alpha (PGC-1α) and beta (PGC-1β) cooperate to promote oxidative metabolism in skeletal muscle and liver via transcription of mitochondrial genes [107, 108]. Furthermore, there are at least four isoforms of PGC-1α that are differentially expressed in response to exercise and confer variable levels of transcriptional activation on associated NRs [109]. Transgenic expression of the truncated PGC-1α4 isoform in mouse skeletal muscles enhances muscle size and strength [110]. In addition, PGC-1α undergoes circadian oscillation of expression in mouse liver nuclei [111], and is subject to post-translational modification by AMP-activated protein kinase (AMPK) [112], one of the most well-established effectors of acute exercise stimulation [113, 114]. It is unclear whether circadian clocks modulate specific isoforms of PGC-1α and/or PGC-1β or whether the time of day impacts their phosphorylation by AMPK, which is also subject to circadian regulation [115], both transcriptionally [71] and post-translationally [116]. The steroid receptor coactivators (SRC-1, −2, and −3) stimulate the transcriptional activity of many NRs and exert broad effects on energy homeostasis. SRC-2 protein exhibits daily oscillation in mouse liver and its interactions with chromatin overlap to a surprisingly large extent with those of BMAL1 [117]. Furthermore, SRC-2 may promote rhythms of chromatin accessibility, thus broadly enabling rhythmic transcription [118].
Among NR co-repressors, a direct role has been demonstrated for nuclear receptor corepressor 1 (NCoR1) in exercise performance, where muscle-specific ablation in mice leads to improved endurance running, associated with increased muscle mass and oxidative capacity, largely attributed to enhanced activities of PPARδ and ERRs [119]. Germline deletion of the clock components and NR co-repressors CRY1 and CRY2 [82] also leads to hyperactivation of PPARδ in muscles, and enhanced exercise capacity [39]. PER2 also seems to repress NRs and its deletion causes abnormal cycling of gluconeogenic genes, hepatic glycogen storage, and adipose tissue metabolism [83, 84], which could also impact exercise physiology. Together these data reveal the widespread crosstalk between NRs, coregulators and molecular clock components; these relationships likely coordinate the response to physiological stressors, such as exercise.
Exercise and circadian clock synchronization both rely on responses to neurohumoral inputs, many of which signal through NRs. One of the major hormonal inputs is circulating glucocorticoids, which oscillate diurnally to mediate fuel catabolism and are increasingly secreted in response to stress. CRYs repress GR-mediated transcription to modulate hepatic glucose production and overall metabolic homeostasis in mice [38]. Since glucocorticoids are released in response to intense exercise and may mediate some of the phase-shifting effects of physical activity in rodents [85], optimal clock control of GR signaling has implications for over-training in athletes and circadian alignment during transmeridian travel. This is supported by the identification of many rhythmic genes in rat skeletal muscle that also happen to be regulated by glucocorticoids. Moreover, a number of these genes encode for exercise-responsive pathways, such as glycolysis, oxidative phosphorylation and glutamine and lipid metabolism [86]. Additional hormones can regulate metabolism and exercise through their respective NRs (e.g. AR and TR), which are also repressed by CRYs [82], but it is less clear how these are modulated by tissue-specific clocks.
A prominent link between the circadian clock and energy metabolism is the Peroxisome proliferator-activated receptor (PPAR) subfamily of NRs. The PPARδ isoform has received much attention given that pharmacological activation or genetic overexpression in mice has been shown to protect against obesity and dramatically improve running endurance. This response seems to involve PGC-1α coactivation, leading to increased FA oxidation in skeletal muscle and a concomitant sparing of glucose [87]. A role for the circadian clock in this axis was revealed by the observation of PPARδ derepression following loss of CRY1/2, subsequently allowing for glycogen storage and improved high-intensity exercise performance [39]. In addition, tissue-specific loss of BMAL1 in skeletal muscle alters the transcriptional programming of PPARδ targets, leading to upregulation of genes involved in lipid transport and oxidation [88]. Oscillations of PPARδ in other peripheral tissues could similarly impact systemic metabolism and exercise capacity; for example, liver PPARδ coordinates hepatic lipogenesis and muscle FA uptake [89]. PPARα and PPARγ are major regulators of of lipid storage and utilization [90, 91] and gluconeogenesis [91], and are also repressed by CRYs [39, 82] and PERs [83, 84, 92].
