Abstract
Introduction:
Umbilical mesenchymal stem cells (USC) have been shown to reduce illness in animal models of necrotizing enterocolitis (NEC), possibly through the paracrine release of hydrogen sulfide (H2S). We hypothesized that animals treated with USCs with inhibited H2S synthesis would exhibit more severe disease.
Methods:
NEC was induced in five-day-old mouse pups by formula feeding and hypoxic and hypothermic stress. Experimental groups received intraperitoneal injection of either saline vehicle or 80,000cells/gram of one of the following cell types: USC, USCs with negative-control siRNA, or USCs with targeted siRNA inhibition of the H2S-producing enzymes. Pups were monitored by clinical assessment and after euthanasia, intestine and lung histologic injury were scored. Tissue was homogenized, and concentrations of IL-6, IL-10, and VEGF were determined by ELISA. For statistical analysis, p<0.05 was considered significant.
Results:
Animals treated with negative-control siRNA USCs were significantly improved compared to vehicle. Clinical sickness scores as well as intestinal and lung histologic injury scores in the targeted siRNA groups were significantly worse when compared to the negative-control siRNA group. IL-6, IL-10, and VEGF had varying patterns of expression in the different groups.
Conclusion:
Inhibition of H2S production in USCs reduces the beneficial effects of these cells during therapy in experimental NEC.
Keywords: Animal model, Necrotizing enterocolitis, Hydrogen sulfide, Umbilical stromal cell, Intestine
INTRODUCTION:
Necrotizing enterocolitis (NEC) remains a major cause of morbidity and mortality in premature infants. These patients often require extensive surgical resection of diseased bowel [1] and mortality rates are as high as 40% [2]. Additionally, these patients suffer systemic illness, and specifically are afflicted with NEC-associated lung disease in addition to gastrointestinal symptoms. Treatments that could prevent the need for intestinal resection as well as limit systemic manifestations of the disease are desperately needed. Stem cells represent a possible answer to this difficult problem.
Umbilical mesenchymal stem cells (USC) are isolated from the umbilical cord and are considered multipotent [3]. They have been used with success in animal models of ischemia and reperfusion [4] as well as NEC [5, 6]. Stem cells are helpful in repairing tissue through multiple mechanisms including migration and engraftment, heterotopic cell fusion, and paracrine effects including exosome release [3]. Though some engraftment likely does occur in the animal model of NEC, it seems likely that the paracrine effect is most influential in intestinal and systemic protection [7].
One specific compound released from USCs that has a potential benefit during ischemia is hydrogen sulfide (H2S), a gasotransmitter that is produced in cells by three enzymes, cystathionine-β- synthase (CBS), cystathionine-γ-lyase (CTH), and 3-mercaptopyruvate sulfurtransferase (3-MPST) [8]. We have previously observed that H2S donors provide protection to intestines during a mouse model of experimental NEC [9]. Previous studies in both in vivo and in vitro settings have also shown that tissue injury secondary to ischemia and reperfusion can be rescued by H2S [10–12].
If USCs are to be used in clinical trials, an understanding of their mechanism of tissue protection is paramount. Fluorescent biomarkers can be used to quantify H2S production, and it is clear that stem cells do release this beneficial gas, especially after exposure to a hypoxic environment [13]. This lends more support to the theory that the paracrine benefits of USCs may be mediated through H2S.
We hypothesized that inhibition of the H2S-producing enzymes in USCs, CBS, CTH, and MPST, would reduce the cells’ protective capacity when administered intraperitoneally during experimental NEC. We expected that pups treated with the H2S-knockdown cells would be more clinically ill and have more significant intestine and lung injury compared to those treated with control cells.
