Abstract
Cardiovascular disease is a leading cause of mortality in the world and is exacerbated by the presence of cardiac fibrosis, defined by the accumulation of non-contractile extracellular matrix proteins. Cardiac fibrosis is directly linked to cardiac dysfunction and increased risk of arrhythmia. Despite its prevalence, there is a lack of efficacious therapies for inhibiting or reversing cardiac fibrosis, largely due to the complexity of the cell types and signaling pathways involved. Ongoing research has aimed to understand the mechanisms of cardiac fibrosis and develop new therapies for treating scar formation. Major approaches include preventing the formation of scar tissue and replacing fibrous tissue with functional cardiomyocytes. While targeting the renin-angiotensin-aldosterone system is currently used as the standard line of therapy for heart failure, there has been increased interest in inhibiting the transforming growth factor-β signaling pathway due its established role in cardiac fibrosis. Significant advances in cell transplantation therapy and biomaterials engineering have also demonstrated potential in regenerating the myocardium. Novel techniques, such as cellular direct reprogramming, and molecular targets, such as non-coding RNAs and epigenetic modifiers, are uncovering novel therapeutic options targeting fibrosis. This review provides an overview of current approaches and discuss future directions for treating cardiac fibrosis.
Keywords: Cardiovascular disease, Cardiac fibrosis, Fibrosis, Therapeutics, Therapies
1. INTRODUCTION
Cardiac fibrosis is a major pathological disorder associated with a multitude of cardiovascular diseases (CVD) and is characterized by excessive extracellular matrix (ECM) protein deposition in the heart1,2. Upon ischemic injury or pressure overload, the heart undergoes a dynamic remodeling process that is driven by a multitude of cells including cardiomyocytes, endothelial cells, immune cells, and cardiac fibroblasts2–4. Cardiomyocytes rapidly become apoptotic and endothelial cells play a critical role in modulating the inflammatory response5,6. In the initial phases of remodeling, immune cells proliferate, infiltrate damaged myocardium to clear dead tissue, and release pro-fibrotic cytokines3,7. In response to these cytokines, cardiac fibroblasts become activated and increase production of ECM proteins such as collagens and fibronectin to form scar tissue1,4,8. Initially, these responses are critical in removing apoptotic CMs and for stabilizing the chamber walls to prevent rupture and the scar that is formed is deemed as reparative fibrosis. However, the persistent presence of non-contractile collagen-rich tissue leads to the maturation of scar tissue and adverse remodeling, the effects of which include an increased risk of arrhythmias and reduced contractility9,10. These effects can have a devastating impact on the clinical outcomes of CVD patients, creating a need to develop strategies to prevent or reverse cardiac fibrosis.
Several obstacles that have limited the development of anti-fibrotic therapies available for CVD patients. First, the regenerative potential of the adult human heart is limited and cardiomyocytes (CMs) are unable to proliferate at a level that can replace damaged myocardium11. This restricts therapies that aim to inhibit fibrosis entirely as the endogenous CMs are unable to replace lost muscle tissue, thus increasing risk of cardiac rupture. Second, the molecular mechanisms driving cardiac fibrosis are complex and not fully understood. Although cardiac fibroblasts are the major contributory cells of cardiac fibrosis, further studies are needed to unravel the mechanistic regulation of these cells. There is a need to understand their mechanisms of activation, the temporal nature of their molecular changes, and whether these cells can be “deactivated” or eliminated12. Finally, the injured heart, particularly after myocardial infarction (MI), is a volatile microenvironment with dramatic levels of CM apoptosis, immune cell infiltration, and fibroblast proliferation4,7,13. This hostile environment may hinder the efficacy of delivering anti-fibrosis therapies. In this review, we aim to describe prominent research areas that are being explored for the treatment of cardiac fibrosis with potential clinical promise.
2. RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM
2.1. Overview of the RAAS System
The renin-angiotensin-aldosterone system (RAAS) plays an integral role in the homeostatic control of arterial pressure, tissue perfusion, and extracellular volume14. This pathway is initiated by the secretion of renin from the juxtaglomerular cells of the kidney in response to various stimuli such as decreased renal perfusion, decreased NaCl concentration, or increased sympathetic activity15,16. Renin goes on to cleave angiotensinogen, to form a biologically inert peptide, Angiotensin (Ang) I. AngI is then hydrolyzed by angiotensin-converting enzyme (ACE) to form an active AngII, which is a potent vasoconstrictor. AngII is the primary effector of a variety of RAAS-induced physiological and pathophysiological actions. Within the cardiovascular system, these effects include vasoconstriction, increased blood pressure, increased cardiac contractility, and vascular and cardiac hypertrophy17. Another important action of AngII include stimulating the production and release of aldosterone from the adrenal cortex. Aldosterone is a major regulator of sodium and potassium balance and thus plays a major role in regulating extracellular volume18. Together, the resulting effects of AngII and aldosterone on their target organs serve to maintain blood pressure and restore renal perfusion. Although the RAAS plays an important role in normal circulatory homeostasis, continued or inappropriate activation of this system is thought to contribute to the pathophysiology of diseases such as hypertension and HF.
2.2. Role of RAAS in Cardiac Fibrosis
In vitro experiments using adult rat cardiac fibroblasts have shown that AngII19–21 and aldosterone19 stimulate collagen synthesis in a dose-dependent manner. AngII additionally suppresses the activity of matrix metalloproteinase-1 (MMP1), a key enzyme of interstitial collagen degradation19, that synergistically leads to progressive collagen accumulation within the myocardial interstitium. AngII induces expression of TGFβ1 within cardiac fibroblasts through the Ang type-I receptor (AT1)22. After an MI, increased wall stress resulting from elevated left ventricular end diastolic pressure (LVEDP) stimulates mechanoreceptors that lead to activation of RAAS. The upregulated AngII increases tissue inflammation, and TGFβ, IL-1β, and TNF-α secretion23–26, leading to enhanced generation of myofibroblasts. Within experimental models of hypertensive heart disease and chronic HF, circulating and local levels of renin-angiotensin-aldosterone promote the development of myocardial fibrosis and diastolic dysfunction27,28. Given the significant role of RAAS in the pathogenesis of cardiac fibrosis, therapies have been developed to antagonize or modulate the activity of various components of this system.
2.3. Direct Renin Inhibitors and Renin Receptor Blockers
Direct renin inhibition may be a promising anti-fibrotic therapy since it attenuates the pro-fibrotic effects of renin in addition to that of other effectors of the renin-angiotensin pathway29. Renin inhibitors interfere with the initial rate limiting step in the synthesis of AngII by binding directly to renin30. Aliskiren is the first orally active renin inhibitor approved by the FDA for the treatment of hypertension in adults31. Zhi et al. showed that aliskiren has direct effects on collagen metabolism in cardiac fibroblasts and prevented myocardial collagen deposition in a non-hypertrophic mouse model of myocardial fibrosis29. Other groups have shown that aliskiren functions through inhibition of AngII-dependent as well as AngII-independent effects mediated via the (pro)renin receptor (PRR)32,33. Cardiac expression of PRR is up-regulated in hypertension and HF and has been shown to be associated with the development of cardiac fibrosis and hypertrophy as well as cardiac dysfunction34–39. Ellmers et al. reported that PRR blockade in a mouse model of MI significantly reduced infarct size and attenuated cardiac fibrosis and adverse remodeling38.
2.4. ACE Inhibitors and Angiotensin Receptor Blockers (ARBs)
ACE inhibitors such as enalapril, lisinopril, and trandolapril, prevent the conversion of inactive AngI into active AngII and are considered first-line therapy for many cardiovascular and renal diseases. There is a large body of evidence that ACE inhibitors regress myocardial fibrosis and are associated with reduction of ventricular arrhythmias and improvement of myocardial function40–45. ARBs are also commonly prescribed clinically and work by preventing the binding of AngII to its receptor (with greater affinity for AT1 than AT2). Wu et al. showed that valsartan, an ARB, improved coronary arterial thickening and perivascular fibrosis in a pressure overload mouse model46. Similarly, Frimm et al. found that rats treated with losartan had a reduction in cardiac infarct size and collagen content one month after experimental MI47. However, despite the efficacy of ACEs and ARBs in a variety of cardiac diseases including heart failure with reduced ejection fraction (HFrEF), recent clinical trials have not shown their benefit in HF patients with preserved ejection fraction (HFpEF)48–50.