Concluding Remarks and Future Perspectives
The increasingly recognized role of the circadian system in exercise physiology has far-reaching consequences for basic research and applied sports medicine. While time-ofday differences in human exercise performance have been a topic of considerable interest, very few studies have directly investigated the underlying regulation by central and tissue-specific clocks. Elucidating the molecular clock-controlled mechanisms of skeletal muscle function will be critical for the future of this field, as well as the necessary integration with other peripheral clocks, the circadian pacemaker and neurohormonal output (see Outstanding Questions). The physiological demands of behavioral practices (particularly intense training schedules, dietary regimens and sleeping habits) will also require careful attention in this process. These personally- and socially-enforced entrainment cues could shape molecular clock function over time through reciprocal feedback. The ability of the circadian clock to coordinate and respond to targeted exercise likely requires complex metabolic signaling, particularly at the outer edges of performance. Centrally placed in this metabolic network are the nuclear receptors, some of which directly control substrate flux and molecular time-keeping (Figure 1). These cellular components are therefore appealing targets for pharma- and nutraceuticals, which could conceivably modulate exercise capacity in humans if delivery is optimized for circadian phase and tissue-specificity. This represents an exciting frontier for circadian biology, as chronotherapeutic strategies are considered in the context of lifestyle and genotype, with the promise of optimizing health, performance, and longevity.
Outstanding Questions.
What are the critical central or peripheral mechanisms that dictate the diurnal variation in exercise performance and response to training? Does this differ between exercise types?
To what extent can the circadian system be modified by long-term exercise habits?
How can existing and prospective ergogenic aids (supplements, light therapy etc.) be optimized to mitigate the decline in performance due to circadian disruption (e.g. sleep loss, jet-lag).
How does circadian clock function and/or the interaction between clocks and metabolism differ between muscle groups and fiber types?
What is the crosstalk between skeletal muscle circadian clocks and other peripheral clocks (e.g. liver, white and brown adipose tissue, cardiovascular, etc.) in coordinating the physiological response to exercise?
Will it someday be possible for military leadership, sports organizations, and public health authorities harness chronobiological data to push the boundaries of human performance and formulate evidence-based exercise recommendations?
Figure 1. Circadian regulation of exercise capacity.
Differences in exercise performance across the day are influenced by several aspects of physiology. The core molecular clock consists of a transcription and translation feedback loop that coordinates systemic and tissue-specific metabolic processes. Clocks are intimately linked to the activity of nuclear hormone receptors (NRs), which are critical mediators of both circadian rhythms and energy metabolism. Circadian modulation of gene expression and physiology has been studied in detail in several organs. We highlight some of the oscillating transcripts that are controlled by circadian clocks in peripheral organs and contribute to daily rhythms in physiological programs that likely influence exercise performance. The circadian molecular mechanisms underlying other rhythmic phenomena remain to be uncovered in future studies.
Trends.
Exercise performance is subject to diurnal variation, with peak capacity generally occurring towards the late-afternoon or early evening. However, this can be modified by training habits and an individual’s inherent chronotype
Time-of-day dependent differences in exercise capacity are associated with diurnal oscillations in core body temperature, cardiorespiratory responses, muscle mechanics and tissue metabolism.
The muscle-specific circadian clock is emerging as an integral mediator of exercise adaptations
Nuclear receptors are based at the crossroads of metabolism and the circadian system; they may become an important consideration for exercise-based interventions and a promising target for chronotherapeutics.