MATERIALS AND METHODS
Cell Culture
Umbilical cord-derived Mesenchymal stromal cells (USCs) were purchased from ATCC (Manassas, VA) and were positive for CD29, CD44, CD73, CD90, CD105, CD166 and negative for CD14, CD31, CD34 and CD45 [14]. Cells were cultured in MesenPro media (Gibco, Waltham, MA) with 2mmol/L L-glutamine (Sigma Aldrich, St. Louis, MO) and 1% penicillin-streptomycin in 225 cm2 polystyrene culture flasks at 37°C in 5% CO2. At 90% confluency, the cells were lifted from the flask with TrypLE Express (Life Technologies, Carlsbad, CA) and passaged or used in experimentation. USCs were used between passages 4 and 9. Cells were counted by hemocytometer with Trypan Blue (Gibco, Waltham, MA) when necessary.
siRNA Transfection
Cells were lifted from culture surface at 90% confluence by TrypLE Express and pelleted at 400g for 5 minutes, resuspended in media, and counted. Cells were plated at a density of 10,000 cells per cm2, or 2.25×106 cells per flask with complete media. The following day, the cells were transfected with pooled siRNA and Dharmafect 1 proprietary reagent (GE Dharmacon, Lafayette, CO) following the protocol for Dharmacon siRNA using antibiotic-free media. The different siRNA products used are called ON-TARGETplus SMARTpool products. They included a non-targeted negative control siRNA (Cat # D001810), as well as three different targeted siRNA pools for CBS (Cat #L-008617), CTH (Cat # L-003481), and MPST (Cat # L-010119). After 24 hours of transfection, a complete media change was performed, and cells were used for experiments 24 hours later (48 hours after transfection initiation). Knockdown of mRNA was confirmed by RT-PCR with band intensities analyzed with ImageJ software (NIH) and compared to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase. Primer sequences are listed in Appendix 1.
In vitro H2S relative quantification
Cells were plated at a density of 10,000 cells per well in 96-well plates with gas-permeable bottoms (Coy Laboratory Products Inc., Grass Lake, MI) and grown to 80–95% confluency. The cells were then treated with H2S-specific fluorophore 7-azido-3-methylcoumarin (AzMC, 25 uM: λex=365nm and λem=450nm; Millipore Sigma, St. Louis, MO). AzMC is an irreversible fluorophore that provides a cumulative index of H2S production. Fluorescence was measured on a SpectraMax M5e plate reader (Molecular Devices, Sunnyvale, CA) according to manufacturer’s recommendations and the cells were then placed in normoxia (21% O2, 5% CO2, and N2 balance) or hypoxia (5% O2, 5% CO2, and N2 balance) to mimic in vivo conditions for up to 24 hours in a model 856 HYPO hypoxia chamber (Plas Labs, Inc., Lansing, MI) at 37°C. The plates were removed at timed intervals, and fluorescence was measured before returning the plates to back to the culture incubator.
Experimental NEC Model:
All protocols and animal use were approved by Indiana University Institutional Animal Care and Use Committee. A previously published and validated model of experimental NEC was utilized [9, 15, 16]. Briefly, experimental groups (n=10) were permanently separated from their mother on postnatal day 5 (P5) through the end of the protocol on postnatal day 9 (P9) and kept in approved satellite housing in a temperature-controlled incubator. They received gavage feeds via 2 French catheter three times daily. Total intake is equal to 300 kcal/kg/day of hyperosmolar formula, supplemented with 8 mg/kg lipopolysaccharide (lipopolysaccharides from Escherichia coli O111:B4, Sigma-Aldrich Company LLC, Dorset, UK). Pups were placed in a hypoxic environment for 10 minutes prior to each feeding, and hypothermic conditions for 10 minutes after the morning and evening feeds as previously described [9].
At the beginning of the protocol, experimental groups received a single injection of phosphate buffered saline (PBS) vehicle or 80,000 cells/g human USCs in PBS. Human cells were used in immune-competent mice, which is consistent with our previous work and other studies which demonstrate that mesenchymal stromal cells have the ability to avoid rejection even after xenotransplantation [17, 18]. Total volume of each injection was 10µL. The dose is weight-based, modified from our previous work in ischemia-reperfusion [4]. Pups were excluded if they died within the first 24 hours. Deaths beyond 24 hours were still included in analysis for all data points including tissue evaluation and all pups were euthanized on the morning of P9.