2.5. Aldosterone Antagonists
Aldosterone is a steroid hormone produced by the zona glomerulosa of the adrenal cortex. It plays a key role in regulating blood pressure and plasma sodium levels through its actions on renal tubules to promote sodium retention and extracellular volume expansion. It has also been reported that aldosterone can be produced within the heart51. This local aldosterone system responds to short- and long-term physiological stimuli, suggesting that the cardiac-generated aldosterone has possibly autocrine or paracrine actions52. Billa et al. demonstrated that chronic administration of aldosterone in the setting of high salt intake causes both interstitial and perivascular fibrosis in the heart53 and that treatment with an aldosterone antagonist, spironolactone, prevents the increase in total and interstitial collagen in rats54,55. Several clinical studies have confirmed survival benefit when aldosterone antagonists are used in HFrEF patients56–59. However, the risk of hyperkalemia requires frequent monitoring60.
Therapies targeting the RAAS have been extensively studied and shown to be effective in preventing collagen deposition and reducing cardiac fibrosis. While RAAS inhibition is the mainstay of clinical care, especially for HFrEF patients, further studies are needed to examine the efficacy and safety of these therapies for patients with HFpEF and other forms of cardiac fibrosis.
3. TGF-β SIGNALING PATHWAY
3.1. Overview of TGF-β Signaling
The Transforming Growth Factor-β (TGFβ) family of peptides is one of the most well-studied regulators of the fibrotic response that plays a central role in the maladaptive remodeling of the heart after injury61–63. The expression of TGFβ in myocardial tissue is markedly increased in both animal experimental models of MI and in heart failure (HF) patients62,64. The targeting of the TGFβ signaling pathway has long been explored as a potential therapy to curtail fibrosis65,66. One of the challenges of studying the TGFβ family includes the complexity of effects that TGFβ peptides can stimulate across multiple cell types and conditions. TGFβ is known to play key roles in regulating inflammation and ECM deposition, two processes that constitute major phases of the fibrotic response. In inflammation, TGFβ signaling is inhibitory and regulates the synthesis of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα)67,68. TGFβ1-null mice demonstrate high levels of autoimmunity, supporting the importance of TGFβ in mediating the inflammatory response69. On the other hand, TGFβ signaling has been shown to induce fibroblast transition into activated myofibroblasts, the major cellular source for ECM protein deposition that make up the fibrotic area4. Due to the multifunctional roles of TGFβ signaling, several studies have revealed that the specificity and timing of targeting this pathway are crucial for effective outcomes70.
3.2. Inhibitors of TGFβ Receptors I and II
TGFβ signaling is activated by the binding of TGFβ to a tetrameric receptor complex made up of two type I (TGFβR1 or ALK5) and two type II (TGFβR2) receptors71. Studies inhibiting either ALK5 or TGFβR2 have shown reduced cardiac fibrosis in mouse models, although adverse effects such as increased mortality and inflammation were observed72,73. Furthermore, long-term inhibition has serious side effect such as cardiac toxicities, which limits its clinical application74. Despite these limitations, there have been promising reports of novel TGFβ receptor inhibitors on treating cardiac fibrosis. GW788388 was recently identified as a more potent inhibitor of both ALK5 and TGFβR2 with an improved pharmacokinetic profile75 and minimal toxic effects76. Multiple studies have demonstrated that GW788388 reduces myocardial fibrosis in murine heart disease models77–79. These studies reveal that GW788388 may be a promising anti-fibrotic agent that requires further exploration.
3.3. Clinical Inhibitors of TGFβ – pirfenidone and tranilast
Pirfenidone and tranilast are two clinically-approved drugs that have a broad range of effects on inflammation and other fibrotic pathways. However, it has additionally been established that these drugs are inhibitory of TGFβ signaling. Both have recently been garnering interest in potentially treating cardiac fibrosis80. Pirfenidone is an oral anti-fibrotic drug that was approved by the FDA in 2014 for the treatment of idiopathic pulmonary fibrosis81. Pirfenidone has been shown to inhibit the transcription of TGFβ and suppress downstream effects of TGFβ signaling, such as ECM protein upregulation82. Several recent studies have additionally demonstrated the anti-fibrotic effects of pirfenidone in cardiac disease. Mirkovic et al. and Nguyen et al. independently showed reduced cardiac scarring after treatment of pirfenidone in hypertensive rats and rats with MI, respectively83,84. Similar effects were seen in murine pressure-overload injury; pirfenidone increased survival and attenuated collagen deposition85,86. Clinical trials are ongoing to explore the anti-fibrotic effects of pirfenidone in patients with HF and preserved ejection fraction (PIROUETTE).
Tranilast was originally used as an antihistamine to treat bronchial asthma, however, since its conception in the 1980s87, investigators have found efficacy of tranilast in other medical conditions. One of the main modes of action of tranilast is the suppression of TGFβ expression and activity87. Several studies have reported that tranilast induces downregulation of collagen production in fibroblasts88–90. Subsequently, the PRESTO (Prevention of REStenosis with Tranilast and its Outcomes) clinical trial which, despite finding little effects of tranilast on restenosis, noted a reduction in the development of MI in patients treated with tranilast91. The effects of tranilast on attenuating myocardial fibrosis have been additionally supported by multiple animal models of cardiomyopathy, including experimental diabetes in rats92 and viral myocarditis in mice93. While the anti-fibrotic effects of tranilast have been attributed to its regulation of TGFβ signaling, Kagitani et al. reported that tranilast treatment is associated with decreased monocyte infiltration, which may also contribute to the reduced fibrosis94. Others have reported the anti-inflammatory effect of tranilast to be related to its ability to inhibit prostaglandin E2, thromboxane B2, or interleukin-8. Additionally, the timing of tranilast administration in relation to time of injury is a significant factor to consider. See et al. showed that early tranilast treatment of rats with left anterior descending artery (LAD) ligation (day 0–7 after injury) exacerbated infarct size, implying a potential hazard when used early after injury95.
Despite the evidences supporting the anti-fibrotic effects of both pirfenidone and tranilast, studies have shown that prolonged dosages of either of these drugs can have hepatic toxicity and may lead to liver failure66. Therefore, more research is warranted to explore alternative methods that can safely, but efficaciously, target TGFβ signaling for reduction of cardiac fibrosis.
4. BIOMATERIAL APPLICATIONS
4.1. Overview of Biomaterials
Biomaterials are natural or engineered substances that interacts with biological systems and are used to replace or repair tissues of the body. There has been a vast array of applications of biomaterials for controlling cardiac fibrosis. In addition to providing a platform for controlled release of anti-fibrotic compounds, biomaterials may also provide mechanical support to the infarcted tissue and decrease elevated wall stress, resulting in improved cardiac function96. Both naturally-derived biomaterials such as collagen97–99, fibrin100–102, and alginate103–105 in addition to synthetic materials including metals and polymers106 have been used in cardiac applications. While natural biomaterials tend to offer better compatibility and low immunogenicity, the main benefits of synthetic materials are their strength and durability107. When combined with cells or cytokines/growth factors, biomaterials may offer enhanced retention of their payload leading to improved engraftment or biological function108. This review will focus on two main classes of biomaterials with cardiovascular applications.
4.2. Injectable Biomaterials
In recent years, injectable biomaterials have seen a significant increase in application towards treating MI108–110. Hydrogels based on alginate and chitosan have been shown to decrease cardiac fibrosis, reduce tissue inflammation, and improve vascularization103,104. Combined with anti-fibrotic/anti-inflammatory compounds or stem cells, the therapeutic potential of injectable biomaterials can be further expanded. In a rat chronic myocarditis model, gelatin hydrogel sheets containing hepatocyte growth factor (HGF) were found to improve cardiac function and fibrosis111. HGF serves as a favorable candidate as it suppresses fibrosis by inhibiting TGFβ (suppressing collagen synthesis) and activating MMP1 to increase collagen degradation112,113. In addition to its anti-fibrotic effects, reports have also indicated their role in angiogenesis114–116 and tissue regeneration117. Other growth factors incorporated with injectable biomaterials include basic fibroblast growth factor118,119, vascular endothelial growth factor120–122, and platelet-derived growth factor123,124. Collectively, there is significant amount of research on the development of injectable biomaterials with anti-fibrotic compounds or biologics to reduce fibrosis and promote healing.