Acknowledgements
This work is supported by National Institutes of Health (DK112927 to K.A.L.).
Glossary:
- Suprachiasmatic nucleus (SCN)
a group of neurons in the anterior hypothalamus which act as the principal circadian pacemaker in mammals. This autonomous “master clock” is necessary for daily rhythms and coordinates peripheral cellular clocks through behavioral, neural and endocrine signals.
- Zeitgeber
any external cue that synchronizes an organism’s circadian clock with its environment e.g. light is the predominant zeitgeber for the SCN. It originates from German, meaning “time-giver.”
- Chronotype
the behavioral manifestation of an individual’s internal circadian clock. It is reflected in the timing of the sleep-wake cycle within the 24-hour period and is commonly determined by a morningness-eveningness questionnaire.
- Myokines
Cytokines and other proteins produced in and released by skeletal muscle. Myokines can influence physiology through autocrine, paracrine or endocrine effects.
- Acrophase
The time at which the peak occurs in a physiological rhythm.
- Peroxisome proliferator-activated receptors (PPARs)
a family of nuclear receptors (NRs) that regulate gene transcription in response to activation by specific ligands. Variants of these receptors exist in different tissues, including liver, adipose tissue, muscle, hepatocytes and endothelial cells, where they play an important role in glucose and lipid metabolism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoo SH et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 101 (15), 5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratmann M and Schibler U (2006) Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms 21 (6), 494–506. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel BM and Zierath JR (2019) Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. [DOI] [PubMed] [Google Scholar]
- 4.Bessot N et al. (2006) The effect of pedal rate and time of day on the time to exhaustion from high-intensity exercise. Chronobiol Int 23 (5), 1009–24. [DOI] [PubMed] [Google Scholar]
- 5.Chtourou H and Souissi N (2012) The effect of training at a specific time of day: a review. J Strength Cond Res 26 (7), 1984–2005. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes AL et al. (2014) Effect of time of day on performance, hormonal and metabolic response during a 1000-M cycling time trial. PLoS One 9 (10), e109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton B et al. (1997) Circadian rhythms have no effect on cycling performance. Int J Sports Med 18 (7), 538–42. [DOI] [PubMed] [Google Scholar]
- 8.Mora-Rodriguez R et al. (2012) Caffeine ingestion reverses the circadian rhythm effects on neuromuscular performance in highly resistance-trained men. PLoS One 7 (4), e33807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teo W et al. (2011) The effects of circadian rhythmicity of salivary cortisol and testosterone on maximal isometric force, maximal dynamic force, and power output. J Strength Cond Res 25 (6), 1538–45. [DOI] [PubMed] [Google Scholar]
- 10.Pallares JG et al. (2015) Pseudoephedrine and circadian rhythm interaction on neuromuscular performance. Scand J Med Sci Sports 25 (6), e603–12. [DOI] [PubMed] [Google Scholar]
- 11.Masmoudi L,GA, Chtourou H, Souissi N (2016) Effect of time of day on soccer specific skills in children: psychological and physiological responses. Biological Rhythm Research 47 (1), 59–68. [Google Scholar]
- 12.Blatter K and Cajochen C (2007) Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav 90 (2–3), 196–208. [DOI] [PubMed] [Google Scholar]
- 13.Kline CE et al. (2007) Circadian variation in swim performance. J Appl Physiol (1985) 102 (2), 641–9. [DOI] [PubMed] [Google Scholar]
- 14.Leatherwood WE and Dragoo JL (2013) Effect of airline travel on performance: a review of the literature. Br J Sports Med 47 (9), 561–7. [DOI] [PubMed] [Google Scholar]
- 15.Song A et al. (2017) How jet lag impairs Major League Baseball performance. Proc Natl Acad Sci U S A 114 (6), 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne JA and Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4 (2), 97–110. [PubMed] [Google Scholar]
- 17.Roenneberg T et al. (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18 (1), 80–90. [DOI] [PubMed] [Google Scholar]
- 18.Kunorozva L et al. (2014) Perception of effort in morning-type cyclists is lower when exercising in the morning. J Sports Sci 32 (10), 917–25. [DOI] [PubMed] [Google Scholar]
- 19.Tamm AS et al. (2009) Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms 24 (3), 211–24. [DOI] [PubMed] [Google Scholar]
- 20.Facer-Childs E and Brandstaetter R (2015) The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol 25 (4), 518–22. [DOI] [PubMed] [Google Scholar]
- 21.Rae DE et al. (2015) Factors to consider when assessing diurnal variation in sports performance: the influence of chronotype and habitual training time-of-day. Eur J Appl Physiol 115 (6), 1339–49. [DOI] [PubMed] [Google Scholar]