Clinical Assessment:
Pups were assessed systematically in a semi-blinded fashion before each feed as previously described [9, 15]. The pups are graded from 0 to 3 on multiple factors: color, activity, responsiveness to stimuli, and movement. It is semi-blinded because the same researcher who administers the therapies is the one to score the animals and record the data. The reported clinical assessment score was the pup’s last score prior to death or euthanasia. A higher score indicates more severe clinical illness, with the possible scores ranging from 0 to 12.
Injury Scores – Macroscopic and Histologic:
After euthanasia the intestine was examined in situ and scored based on previously published macroscopic injury score assessing color, consistency, and dilation of the small intestine. A higher score indicates more severe injury, with the possible scores ranging from 0 to 6 [9, 15].
Terminal ileum and right lower lung lobes were resected following euthanasia of experimental groups and fixed for 24 hours in 4% paraformaldehyde at 4°C, followed by dehydration with 70% ethanol, paraffin embedding, sectioning and hematoxylin and eosin staining. Specimens were scored in a blinded manner by 2 observers using previously described scales [9, 19]. The highest possible score for intestinal injury is 4 with the scores ranging from 0 to 4. For lung injury, the highest possible score is 12 with scores ranging from 0 to 12.
Statistical Analysis:
Ordinal data was reported using median and interquartile range (IQR). Continuous variables were reported as mean with standard error of the mean (SEM). Non-parametric data was compared using the Mann-Whitney U test, while parametric data was compared with student’s t-test. GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for all statistical analysis and figures. P values less than 0.05 were considered statistically significant.
RESULTS
USCs produce more H2S in hypoxia compared to normoxia, and transfection with targeted siRNA for H2S-producing enzymes results in depression of USCs H2S gas production in vitro.
After transfection, mRNA expression for H2S-producing enzymes in the USCs were not significantly changed in the cells treated with negative control siRNA (<15% difference in band intensity on imageJ software for all three enzymes). For those transfected with targeted siRNA, the CBS, CTH, and MPST mRNA expression were knocked down <50% compared to the negative control siRNA cells (Figure 1A).
Figure 1:
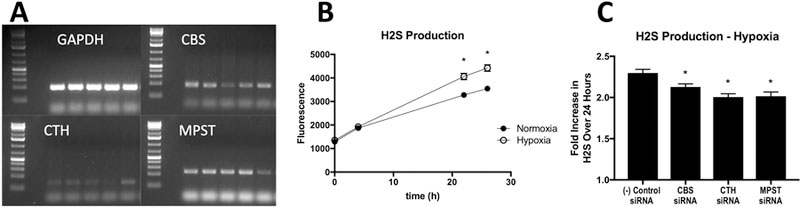
A- Representative RT-PCR gel demonstrating >50% decrease after siRNA treatment. Each panel demonstrates an amplification for the specified gene, and for each one, from left to right the lanes are as follows: USCs (no transfection), negative control siRNA cells, CBS siRNA, CTH siRNA, MPST siRNA. B- H2S production over 24 hours in USCs in normoxia and hypoxia (error bars are smaller than data points). *: p<0.0001 at the time point. C- H2S production expressed as a fold change from baseline over 24 hours in hypoxic conditions. *: p<0.05 vs. (−) control siRNA
AzMC fluorescence, indicative of cumulative H2S production was significantly increased in hypoxic control cells over 24 hours (p<0.0001) as compared to cells in normoxic conditions (Figure 1B). Further, in hypoxic cells, there was a significant decrease in H2S production in the cells treated with targeted siRNA compared to the negative control siRNA cells. The negative control siRNA USCs produced 2.30 (±0.04) times as much H2S at 24 hours as at baseline (time zero). The siRNA knockdown groups each had a significant decrease in production over time with multipliers being 2.12 (±0.03, p=0.0300) in the CBS-knockdown, 2.01 (±0.04, p<0.0001) in CTH-knockdown, and 2.02 (±0.05, p<0.0001) in MPST-knockdown USCs (Figure 1B). Each well’s production was compared to its own baseline to correct for small and unknown differences in the numbers of healthy cells per well.