4.3. Cardiac Patches
Cardiac patches have generally contained cells combined with a natural or synthetic biomaterial although acellular patch therapies and cell sheets have also been investigated. While many in vivo studies in small animals have shown an improvement in cardiac function, one limitation with this application is the thickness of the material due to diffusion limitations125,126. The use of a collagen scaffold for cardiac patch has been well studied in combination with a variety of cell types98,127–129. Fibrin cardiac patches have also contributed to improved cell delivery and cardiac function in large animal models100,130,131. Processed decellularized cardiac ECM has also shown promise as an injectable hydrogel132–134 and patch135,136. This is a naturally-derived matrix that provides cells with tissue-specific biochemical cues important for cell migration and differentiation, and tissue regeneration. Pieces of the myocardium (or the entire heart) may be chemically or enzymatically digested to obtain cardiac ECM132. The major composition of decellularized cardiac ECM include collagen, elastin, and fibronectin. It should be noted that although fibronectin has been shown to activate cardiac fibroblasts into myofibroblasts137, it is thought that other factors or cytokines within the cardiac ECM matrix may offset this activation and lead to overall benefit112. Other clinical studies on the use of ECM are underway138–140.
Injectable biomaterials and cardiac patches for the treatment of MI have recently been launched in clinical trials. While many promising studies have been completed in rodent and large animal models, further studies are needed to better understand the mechanisms behind their observed effects as well as utility for clinical applications.
5. CELL TRANSPLANTATION THERAPY
5.1. Overview of Cardiac Cell Therapy
Reduction of blood flow and oxygen to the heart resulting from ischemia can lead to irreversible loss of CMs and replacement with fibrotic scar tissue. Although traditional medical therapies are beneficial, many patients eventually progress to end-stage HF, with cardiac transplantation as the only definitive option. Due to the limited supply of donor hearts and potential complications from chronic immunosuppressive therapy, investigators have turned to therapeutic approaches aimed at improving myocardial function by cell transplantation141–143. The inception of the use of stem cells as a form of cardiac therapy initially emerged in animal studies over 20 years ago144 and reached clinical trials 10 years thereafter145. Despite early promises, there is no evidence to suggest that current approaches for cardiac cell therapy offer any clinical benefit. Although there are many strategies of cell therapy, this review will focus on two main avenues: 1) direct remuscularization of injured heart and 2) targeting endogenous mechanisms of repair.
5.2. Direct Remuscularization of Fibrotic Tissue in Injured Heart
The concept behind this approach is that transplantation of cells into the injured area leads to possible integration with viable cells in the host myocardium thereby improving cardiac contraction and reducing the risk of ventricular rupture or aneurysms. For that reason, many different cell types have been explored as a source for cell transplantation, including autologous skeletal myoblasts146, bone marrow-derived CD34+ cells (endothelial progenitor cells)147, C-kit surface antigen-selected cells148, ESC/iPSC-derived CM precursors149–153, and ESC/iPSC-derived CM154–156.
Clinical application of skeletal myoblasts failed due to concerns over arrhythmias generated by the transplanted cells157. C-kit surface antigen-selected cardiac progenitor cells initially showed some promise owing to their potential to proliferate and differentiate into the new myocardium, although later studies have challenged the existence of such cells that could generate new cardiomyocytes158,159. Since 1998 which human pluripotent stem cells (hPSC) were characterized160, they have been used to generate CMs in vitro and in vivo. Nevertheless, injection of hPSC-derived CMs or progenitors in large animal models after an acute MI have raised safety concerns such as ventricular fibrillation/tachycardia154,161,162. One possibility is the potential contamination of non-cardiac or pacemaker cells in the hPSC-derived population capable of inducing arrhythmias. Another possibility is failure of transplanted hPSC-derived cardiac cells to physiologically couple with endogenous CMs, leading to disruption of cardiac action potential propagation. Alternative to direct injection of cells into the injured myocardium, others have used bioengineering approaches such as scaffolds or patches for cell therapy (discussed in detail above). Menasch and others developed a sheet of C-derived CMs and applied it onto the surface of the scar and border zone in MI hearts149. This single case report study was the first clinical application of hPSC-derived cardiac cell therapy and no safety issues were reported. However, epicardially-administered cells are unlikely to engraft, migrate, and integrate into the host myocardial tissue. Despite the promising advancement in developing contractile cardiac cell products and successfully applying them in animal models153, further studies are still needed to optimize this strategy to enhance the safety and long-term engraftment of transplanted cells163.
5.3. Stimulation of Endogenous Cardiovascular Progenitors and/or CMs for Regenerative Therapy
This approach aims to use cells or their by-products to induce endogenous progenitors or CMs to proliferate and replace fibrotic tissue in the injured myocardium via paracrine-mediated effects. However, no study has yet provided unequivocal evidence for the existence of cardiac progenitors in adult human hearts. Studies have shown that certain cells have myogenic differentiation capacity164 or release by-products, such as exosomes165, that may stimulate cardiac regeneration. Other studies have used adult, undifferentiated progenitor cells such as bone marrow aspirated mononuclear cells166, marrow-derived mesenchymal stem cells (MSCs)167,168, and resident adult cardiac progenitors (CPCs)169 to stimulate endogenous pathway for regenerative therapy. A clinical trial involving the use of MSCs is ongoing, despite its uncertain efficacy170. Some studies have revealed an improvement in scar size171, while others have shown no benefit172. Pre-clinical studies of CPCs suggested that these cells possess myogenic differentiation capacity, however, further mechanistic studies revealed their anti-inflammatory and antifibrotic properties, as well as stimulation of endogenous cardiac progenitor and CMs173–176. Other genetic fate-mapping studies have shown that endogenous CPCs158,177 and MSCs173,178,179 produce new CMs, although the percentage of CMs emerging from the CPCs and MSCs was extremely low. Altogether, engraftment of MSCs and CPCs is lower compared to ESC/iPSC-based strategies but leads to significant improvement in left ventricular function and reduction of scar size153,169,180. These findings promised a paradigm shift in cardiac biology and new opportunities for future regenerative therapy. However, several years after these findings, a consensus on the biological role of these populations remains obscure.
In summary, although significant advancements have been made in cardiac regenerative medicine and engineering, several critical issues remain. In order to expedite clinical application of cell therapy, a better understanding of the mechanism of action, improvement in cellular delivery and retention, purification of the desired cell types, and functional integration of transplanted cells need to be addressed.
6. DIRECT REPROGRAMMING OF FIBROBLASTS
6.1. Overview of Cardiac Direct Reprogramming
The development of cardiac fibrosis is driven by the proliferation and activation of cardiac fibroblasts, which become the main cellular components of scar tissue1,4,8. Considering the abundance of this cell type within the fibrotic region, they may be an ideal target for direct reprogramming to generate CMs to replace the scar and restore cardiac function181. In 2010, Ieda et al. demonstrated the ability to reprogram postnatal murine dermal and cardiac fibroblasts into induced CM-like cells by transducing the cells with three factors (Gata4, Mef2c, and Tbx5, hereafter collectively referred to as GMT)182. The resulting cells expressed CM-specific markers such as cardiac troponin T (cTnT) and α-myosin heavy chain (αMHC)182 Wada et al. further demonstrated that transduction with GMT plus additional factors Mesp1 and Myocardin could produce induced CM-like cells from human fibroblasts183. This has generated enthusiasm for improving and utilizing direct reprogramming for potential therapeutic purposes.