- 22.Brown FM et al. (2008) Collegiate rowing crew performance varies by morningness-eveningness. J Strength Cond Res 22 (6), 1894–900. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer M et al. (2015) Owls and larks in mice. Front Neurol 6, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aschoff J (1983) Circadian control of body temperature Journal of Thermal Biology 8 (1–2), 143–147. [Google Scholar]
- 25.Souissi N et al. (2004) Circadian rhythms in two types of anaerobic cycle leg exercise: force-velocity and 30-s Wingate tests. Int J Sports Med 25 (1), 14–9. [DOI] [PubMed] [Google Scholar]
- 26.Deschodt VJ and Arsac LM (2004) Morning vs. evening maximal cycle power and technical swimming ability. J Strength Cond Res 18 (1), 149–54. [DOI] [PubMed] [Google Scholar]
- 27.Taylor K et al. (2011) Warm-up affects diurnal variation in power output. Int J Sports Med 32 (3), 185–9. [DOI] [PubMed] [Google Scholar]
- 28.Robinson WR et al. (2013) Does lowering evening rectal temperature to morning levels offset the diurnal variation in muscle force production? Chronobiol Int 30 (8), 998–1010. [DOI] [PubMed] [Google Scholar]
- 29.Ranatunga KW (1982) Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol 329, 465–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benavides A et al. (1998) Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am J Physiol 275 (3 Pt 2), R811–7. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi H et al. (2015) (1)(3)C MRS reveals a small diurnal variation in the glycogen content of human thigh muscle. NMR Biomed 28 (6), 650–5. [DOI] [PubMed] [Google Scholar]
- 32.Macauley M et al. (2015) Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin Sci (Lond) 128 (10), 707–13. [DOI] [PubMed] [Google Scholar]
- 33.Skein M et al. (2011) Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc 43 (7), 1301–11. [DOI] [PubMed] [Google Scholar]
- 34.Kjaer M et al. (1985) Effect of exercise on epinephrine turnover in trained and untrained male subjects. J Appl Physiol (1985) 59 (4), 1061–7. [DOI] [PubMed] [Google Scholar]
- 35.Bergman BC et al. (2000) Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278 (2), E244–51. [DOI] [PubMed] [Google Scholar]
- 36.MacRae HH et al. (1995) Effects of endurance training on lactate removal by oxidation and gluconeogenesis during exercise. Pflugers Arch 430 (6), 964–70. [DOI] [PubMed] [Google Scholar]
- 37.Scheer FA et al. (2010) Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 107 (47), 20541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamia KA et al. (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480 (7378), 552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan SD et al. (2017) CRY1/2 Selectively Repress PPARdelta and Limit Exercise Capacity. Cell Metab 26 (1), 243–255 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chtourou H, et al. (2013) Effect of Time-of-Day on Muscle Fatigue: A Review. J. Nov Physiother 3 (160), 1–10. [Google Scholar]
- 41.Elherik K et al. (2002) Circadian variation in vascular tone and endothelial cell function in normal males. Clin Sci (Lond) 102 (5), 547–52. [PubMed] [Google Scholar]
- 42.Etsuda H et al. (1999) Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: possible connection to morning peak of cardiac events? Clin Cardiol 22 (6), 417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conroy DA et al. (2005) Daily rhythm of cerebral blood flow velocity. J Circadian Rhythms 3 (1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston JG and Pollock DM (2018) Circadian regulation of renal function. Free Radic Biol Med 119, 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guette M et al. (2005) Time-of-day effect on the torque and neuromuscular properties of dominant and non-dominant quadriceps femoris. Chronobiol Int 22 (3), 541–58. [DOI] [PubMed] [Google Scholar]
- 46.Chtourou H et al. (2011) Diurnal variation in Wingate-test performance and associated electromyographic parameters. Chronobiol Int 28 (8), 706–13. [DOI] [PubMed] [Google Scholar]
- 47.Damiola F et al. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14 (23), 2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroeder AM et al. (2012) Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol 590 (23), 6213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youngstedt SD et al. (2016) Circadian Phase-Shifting Effects of Bright Light, Exercise, and Bright Light + Exercise. J Circadian Rhythms 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buxton OM et al. (2003) Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol 284 (3), R714–24. [DOI] [PubMed] [Google Scholar]