Mice treated with H2S-knockdown USCs developed more severe clinical illness.
Mice treated only with saline vehicle had a median clinical sickness score of 3 (IQR 2–5), and those treated with both non-transfected USCs (median 1, IQR 0.25–2.75, p=0.0262) and negative control siRNA USCs (median 1.5, IQR 1–2, p=0.0135) demonstrated significantly improved clinical status in comparison. After knockdown of each H2S-producing enzyme, a clinical difference was evident in mice treated with these USCs compared to the control cells. The CBS-knockdown group had a median score of 4 (IQR 1.25–6, p=0.0389 vs. negative control), and the CTH- and MPST-knockdown groups had scores of 5 (IQR 4–5, p=0.0008) and 2 (IQR 2–4, p=0.0300) respectively (Figure 2A).
Figure 2:
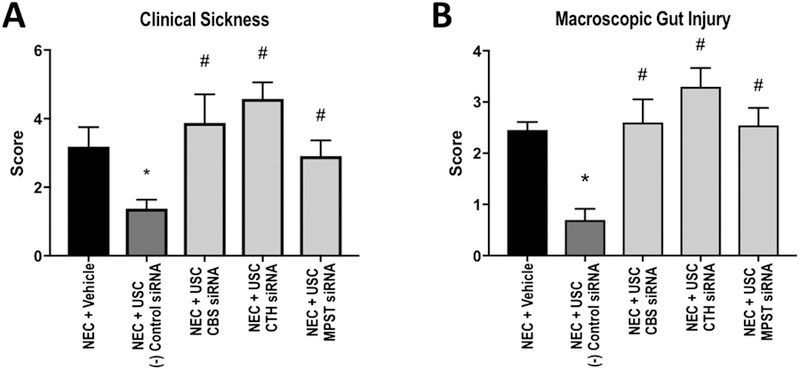
A- Clinical sickness scores for each group. Higher score indicates a sicker animal. B-Macroscopic injury score for each group. Higher score indicates more severe injury. *: p<0.05 vs. vehicle, #: p<0.05 vs. (−) control siRNA.
Intestinal injury was more severe in animals treated with H2S-knockdown USCs compared to control cells.
Macroscopically, it was evident that the animals treated with the inhibited cells suffered more severe intestinal compromise compared to those treated with negative control siRNA cells. The vehicle group had a median macroscopic injury score of 2 (IQR 2–3) while the negative control siRNA cell treated group had a median score of 1 (IQR 0–1, p<0.0001). The CBS-, CTH-, and MPST-knockdown groups had median scores of 2.5 (IQR 1–4, p=0.0021), 3.5 (IQR 2.75–4, p=0.0001), and 3, (IQR 1–3, p=0.0006) respectively (Figure 2B).
Treatment with negative control siRNA USCs resulted in a median intestinal histologic injury score of 1.25 (IQR 0.625–1.5) compared to the vehicle-treated animals with a score of 2.5 (IQR 1.5–3, p=0.0022). Compared to treatment with the negative control siRNA cells, the animals treated with the H2S knockdown cells each had significantly worse overall histologic injury with median scores of 2.5 (IQR 2–3, p=0.0022), 2.5 (IQR 2–3.5, p<0.0001), and 2.5 (IQR 2–4, p=0.0157, Figure 3A). Additionally, treatment with the siRNA-knockdown USCs resulted in higher incidence of severe NEC (injury grades 3 and 4) compared to the group treated with the control cells (Figure 3B).