6.2. In vivo Direct Reprogramming by Retroviral Delivery of Transcription Factors
The potential of direct reprogramming to be used for treating ischemic heart disease was first explored in 2012 by several groups that attempted to apply successful in vitro results to an in vivo setting. Qian et al. and Song et al. independently reported successful reprogramming of resident fibroblasts to induced CMs in murine hearts after LAD ligation by retroviral transduction of GMT and GMT plus Hand2, respectively184,185. Both groups observed increased reprogramming efficiency in vivo compared to in vitro, suggesting that the cardiac environment may influence the reprogramming process. However, the percentage of cells that were successfully reprogrammed remained low. Inagawa et al. reported an improvement in the reprogramming efficiency with GMT by using a retroviral polycistronic vector186. All three studies demonstrated that retroviral delivery of GMT or GMHT into the murine heart after an experimental MI could reduce the extent of cardiac fibrosis, solidifying the therapeutic potential for direct reprogramming.
While numerous groups have reported successful direct reprogramming of cardiac fibroblasts in murine ischemic heart disease models, the clinical translation of this approach has not been fully addressed. Retroviral delivery involves random insertion of DNA into the host cell’s genome which make this mechanism of reprogramming potentially pathogenic187. Therefore, non-integrative methods of reprogramming that can be safely applied to human patients are warranted. There is an additional need to verify the safety and efficacy of direct reprogramming of cardiac fibroblasts to induced cardiomyocytes-like cells in large animal models as a preclinical prelude to future human studies.
6.3. Potential Clinical Applications of Direct Reprogramming
Non-integrative viral vectors such as Adenovirus (AdV), Adeno-associated viruses (AAV), and Sendai virus (SeV) have recently garnered interest in the reprogramming field. AdVs are a widely used research tool that can transduce a variety of cells with high efficiency. A recent report demonstrated that AdVs encoding GMT were able to induce cardiac reprogramming in a rat infarct model to a similar degree as an integrative viral vector (lentivirus)188. However, clinical applications of AdVs have been dampened by their high immunogenicity189. AAVs, on the other hand, are a more viable option as they are able to target various cell types similar to AdVs but exhibit significantly reduced immunogenicity190. Clinical trials investigating the use of AAVs for gene therapy in various conditions are currently underway191. Yoo et al. demonstrated that chimeric-AAVs encoding GMT are able to induce direct cardiac reprogramming and reduce infarct size after LAD ligation in mice192. Finally, SeVs are a relatively new tool for gene therapy that is gaining attention due to their lack of integration and high expression of viral genes193. Indeed, SeV vectors expressing GMT have been shown to significantly increase the efficiency of cardiac reprogramming in mouse infarct hearts, compared to retroviral vectors, and resulted in lower levels of fibrosis194,195.
Several studies have also explored the potential to directly reprogram fibroblasts into CMs by non-viral methods. Recent reports have shown the ability to reprogram mouse fibroblasts into CMs by addition of small molecules in vitro196,197. Additionally, another group has demonstrated the capabilities of miRNA transfection in cardiac reprogramming in vivo198. These advancements have laid a framework for a future in vivo reprogramming without the need for viral transduction.
7. EMERGING NOVEL ANTI-FIBROTIC THERAPEUTIC STRATEGIES
7.1. Non-coding RNAs in Cardiac Fibrosis
There have been several exciting findings for other novel anti-fibrotic therapeutic strategies. Several studies have identified a variety of non-coding RNAs (miRNAs and lncRNA) which may modulate fibrosis199,200. miRNA-21201, miRNA-29202, and miRNA-34203 are a few of the identified miRNAs that are being extensively characterized for their role in regulating cardiac fibrosis. Silencing of miRNA-21 and miRNA-34 reduced fibrosis while down-regulation of miRNA-29 exacerbated collagen production. These data suggest that a variety of miRNAs possess both anti-fibrotic and pro-fibrotic roles. Additionally, lncRNAs have gained interest as another family of regulatory non-coding RNAs in cardiac fibrosis. Wisper and MIAT are two recently identified lncRNAs that function to regulate fibrosis-related genes204,205. There remain challenges in targeting miRNAs and lncRNAs for therapy due to their broad and non-specific effects. Ongoing efforts to identify the molecular targets of these non-coding RNAs will undoubtedly shed light on this novel therapeutic approach.
7.2. Epigenetic Modifiers in Cardiac Fibrosis
The contributions of epigenetics to the development of cardiac fibrosis is an additional growing field. Evidences have shown that modifications to the epigenetic landscape of various cell types can arise from different stimuli and stresses. These changes can regulate the expression of pro-inflammatory and pro-fibrotic genes in immune cells and cardiac fibroblasts206. Therefore, therapies targeting epigenetic modifiers may be promising in reversing pathological symptoms in cardiac fibrosis. Preliminary studies have shown that histone deacetylase inhibitors, such as Mocetinostat, can reverse cardiac fibrosis by targeting cardiac fibroblast activation207,208. Additionally, inhibition of the epigenetic reader BRD4 was shown to reduce fibrosis in mice undergoing MI209. These findings have been mainly from pre-clinical studies and require further exploration as a promising tool for treating cardiac fibrosis in the future.
8. CONCLUSIONS
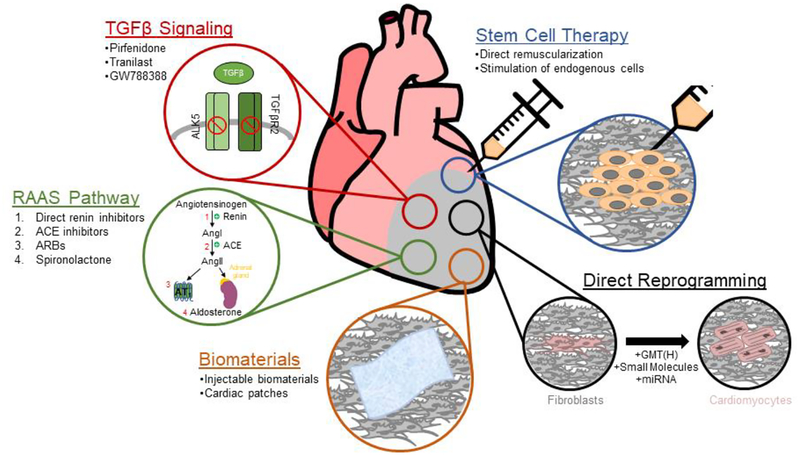
In this review, we discussed several potential therapeutic options for preventing or reducing cardiac fibrosis (Figure 1). While the research conducted in these fields have exhibited great promise, there remain challenges for translating these data into clinical practice. Both the RAAS and TGFβ pathway are major signaling cascades that significantly regulate the development of cardiac fibrosis. Inhibitors of components from either of these pathways have shown strong evidences of reducing fibrosis in animal models, although their applications in the clinic require further investigation. The goal of cell transplantation has been to replenish cardiac muscle and replace fibrotic tissue. Questions remain regarding the most suitable cell type for transplantation and how to promote functional integration of transplanted cells into the recipient hearts. The development of engineered biomaterials in the form of hydrogels or cardiac patches have begun to address some of these limiting factors. It is likely that the future success of cell therapy will ultimately involve a combinatorial approach where the ideal cell types are embedded within a scaffold for optimal cell survival, differentiation, and functional integration into the host myocardium while replacing the scar tissue. Direct reprogramming provides a novel method of replacing pathological fibroblasts with induced CMs. However, the safety of in vivo reprogramming still requires validation in large animal models. It is likely that a combination of various therapies will be necessary to address the complex pathology of cardiac fibrosis.
Figure 1:
Schematic diagram depicting potential therapeutic strategies for targeting cardiac fibrosis.
An obstacle not discussed in detail in this review is the significant difficulty in translating results from animal studies to human subjects. The majority of translational research is conducted in rodents (mice or rats), which exhibit significantly different characteristics in cardiac physiology compared with humans (Table 1). These differences have been reflected by poor clinical trial outcomes despite promising pre-clinical data. There has been a movement in recent pre-clinical work to be conducted in larger animal models (pigs and non-human primates), which more closely resemble human physiology. However, there are still species-specific differences that can hinder the development of efficacious therapies. Continued research, considering these factors, on potential anti-fibrosis therapeutic strategies will help to progress these therapies to the clinic.