- 51.Youngstedt SD et al. (2019) Human circadian phase-response curves for exercise. J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R et al. (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111 (45), 16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodge BA et al. (2015) The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terry EE et al. (2018) Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyar KA et al. (2015) The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol Metab 4 (11), 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyar KA et al. (2018) Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 174 (6), 1571–1585 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrin L et al. (2018) Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perrin L et al. (2015) Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab 4 (11), 834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Moorsel D et al. (2016) Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab 5 (8), 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dyar KA et al. (2014) Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 3 (1), 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harfmann BD et al. (2016) Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peek CB et al. (2017) Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab 25 (1), 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bae K et al. (2006) Differential effects of two period genes on the physiology and proteomic profiles of mouse anterior tibialis muscles. Mol Cells 22 (3), 275–84. [PubMed] [Google Scholar]
- 64.Delezie J et al. (2012) The nuclear receptor REV-ERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J 26 (8), 3321–35. [DOI] [PubMed] [Google Scholar]
- 65.Woldt E et al. (2013) Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19 (8), 1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zwighaft Z et al. (2016) The Liver in the Eyes of a Chronobiologist. J Biol Rhythms 31 (2), 115–24. [DOI] [PubMed] [Google Scholar]
- 67.Lamia KA et al. (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A 105 (39), 15172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsey KM et al. (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324 (5927), 651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peek CB et al. (2013) Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342 (6158), 1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White AT and Schenk S (2012) NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 303 (3), E308–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamia KA et al. (2009) AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326 (5951), 437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neufeld-Cohen A et al. (2016) Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S A 113 (12), E1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bugge A et al. (2012) Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev 26 (7), 657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobi D et al. (2015) Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab 22 (4), 709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu C et al. (2016) Circadian Clock Regulates Bone Resorption in Mice. J Bone Miner Res 31 (7), 1344–55. [DOI] [PubMed] [Google Scholar]
- 76.Yang N and Meng QJ (2016) Circadian Clocks in Articular Cartilage and Bone: A Compass in the Sea of Matrices. J Biol Rhythms 31 (5), 415–27. [DOI] [PubMed] [Google Scholar]
- 77.Paschos GK et al. (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18 (12), 1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henriksson E and Lamia KA (2015) Adipose Clocks: Burning the Midnight Oil. J Biol Rhythms 30 (5), 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerhart-Hines Z et al. (2013) The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 503 (7476), 410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christou S et al. (2019) Circadian regulation in human white adipose tissue revealed by transcriptome and metabolic network analysis. Sci Rep 9 (1), 2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X et al. (2007) Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb Symp Quant Biol 72, 387–94. [DOI] [PubMed] [Google Scholar]
- 82.Kriebs A et al. (2017) Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc Natl Acad Sci U S A 114 (33), 8776–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grimaldi B et al. (2010) PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab 12 (5), 509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmutz I et al. (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24 (4), 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sasaki H et al. (2016) Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci Rep 6, 27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almon RR et al. (2008) Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 295 (4), R1031–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan W et al. (2017) PPARdelta Promotes Running Endurance by Preserving Glucose. Cell Metab 25 (5), 1186–1193 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dyar KA et al. (2018) Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol 16 (8), e2005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S et al. (2013) A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502 (7472), 550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muoio DM et al. (2002) Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51 (4), 901–9. [DOI] [PubMed] [Google Scholar]