Figure 3:
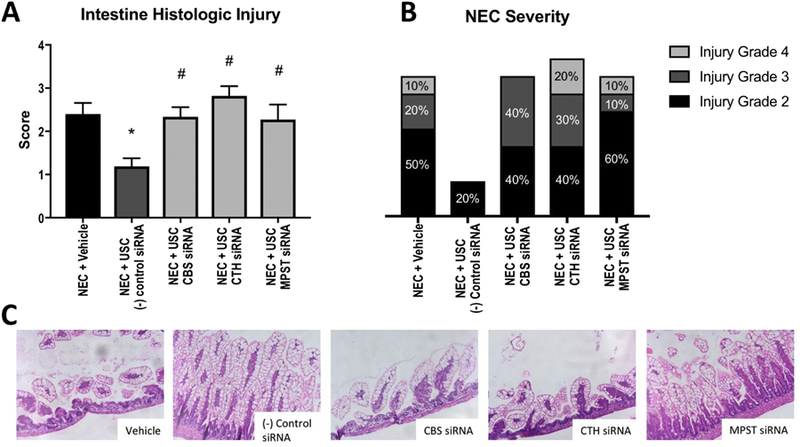
A- Intestinal histologic injury scores for each group. Higher score indicates more significant injury. *: p<0.05 vs. vehicle, #: p<0.05 vs. (−) control siRNA. B- Incidence of each grade of injury corresponding with NEC. Grade 3 and 4 indicate severe NEC. C- Representative images of histologic appearance for each group.
Lung injury was more severe in animals treated with H2S-knockdown USCs compared to control cells.
Compared to the vehicle group, which had a median lung histologic injury score of 6.5 (IQR 5–7.5), the group treated with negative control siRNA USCs had a score of 3 (IQR 2.25–3.75, p=). Treatment with CBS-knockdown cells resulted in a significantly more severe median score of 5 (IQR 3–6, p=0.0177), as did treatment with CTH-knockdown (6, IQR 4–7, p=0.0008) and MPST-knockdown cells (6, IQR 4–7, p=0.0071, Figure 4).
Figure 4:
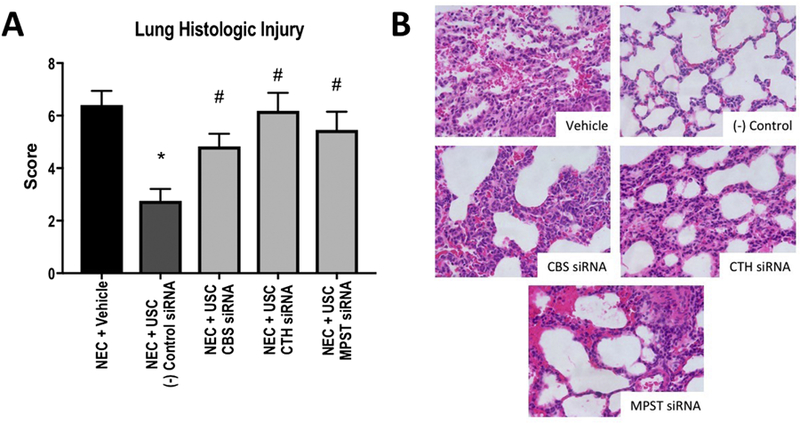
A- Lung histologic injury scores for each group. Higher score indicates more severe injury. *: p<0.05 vs. vehicle, #: p<0.05 vs. (−) control siRNA . B- Representative images of histologic appearance for each group.
DISCUSSION
Researchers in the field of experimental NEC have demonstrated that stem cells are beneficial in therapy for this condition [3, 5, 20, 21]. The question remains as to how these cells are working – whether it is by paracrine effects or by integration into the damaged tissue with regeneration. Our hypothesis was that USCs were working at least in part through release of H2S gas as a paracrine mediator, and that inhibiting this release would result in more severe outcomes during experimental NEC.
First, it was interesting to see that in hypoxic conditions, the USCs produced more H2S over 24 hours than when kept at ambient O2 levels. It has previously been shown that cellular H2S production increases as a mechanism of O2 sensing and signaling in hypoxia [22], and this is likely why these cells are effective in the NEC model in vivo.