Table 1:
Animal and clinical studies assessing cardiac fibrosis therapies.
| Therapeutic Target | Strategy | Year | Model of Fibrosis | Species | Ref |
|---|---|---|---|---|---|
| RAAS | Renin inhibition (aliskiren)* | 2013 | Hyperhomocysteinemia- induced myocardial fibrosis |
Mice | 29 |
| Pro-renin receptor blockade | 2016 | Myocardial infarction | Mice | 38 | |
| ACE inhibition (lisinopril)* | 1991 | Spontaneous hypertension | Rat | 40 | |
| ACE inhibition (trandolapril)* | 1995 | Spontaneous hypertension | Rat | 43 | |
| ACE inhibition (captopril)* | 1997 | Spontaneous hypertension | Rat | 44 | |
| ACE inhibition (lisinopril)* | 2000 | Patients with primary HTN, LV hypertrophy, and LV diastolic dysfunction | Human | 42 | |
| ACE inhibition (perindopril)* | 2006 | HFpEF | Human | 48 | |
| ACE inhibition (captopril)* | 2017 | LPS-induced inflammation | Rat | 45 | |
| ARB (losartan)* | 1997 | Myocardial infarction | Rat | 47 | |
| ARB (valsartan)* | 2002 | Transaortic constriction | Mice | 46 | |
| ARB (candesartan)* | 2003 | HFpEF | Human | 49 | |
| ARB (irbesartan)* | 2008 | HFpEF | Human | 50 | |
| Aldosterone antagonist (spironolactone)* | 1992 | Renovascular hypertension | Rat | 54 | |
| Aldosterone antagonist (spironolactone)* | 1996 | Chronic HF patients | Human | 58 | |
| Aldosterone antagonist (spironolactone)* | 1999 | HFrEF | Human | 59 | |
| Aldosterone antagonist (spironolactone)* | 2014 | HFpEF | Human | 57 | |
| Aldosterone antagonist (spironolactone)* | 2018 | HFpEF | Human | 60 | |
| TGFβ Signaling | TGFβRII plasmid transfection | 2004 | Myocardial infarction | Mice | 73 |
| SM16 (inhibitor of ALK5) | 2014 | Pressure overload | Mice | 72 | |
| GW788388 (inhibitor of ALK5 and TGFpRII) | 2010 | Myocardial infarction | Rats | 79 | |
| GW788388 (inhibitor of ALK5 and TGFpRII) | 2012 | Chagas | Mice | 77 | |
| GW788388 (inhibitor of ALK5 and TGFpRII) | 2017 | Scn5a+/− | Mice | 78 | |
| Pirfenidone | 2002 | DOCA-salt hypertension | Rats | 83 | |
| Pirfenidone | 2010 | Myocardial infarction | Rats | 84 | |
| Pirfenidone | 2013 | Pressure overload | Mice | 85 | |
| Pirfenidone | 2015 | Pressure overload | Mice | 86 | |
| Tranilast | 2004 | DOCA-salt hypertension | Rats | 94 | |
| Tranilast | 2013 | Myocardial infarction | Rats | 95 | |
| Tranilast | 2016 | Viral myocarditis | Mice | 93 | |
| Biomaterials | Hydrogel (alginate) | 2009 | Myocardial infarction | Porcine | 104 |
| Hydrogel (polyester-VEGF) | 2011 | Myocardial infarction | Rats | 121 | |
| Hydrogel (decellularized ECM) | 2012 | Myocardial infarction | Rats | 133 | |
| Hydrogel (alginate-chitosan) | 2014 | Myocardial infarction | Rats | 103 | |
| Hydrogel (gelatin-HGF) | 2014 | Chronic myocarditis | Rats | 111 | |
| Hydrogel (CorMatrix®-ECM) | ongoing | CABG after myocardial infarction | Human | 138 | |
| Hydrogel (VentriGel) | ongoing | STEMI undergoing PCI | Human | 140 | |
| Patch (alginate-neonatal rat CMs) | 2009 | Myocardial infarction | Rats | 120 | |
| Patch (hiPS-CMs) | 2012 | Myocardial infarction | Porcine | 130 | |
| Patch (decellularized ECM) | 2016 | Ischemia-reperfusion | Porcine | 136 | |
| Glue (fibrin-FGF) | 2010 | Myocardial infarction | Canine | 118 | |
| Microspheres | 2006 | Myocardial infarction | Canine | 119 | |
| Self-assembling peptides (skeletal myoblasts-PDGF) | 2008 | Myocardial infarction | Rats | 123 | |
| Self-assembling peptides (PDGF-FGF) | 2011 | Myocardial infarction | Rats | 124 | |
| Scaffold (fibrin-ECs-SMCs) | 2011 | Myocardial infarction | Porcine | 100 | |
| Cell Transplantation | Direct Remuscularization skeletal (myoblasts) | 1993 | Mice | 146 | |
| Direct Remuscularization (BM-MSCs) | 2004 | Myocardial infarction | Human | 145 | |
| Direct Remuscularization | 2005 | Myocardial infarction | Human | 141 | |
| Direct Remuscularization (hematopoietic BM stem cell) | 2006 | Myocardial infarction | Human | 142 | |
| Direct Remuscularization (BM-derived progenitor cells) | 2006 | Myocardial infarction | Human | 143 | |
| Direct Remuscularization (myoblast) | 2008 | Ischemic Cardiomyopathy | Human | 157 | |
| Direct Remuscularization (cardiac stem cell) | 2012 | Ischemic Cardiomyopathy | Human | 210 | |
| Direct Remuscularization (hESC-CMs) | 2014 | Myocardial infarction | Monkey | 154 | |
| Direct Remuscularization (hESC-CMs) | 2014 | Myocardial infarction | Pig | 156 | |
| Direct Remuscularization (hESC-CMs and hESC-CVPs) | 2015 | Myocardial infarction | Rat | 153 | |
| Direct Remuscularization (hESC-CVPs) | 2015 | Severe heart failure | Human | 149 | |
| Direct Remuscularization (hESC-CMs) | 2015 | Myocardial infarction | Rat | 155 | |
| Direct Remuscularization (CD34+ Cell) | 2016 | Refractory Angina | Human | 147 | |
| Direct Remuscularization (myoblast) | 2016 | Myocardial infarction | Monkey | 161 | |
| Stimulation of Endogenous CVPs | 2001 | Myocardial infarction | Mice | 166 | |
| Stimulation of Endogenous CVPs (BM-MSCs) | 2008 | Ischemic heart disease | Human | 168 | |
| Stimulation of Endogenous CVPs (BM-MSCs) | 2009 | Myocardial infarction | Pig | 178 | |
| Stimulation of Endogenous CVPs (BM-MSCs) | 2010 | Myocardial infarction | Pig | 173 | |
| Stimulation of Endogenous CVPs (cardiac stem cell c-kit+) | 2011 | Myocardial infarction | Mice | 174 | |
| Stimulation of Endogenous CVPs (cardiac stem cell c-kit+) | 2012 | Heart failure due to Ischemia | Human | 169 | |
| Stimulation of Endogenous CVPs (BM-MSCs) | 2013 | Myocardial infarction & Ischemic heart disease |
Human | 171 | |
| Stimulation of Endogenous CVPs (cardiosphere-derived cells) | 2013 | Myocardial infarction | Mice | 175 | |
| Stimulation of Endogenous CVPs (MSCs and cardiac progenitor cells) | 2015 | Myocardial infarction | Pig | 176 | |
| Stimulation of Endogenous CVPs | 2017 | Myocardial infarction | Pig | 165 | |
| Direct Reprogramming | GMT (retrovirus/lentivirus) | 2010 | N/A |
In vitro -> in vivo (Mice) |
182 |
| GMT/GMTMM (retrovirus/lentivirus) | 2013 | N/A | in vitro (Human) | 183 | |
| Small molecule cocktail + Oct4 | 2014 | N/A |
In vitro (Mouse) |
196 | |
| Chemical cocktail | 2015 | N/A | In vitro (Mouse) | 197 | |
| GMT (retrovirus) | 2012 | Myocardial infarction | Mice | 184 | |
| GMHT (retrovirus) | 2012 | Myocardial infarction | Mice | 185 | |
| GMHT (retrovirus, polycistronic) | 2012 | Myocardial infarction | Mice | 186 | |
| miRNAs (1, 133, 208, and 499) | 2015 | Myocardial infarction | Mice | 198 | |
| GMT (adenovirus) | 2017 | Myocardial infarction | Rats | 188 | |
| GMT (chimeric AAV) | 2018 | Myocardial infarction | Mice | 192 | |
| GMT (sendai virus) | 2018 | Myocardial infarction | Mice | 194 |
currently used clinical therapy
FUNDING
Park, S is supported by the Ruth L. Kirschstein National Research Service Award (NRSA) T32HL69766. Nguyen, NB is supported by the Ruth L. Kirschstein NRSA Predoctoral Fellowship F31HL144057. Pezhouman, A is supported by the Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research Training Program. Ardehali, R is supported in part by the National Institute of Health DP2 (HL127728) and the California Institute for Regenerative Medicine (RN3–06378).