- 91.Kersten S et al. (1999) Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 103 (11), 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Costa MJ et al. (2011) Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J Biol Chem 286 (11), 9063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Partch CL et al. (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24 (2), 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Preitner N et al. (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110 (2), 251–60. [DOI] [PubMed] [Google Scholar]
- 95.Sato TK et al. (2004) A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43 (4), 527–37. [DOI] [PubMed] [Google Scholar]
- 96.Archer SN et al. (2003) A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26 (4), 413–5. [DOI] [PubMed] [Google Scholar]
- 97.Patke A et al. (2017) Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 169 (2), 203–215 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kunorozva L et al. (2012) Chronotype and PERIOD3 variable number tandem repeat polymorphism in individual sports athletes. Chronobiol Int 29 (8), 1004–10. [DOI] [PubMed] [Google Scholar]
- 99.Anderson A et al. (2018) Circadian Effects on Performance and Effort in Collegiate Swimmers. J Circadian Rhythms 16, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lastella M et al. (2016) The Chronotype of Elite Athletes. J Hum Kinet 54, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henst RH et al. (2015) A chronotype comparison of South African and Dutch marathon runners: The role of scheduled race start times and effects on performance. Chronobiol Int 32 (6), 858–68. [DOI] [PubMed] [Google Scholar]
- 102.Sedliak M et al. (2018) Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training. Chronobiol Int 35 (4), 450–464. [DOI] [PubMed] [Google Scholar]
- 103.Mitchell CJ et al. (2013) Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One 8 (10), e78636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jouffe C et al. (2013) The circadian clock coordinates ribosome biogenesis. PLoS Biol 11 (1), e1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shavlakadze T et al. (2013) Impact of fasting on the rhythmic expression of myogenic and metabolic factors in skeletal muscle of adult mice. Am J Physiol Cell Physiol 305 (1), C26–35. [DOI] [PubMed] [Google Scholar]
- 106.Thalacker-Mercer A et al. (2013) Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45 (12), 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamei Y et al. (2003) PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A 100 (21), 12378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin J et al. (2003) PGC-1beta in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278 (33), 30843–8. [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Redondo V et al. (2015) The hitchhiker’s guide to PGC-1alpha isoform structure and biological functions. Diabetologia 58 (9), 1969–77. [DOI] [PubMed] [Google Scholar]
- 110.Ruas JL et al. (2012) A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151 (6), 1319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu C et al. (2007) Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447 (7143), 477–81. [DOI] [PubMed] [Google Scholar]
- 112.Jager S et al. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104 (29), 12017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gabriel BM and Zierath JR (2017) The Limits of Exercise Physiology: From Performance to Health. Cell Metab 25 (5), 1000–1011. [DOI] [PubMed] [Google Scholar]
- 114.Mounier R et al. (2015) Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab 26 (6), 275–86. [DOI] [PubMed] [Google Scholar]
- 115.Jordan SD and Lamia KA (2013) AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol 366 (2), 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henriksson E et al. (2017) The Liver Circadian Clock Modulates Biochemical and Physiological Responses to Metformin. J Biol Rhythms 32 (4), 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stashi E et al. (2014) SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Rep 6 (4), 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu B et al. (2015) Coactivator-Dependent Oscillation of Chromatin Accessibility Dictates Circadian Gene Amplitude via REV-ERB Loading. Mol Cell 60 (5), 769–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamamoto H et al. (2011) NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 147 (4), 827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]