Measuring H2S has been historically difficult [13], but after confirmation of the knockdown of the H2S-producing enzymes, it was important to show that the gas was actually decreased, and that the other enzymes did not overcompensate and continue to produce the same amount of H2S. Inhibition of H2S synthesis did lead to a significant decrease in gas release in hypoxic conditions and likely rendered the cells unable to produce adequate H2S levels that the tissues required for protection.
After demonstrating that inhibiting the H2S-producing enzymes resulted in lower H2S production, the increased severity of clinical illness score was as expected. The negative control siRNA cells were treated with a non-targeting siRNA to control for the possibility that altering the cells would affect the results, and a significant difference was still noted in the animals treated with the inhibited cells.
Multiple animal studies have demonstrated improved intestinal architecture with stem cell therapy, often directly injected into the peritoneal cavity [5, 6]. Inhibiting the paracrine action of H2S in these cells is novel and demonstrates that release of this gasotransmitter is a possible mechanism for intestinal protection. All 3 groups that were treated with the inhibited USCs demonstrated more severe intestinal injury, both macroscopically and histologically injury. In some cases, the histologic injury actually trended toward being even more severe than the vehicle group, but these differences were not statistically significant. This indicates that inhibiting the H2S-producing enzymes critically impacts the cells’ abilities to protect the intestine from injury during experimental NEC.
The lung injury followed a similar pattern, as this was thought to be related to systemic illness, similar to NEC-associated lung injury in human neonates [23]. This pathology represents an important possibility for intervention, to prevent ongoing systemic illness in the face of intestinal dysfunction. Inhibiting H2S production in the USCs before treatment of the pups with these cells resulted in more severe lung histologic injury scores in all 3 groups, indicating that a paracrine mechanism of action is at least partly responsible for the capacity of the gas to protect the lung in this condition.
While the full extent of the benefits of USCs on the intestine in experimental NEC is not understood, the inhibition of H2S-production in the cells certainly impacts the disease course, and therefore is likely to play a role in the paracrine function of these cells. Further studies surrounding the downstream effects of stem cell mediated hydrogen sulfide release are warranted to more fully understand their mechanistic pathways.
LIMITATIONS
This study is limited by the nature of the animal model. It is labor intensive, and only about 70% effective in producing NEC in pups, Furthermore, the presence of the pathology cannot be determined until euthanasia. In future work, we hope to be able to actually detect NEC-like disease development and treat with the stem cells at that point, rather than prior to the start of the protocol. We plan for further experiments where we identify worsening clinical scores throughout the protocol and administer the USC at that time. This would be more clinically applicable as a treatment model, rather than a prevention model.
An additional limitation is that the measurements of H2S take place over 24 hours. Unfortunately, we have been unable to get meaningful results with longer incubations due to cell death. It has been demonstrated that stem cells transplanted into healthy hosts live at least 3 days, and that they migrate to other areas, including the lungs, so knowing the longer term production of H2S would be helpful [24]. Finally, it is not ideal to use cells from another species. We use human cells in order to treat this as pre-clinical experiment and because it has been well-demonstrated that mesenchymal cells can be xenotransplanted without detection or rejection [17, 18]
CONCLUSION
Umbilical stem cells are promising for the therapy of necrotizing enterocolitis by reducing injury in both the intestine and the lung. This benefit is likely mediated through a paracrine mechanism, as inhibition of H2S release reduces the protective effects of USC.