Abbreviations:
- AAV
adeno-associated viruses
- AdV
adenovirus
- Ang
angiotensin
- CM
cardiomyocyte
- CPC
cardiac progenitor cell
- cTnT
cardiac troponin T
- CVD
cardiovascular diseases
- ECM
extracellular matrix
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HGF
hepatocyte growth factor
- hPSC
human pluripotent stem cell
- LAD
left anterior descending artery
- LVEDP
left ventricular end diastolic pressure
- MI
myocardial infarction
- MMP1
matrix metalloproteinase-1
- MSC
mesenchymal stem cell
- PRR
(pro)renin receptor
- RAAS
renin-angiotensin-aldosterone system
- SeV
sendai virus
- TGFβ
transforming growth factor β
- TNFα
tumor necrosis factor α
- αMHC
α-myosin heavy chain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & Tissue Repair. 2012;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Sciences. 2014;71(4):549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoletti A, Michel J-B. Cardiac fibrosis and inflammationinteraction with hemodynamic and hormonal factors. Cardiovascular Research. 1999;41(3):532–543. [DOI] [PubMed] [Google Scholar]
- 4.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circulation research. 2016;118(6):1021–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumont Ewald AWJ, Hofstra L, van Heerde Waander L, et al. Cardiomyocyte Death Induced by Myocardial Ischemia and Reperfusion. Circulation. 2000;102(13):1564–1568. [DOI] [PubMed] [Google Scholar]
- 6.Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc Dis Prev. 2010;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. The inflammatory response in myocardial injury, repair and remodeling. Nature reviews Cardiology. 2014;11(5):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krenning G, Zeisberg EM, Kalluri R. The Origin of Fibroblasts and Mechanism of Cardiac Fibrosis. Journal of cellular physiology. 2010;225(3):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baicu CF, Stroud JD, Livesay VA, et al. Changes in extracellular collagen matrix alter myocardial systolic performance. American Journal of Physiology Heart and Circulatory Physiology. 2003;284(1):H122–132. [DOI] [PubMed] [Google Scholar]
- 11.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Molecular Medicine. 2011;3(12):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac Fibroblast Activation Post-Myocardial Infarction: Current Knowledge Gaps. Trends in Pharmacological Sciences. 2017;38(5):448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akasaka Y, Morimoto N, Ishikawa Y, et al. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Modern Pathology. 2006;19:588. [DOI] [PubMed] [Google Scholar]
- 14.Atlas SA. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. Journal of Managed Care Pharmacy. 2007;13(8 Supp B):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J Brown M Direct renin inhibition - A new way of targeting the renin system. Vol 72006. [Google Scholar]
- 16.Laragh JH, Sealey JE. Renin–Angiotensin–Aldosterone System and the Renal Regulation of Sodium, Potassium, and Blood Pressure Homeostasis. Comprehensive Physiology. 2011. [Google Scholar]
- 17.Carey RM, Siragy HM. Newly Recognized Components of the Renin-Angiotensin System: Potential Roles in Cardiovascular and Renal Regulation. Endocrine Reviews. 2003;24(3):261–271. [DOI] [PubMed] [Google Scholar]
- 18.Funder JW. New biology of aldosterone, and experimental studies on the selective aldosterone blocker eplerenone. American Heart Journal. 2002;144(5, Supplement):S8–S11. [DOI] [PubMed] [Google Scholar]
- 19.Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen Metabolism in Cultured Adult Rat Cardiac Fibroblasts: Response to Angiotensin II and Aldosterone. Journal of Molecular and Cellular Cardiology. 1994;26(7):809–820. [DOI] [PubMed] [Google Scholar]
- 20.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation. 1993;88(6):2849–2861. [DOI] [PubMed] [Google Scholar]
- 21.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circulation Research. 1993;73(3):413–423. [DOI] [PubMed] [Google Scholar]
- 22.Campbell SE, Katwa LC. Angiotensin II Stimulated Expression of Transforming Growth Factor-β1in Cardiac Fibroblasts and Myofibroblasts. Journal of Molecular and Cellular Cardiology. 1997;29(7):1947–1958. [DOI] [PubMed] [Google Scholar]
- 23.Sutton Martin GSJ, Sharpe N. Left Ventricular Remodeling After Myocardial Infarction. Circulation. 2000;101(25):2981–2988. [DOI] [PubMed] [Google Scholar]
- 24.Lahera V, Cachofeiro V, de las Heras N. Interplay of Hypertension, Inflammation, and Angiotensin II. American Journal of Hypertension. 2011;24(10):1059–1059. [DOI] [PubMed] [Google Scholar]
- 25.Haudek SB, Cheng J, Du J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. Journal of Molecular and Cellular Cardiology. 2010;49(3):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodiga S, Zhong JC, Wang W, et al. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47phox NADPH oxidase subunit. Cardiovascular Research. 2011;91(1):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circulation Research. 1990;67(6):1355–1364. [DOI] [PubMed] [Google Scholar]
- 28.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83(6):1849–1865. [DOI] [PubMed] [Google Scholar]
- 29.Zhi H, Luptak I, Alreja G, et al. Effects of direct Renin inhibition on myocardial fibrosis and cardiac fibroblast function. PloS one. 2013;8(12):e81612–e81612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinert HD. Hemodynamic Effects of Renin Inhibitors. American Journal of Nephrology. 1996;16(3):252–260. [DOI] [PubMed] [Google Scholar]
- 31.Staessen JA, Li Y, Richart T. Oral renin inhibitors. The Lancet. 2006;368(9545):1449–1456. [DOI] [PubMed] [Google Scholar]
- 32.Gross O, Girgert R, Rubel D, Temme J, Theissen S, Müller G-A. Renal Protective Effects of Aliskiren Beyond Its Antihypertensive Property in a Mouse Model of Progressive Fibrosis. American Journal of Hypertension. 2011;24(3):355–361. [DOI] [PubMed] [Google Scholar]
- 33.Montes E, Ruiz V, Checa M, et al. Renin is an angiotensin-independent profibrotic mediator: role in pulmonary fibrosis. European Respiratory Journal. 2012;39(1):141. [DOI] [PubMed] [Google Scholar]
- 34.Danser AHJ, van Kesteren Catharina AM, Bax Willem A, et al. Prorenin, Renin, Angiotensinogen, and Angiotensin-Converting Enzyme in Normal and Failing Human Hearts. Circulation. 1997;96(1):220–226. [DOI] [PubMed] [Google Scholar]
- 35.Ichihara A, Kaneshiro Y, Takemitsu T, et al. Nonproteolytic Activation of Prorenin Contributes to Development of Cardiac Fibrosis in Genetic Hypertension. Hypertension. 2006;47(5):894–900. [DOI] [PubMed] [Google Scholar]
- 36.Hirose T, Mori N, Totsune K, et al. Gene expression of (pro)renin receptor is upregulated in hearts and kidneys of rats with congestive heart failure. Peptides. 2009;30(12):2316–2322. [DOI] [PubMed] [Google Scholar]
- 37.Moilanen A-M, Rysä J, Serpi R, et al. (Pro)renin Receptor Triggers Distinct Angiotensin II-Independent Extracellular Matrix Remodeling and Deterioration of Cardiac Function. PLOS ONE. 2012;7(7):e41404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellmers LJ, Rademaker MT, Charles CJ, Yandle TG, Richards AM. (Pro)renin Receptor Blockade Ameliorates Cardiac Injury and Remodeling and Improves Function After Myocardial Infarction. Journal of Cardiac Failure. 2016;22(1):64–72. [DOI] [PubMed] [Google Scholar]
- 39.The Nguyen G. (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Current Opinion in Nephrology and Hypertension. 2007;16(2):129–133. [DOI] [PubMed] [Google Scholar]
- 40.Brilla CG, Janicki JS, Weber KT. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circulation Research. 1991;69(1):107–115. [DOI] [PubMed] [Google Scholar]
- 41.Brilla CG, Janicki JS, Weber KT. Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation. 1991;83(5):1771–1779. [DOI] [PubMed] [Google Scholar]
- 42.Brilla Christian G, Funck Reinhard C, Rupp H. Lisinopril-Mediated Regression of Myocardial Fibrosis in Patients With Hypertensive Heart Disease. Circulation. 2000;102(12):1388–1393. [DOI] [PubMed] [Google Scholar]
- 43.Chevalier B, Heudes D, Heymes C, et al. Trandolapril Decreases Prevalence of Ventricular Ectopic Activity in Middle-Aged SHR. Circulation. 1995;92(7):1947–1953. [DOI] [PubMed] [Google Scholar]
- 44.Brooks Wesley W, Bing Oscar HL, Robinson Kathleen G, Slawsky Mara T, Chaletsky David M, Conrad Chester H. Effect of Angiotensin-Converting Enzyme Inhibition on Myocardial Fibrosis and Function in Hypertrophied and Failing Myocardium From the Spontaneously Hypertensive Rat. Circulation. 1997;96(11):4002–4010. [DOI] [PubMed] [Google Scholar]
- 45.Abareshi A, Norouzi F, Asgharzadeh F, et al. Effect of angiotensin-converting enzyme inhibitor on cardiac fibrosis and oxidative stress status in lipopolysaccharide-induced inflammation model in rats. International Journal of Preventive Medicine. 2017;8(1):69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Iwai M, Nakagami H, et al. Effect of Angiotensin II Type 1 Receptor Blockade on Cardiac Remodeling in Angiotensin II Type 2 Receptor Null Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(1):49–54. [DOI] [PubMed] [Google Scholar]
- 47.De Carvalho Frimm C, Sun Y, Weber KT. Angiotensin II receptor blockade and myocardial fibrosis of the infarcted rat heart. The Journal of Laboratory and Clinical Medicine. 1997;129(4):439–446. [DOI] [PubMed] [Google Scholar]
- 48.Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European Heart Journal. 2006;27(19):2338–2345. [DOI] [PubMed] [Google Scholar]
- 49.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet. 2003;362(9386):777–781. [DOI] [PubMed] [Google Scholar]
- 50.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. New England Journal of Medicine. 2008;359(23):2456–2467. [DOI] [PubMed] [Google Scholar]
- 51.Silvestre JS, Robert V, Heymes C, et al. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273(9):4883–4891. [DOI] [PubMed] [Google Scholar]
- 52.Lijnen P, Petrov V. Induction of Cardiac Fibrosis by Aldosterone. Journal of Molecular and Cellular Cardiology. 2000;32(6):865–879. [DOI] [PubMed] [Google Scholar]
- 53.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. The journal of laboratory and clinical medicine. 1992;120(6):893–901. [PubMed] [Google Scholar]
- 54.Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovascular Research. 1992;26(7):671–677. [DOI] [PubMed] [Google Scholar]
- 55.Brilla CG, Matsubara LS, Weber KT. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. American Journal of Cardiology. 1993;71(3):A12–A16. [DOI] [PubMed] [Google Scholar]
- 56.Brewster UC, Perazella MA, Setaro JF. The Renin-Angiotensin-Aldosterone System: Cardiorenal Effects and Implications for Renal and Cardiovascular Disease States. The American Journal of the Medical Sciences. 2003;326(1):15–24. [DOI] [PubMed] [Google Scholar]
- 57.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. New England Journal of Medicine. 2014;370(15):1383–1392. [DOI] [PubMed] [Google Scholar]
- 58.Effectiveness of Spironolactone Added to an Angiotensin-Converting Enzyme Inhibitor and a Loop Diuretic for Severe Chronic Congestive Heart Failure (The Randomized Aldactone Evaluation Study [RALES]) *. American Journal of Cardiology. 1996;78(8):902–907. [DOI] [PubMed] [Google Scholar]
- 59.Pitt B, Zannad F, Remme WJ, et al. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. New England Journal of Medicine. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 60.Desai AS, Liu J, Pfeffer MA, et al. Incident Hyperkalemia, Hypokalemia, and Clinical Outcomes During Spironolactone Treatment of Heart Failure With Preserved Ejection Fraction: Analysis of the TOPCAT Trial. Journal of Cardiac Failure. 2018;24(5):313–320. [DOI] [PubMed] [Google Scholar]
- 61.Meng X-M, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nature Reviews Nephrology. 2016;12(6):325–338. [DOI] [PubMed] [Google Scholar]
- 62.Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Research and Clinical Practice. 2017;133:124–130. [DOI] [PubMed] [Google Scholar]
- 63.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovascular Research. 2007;74(2):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan S, Joyce J, Margulies KB, Tsuda T. Enhanced bioactive myocardial transforming growth factor-β in advanced human heart failure. Circulation Journal: Official Journal of the Japanese Circulation Society. 2014;78(11):2711–2718. [DOI] [PubMed] [Google Scholar]
- 65.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β Mediated MAD ignaling for the Prevention of Fibrosis. Frontiers in Pharmacology. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang L, Murphy AJ, Dart AM. A Clinical Perspective of Anti-Fibrotic Therapies for Cardiovascular Disease. Front Pharmacol. 2017;8:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annual Review of Immunology. 1998;16:137–161. [DOI] [PubMed] [Google Scholar]
- 68.Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(16):5788–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaswen L, Kulkarni AB, Fredrickson T, et al. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood. 1996;87(4):1439–1445. [PubMed] [Google Scholar]
- 70.Liao R. Yin and Yang of myocardial transforming growth factor-beta1: timing is everything. Circulation. 2005;111(19):2416–2417. [DOI] [PubMed] [Google Scholar]
- 71.Massagu J TGFβ signalling in context. Nature Reviews Molecular Cell Biology. 2012;13(10):616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engebretsen KVT, Skårdal K, Bjørnstad S, et al. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. Journal of Molecular and Cellular Cardiology. 2014;76:148–157. [DOI] [PubMed] [Google Scholar]
- 73.Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovascular Research. 2004;64(3):526–535. [DOI] [PubMed] [Google Scholar]
- 74.Herbertz S, Sawyer JS, Stauber AJ, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Design, Development and Therapy. 2015;9:4479–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gellibert F, de Gouville AC, Woolven J, et al. Discovery of 4-{4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl}-N-(tetrahydro-2H-pyran-4-yl)benzamide (GW788388): a potent, selective, and orally active transforming growth factor-beta type I receptor inhibitor. J Med Chem. 2006;49(7):2210–2221. [DOI] [PubMed] [Google Scholar]
- 76.Petersen M, Thorikay M, Deckers M, et al. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73(6):705–715. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira FLd, Araújo-Jorge TC, Souza EMd, et al. Oral Administration of GW788388, an Inhibitor of Transforming Growth Factor Beta Signaling, Prevents Heart Fibrosis in Chagas Disease. PLOS Neglected Tropical Diseases. 2012;6(6):e1696. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Derangeon M, Montnach J, Cerpa CO, et al. Transforming growth factor β receptor inhibition prevents ventricular fibrosis in a mouse model of progressive cardiac conduction disease. Cardiovascular Research. 2017;113(5):464–474. [DOI] [PubMed] [Google Scholar]
- 79.Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2010;298(5):H1415–1425. [DOI] [PubMed] [Google Scholar]
- 80.Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-β. Cardiovascular Therapeutics. 2012;30(1):e30–40. [DOI] [PubMed] [Google Scholar]
- 81.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. The New England Journal of Medicine. 2014;370(22):2083–2092. [DOI] [PubMed] [Google Scholar]
- 82.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. The Journal of Pharmacology and Experimental Therapeutics. 1999;291(1):367–373. [PubMed] [Google Scholar]
- 83.Mirkovic S, Seymour A-ML, Fenning A, et al. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. British Journal of Pharmacology. 2002;135(4):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7(10):1438–1445. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Wu Y, Chen J, Zhao S, Li H. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology. 2013;126(1):1–11. [DOI] [PubMed] [Google Scholar]