Acknowledgments
Funding:
- Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number K08DK113226 (TAM). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
- IOS 1051627 (KRO) from the National Science Foundation and Indiana University BRG Grant (KRO)
- Indiana University Health, Indianapolis, IN
Appendix 1. Primers (5’→3’)
| CBS | Forward | GTCAGACCAAGTTGGCAAAGT |
| Reverse | CACCCCGAACACCATCTGC | |
| CTH | Forward | CATGAGTTGGTGAAGCGTCAG |
| Reverse | AGCTCTCGGCCAGAGTAAATA | |
| MPST | Forward | CGCCGTGTCACTGCTTGAT |
| Reverse | CAGGTTCAATGCCGTCTCG | |
| GAPDH | Forward | GGAGCGAGATCCCTCCAAAAT |
| Reverse | GGCTGTTGTCATACTTCTCATGG |
Footnotes
Disclosures: The authors have no relevant financial conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lin HC, Wu SF, Underwood M. Necrotizing enterocolitis. The New England journal of medicine 2011;364(19):1878–9; author reply 9. [DOI] [PubMed] [Google Scholar]
- [2].Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine 2011;364(3):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Drucker NA, McCulloh CJ, Li B, Pierro A, Besner GE, Markel TA. Stem cell therapy in necrotizing enterocolitis: Current state and future directions. Semin Pediatr Surg 2018;27(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. J Surg Res 2016;204(2):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res 2017;214:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg 2017;52(6):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tayman C, Uckan D, Kilic E, Ulus AT, Tonbul A, Murat Hirfanoglu I, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res 2011;70(5):489–94. [DOI] [PubMed] [Google Scholar]
- [8].Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Yoder MC, DeLeon ER, et al. Hydrogen Sulfide: A Potential Novel Therapy for the Treatment of Ischemia. Shock 2017;48(5):511–24. [DOI] [PubMed] [Google Scholar]
- [9].Drucker NA, Jensen AR, Ferkowicz M, Markel TA. Hydrogen sulfide provides intestinal protection during a murine model of experimental necrotizing enterocolitis. J Pediatr Surg 2018;53(9):1692–8. [DOI] [PubMed] [Google Scholar]
- [10].Li B, Zani A, Martin Z, Lee C, Zani-Ruttenstock E, Eaton S, et al. Intestinal epithelial cell injury is rescued by hydrogen sulfide. J Pediatr Surg 2016;51(5):775–8. [DOI] [PubMed] [Google Scholar]
- [11].Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. The Journal of thoracic and cardiovascular surgery 2009;138(4):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, et al. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain research 2013;1491:188–96. [DOI] [PubMed] [Google Scholar]
- [13].Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 2012;17(1):32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jensen AR, Drucker NA, Ferkowicz MJ, Markel TA. Umbilical mesenchymal stromal cells provide intestinal protection through nitric oxide dependent pathways. J Surg Res 2018;224:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zani A, Cordischi L, Cananzi M, De Coppi P, Smith VV, Eaton S, et al. Assessment of a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 2008;18(6):423–6. [DOI] [PubMed] [Google Scholar]
- [16].Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. Journal of immunology 2006;177(5):3273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. J Surg Res 2015;199(1):56–66. [DOI] [PubMed] [Google Scholar]
- [18].Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev 2012;21(15):2770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Drucker NA, Jensen AR, Te Winkel JP, Ferkowicz MJ, Markel TA. Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J Pediatr Surg 2018;53(6):1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eaton S, Zani A, Pierro A, De Coppi P. Stem cells as a potential therapy for necrotizing enterocolitis. Expert Opin Biol Ther 2013;13(12):1683–9. [DOI] [PubMed] [Google Scholar]
- [21].Zani A, Cananzi M, Lauriti G, Fascetti-Leon F, Wells J, Siow B, et al. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 2014;24(1):57–60. [DOI] [PubMed] [Google Scholar]
- [22].Olson KR. Hydrogen sulfide as an oxygen sensor. Antioxid Redox Signal 2015;22(5):377–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jia H, Sodhi CP, Yamaguchi Y, Lu P, Martin LY, Good M, et al. Pulmonary Epithelial TLR4 Activation Leads to Lung Injury in Neonatal Necrotizing Enterocolitis. J Immunol 2016;197(3):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation 2004;78(4):503–8. [DOI] [PubMed] [Google Scholar]