- 86.Yamagami K, Oka T, Wang Q, et al. Pirfenidone exhibits cardioprotective effects by regulating myocardial fibrosis and vascular permeability in pressure-overloaded hearts. American Journal of Physiology Heart and Circulatory Physiology. 2015;309(3):H512–522. [DOI] [PubMed] [Google Scholar]
- 87.Darakhshan S, Pour AB. Tranilast: A review of its therapeutic applications. Pharmacological Research. 2015;91:15–28. [DOI] [PubMed] [Google Scholar]
- 88.Isaji M, Aruga N, Naito J, Miyata H. Inhibition by tranilast of collagen accumulation in hypersensitive granulomatous inflammation in vivo and of morphological changes and functions of fibroblasts in vitro. Life Sciences. 1994;55(15):PL287–292. [DOI] [PubMed] [Google Scholar]
- 89.Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Japanese Journal of Pharmacology. 1992;60(2):91–96. [DOI] [PubMed] [Google Scholar]
- 90.Ikeda H, Inao M, Fujiwara K. Inhibitory effect of tranilast on activation and transforming growth factor beta 1 expression in cultured rat stellate cells. Biochemical and Biophysical Research Communications. 1996;227(2):322–327. [DOI] [PubMed] [Google Scholar]
- 91.Holmes DR, Savage M, LaBlanche JM, et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2002;106(10):1243–1250. [DOI] [PubMed] [Google Scholar]
- 92.Martin J, Kelly DJ, Mifsud SA, et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-beta. Cardiovascular Research. 2005;65(3):694–701. [DOI] [PubMed] [Google Scholar]
- 93.Wen C, Xie G, Zeng P, Huang L-F, Chen C-Y. [Tranilast inhibits myocardial fibrosis in mice with viral myocarditis]. Zhongguo Dang Dai Er Ke Za Zhi = Chinese Journal of Contemporary Pediatrics. 2016;18(5):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kagitani S, Ueno H, Hirade S, Takahashi T, Takata M, Inoue H. Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. Journal of Hypertension. 2004;22(5):1007–1015. [DOI] [PubMed] [Google Scholar]
- 95.See F, Watanabe M, Kompa AR, et al. Early and Delayed Tranilast Treatment Reduces Pathological Fibrosis Following Myocardial Infarction. Heart, Lung and Circulation. 2013;22(2):122–132. [DOI] [PubMed] [Google Scholar]
- 96.Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Advanced Drug Delivery Reviews. 2010;62(7):784–797. [DOI] [PubMed] [Google Scholar]
- 97.Blackburn NJR, Sofrenovic T, Kuraitis D, et al. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials. 2015;39:182–192. [DOI] [PubMed] [Google Scholar]
- 98.Pozzobon M, Bollini S, Iop L, et al. Human Bone Marrow-Derived CD133+ Cells Delivered to a Collagen Patch on Cryoinjured Rat Heart Promote Angiogenesis and Arteriogenesis. Cell Transplantation. 2010;19(10):1247–1260. [DOI] [PubMed] [Google Scholar]
- 99.Dai W, Hale SL, Kay GL, Jyrala AJ, Kloner RA. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology. Regenerative medicine. 2009;4(3):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiong Q, Hill KL, Li Q, et al. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem cells (Dayton, Ohio). 2011;29(2):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamuta JS, Danoviz ME, Marques FLN, et al. Cell Therapy Attenuates Cardiac Dysfunction Post Myocardial Infarction: Effect of Timing, Routes of Injection and a Fibrin Scaffold. PLOS ONE. 2009;4(6):e6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, Wang H, Ma X, et al. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Experimental Biology and Medicine. 2010;235(12):1505–1515. [DOI] [PubMed] [Google Scholar]
- 103.Deng B, Shen L, Wu Y, et al. Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. Journal of Biomedical Materials Research Part A. 2014;103(3):907–918. [DOI] [PubMed] [Google Scholar]
- 104.Leor J, Tuvia S, Guetta V, et al. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. Journal of the American College of Cardiology. 2009;54(11):1014–1023. [DOI] [PubMed] [Google Scholar]
- 105.Landa N, Miller L, Feinberg Micha S, et al. Effect of Injectable Alginate Implant on Cardiac Remodeling and Function After Recent and Old Infarcts in Rat. Circulation. 2008;117(11):1388–1396. [DOI] [PubMed] [Google Scholar]
- 106.Bridges AW, García AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. Journal of diabetes science and technology. 2008;2(6):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lam MT, Wu JC. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert review of cardiovascular therapy. 2012;10(8):1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segers Vincent FM, Lee Richard T, Dimmeler S, Losordo D. Biomaterials to Enhance Stem Cell Function in the Heart. Circulation Research. 2011;109(8):910–922. [DOI] [PubMed] [Google Scholar]
- 109.Rane AA, Christman KL. Biomaterials for the Treatment of Myocardial Infarction: A 5-Year Update. Journal of the American College of Cardiology. 2011;58(25):2615–2629. [DOI] [PubMed] [Google Scholar]
- 110.Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. Journal of the Royal Society, Interface. 2012;9(66):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakano J, Marui A, Muranaka H, et al. Effects of hepatocyte growth factor in myocarditis rats induced by immunization with porcine cardiac myosin. Interactive cardiovascular and thoracic surgery. 2014;18(3):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288(5):H2131–H2139. [DOI] [PubMed] [Google Scholar]
- 113.Taniyama Y, Morishita R, Aoki M, et al. Angiogenesis and Antifibrotic Action by Hepatocyte Growth Factor in Cardiomyopathy. Hypertension. 2002;40(1):47–53. [DOI] [PubMed] [Google Scholar]
- 114.Aoki M, Morishita R, Taniyama Y, et al. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Therapy. 2000;7:417. [DOI] [PubMed] [Google Scholar]
- 115.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. The Journal of cell biology. 1992;119(3):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Belle E, Witzenbichler B, Chen D, et al. Potentiated Angiogenic Effect of Scatter Factor/Hepatocyte Growth Factor via Induction of Vascular Endothelial Growth Factor. Circulation. 1998;97(4):381–390. [DOI] [PubMed] [Google Scholar]
- 117.Balkovetz DF, Lipschutz JH. Hepatocyte Growth Factor and the Kidney: It Is Not Just for the Liver In: Jeon KW, ed. International Review of Cytology. Vol 186 Academic Press; 1998:225–260. [DOI] [PubMed] [Google Scholar]
- 118.Nie S-p, Wang X, Qiao S-b, et al. Improved myocardial perfusion and cardiac function by controlled-release basic fibroblast growth factor using fibrin glue in a canine infarct model. Journal of Zhejiang University Science B. 2010;11(12):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y, Sun L, Huan Y, Zhao H, Deng J. Effects of basic fibroblast growth factor microspheres on angiogenesis in ischemic myocardium and cardiac function: analysis with dobutamine cardiovascular magnetic resonance tagging. European Journal of Cardio-Thoracic Surgery. 2006;30(1):103–107. [DOI] [PubMed] [Google Scholar]
- 120.Dvir T, Kedem A, Ruvinov E, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(35):14990–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu J, Zeng F, Huang X-P, et al. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials. 2011;32(2):579–586. [DOI] [PubMed] [Google Scholar]
- 122.Simón-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics. 2012;2(6):541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dubois G, Segers VFM, Bellamy V, et al. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;87B(1):222–228. [DOI] [PubMed] [Google Scholar]
- 124.Kim JH, Jung Y, Kim S-H, et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials. 2011;32(26):6080–6088. [DOI] [PubMed] [Google Scholar]
- 125.Christman KL, Lee RJ. Biomaterials for the Treatment of Myocardial Infarction. Journal of the American College of Cardiology. 2006;48(5):907–913. [DOI] [PubMed] [Google Scholar]
- 126.Sarig U, Machluf M. Engineering cell platforms for myocardial regeneration. Expert Opinion on Biological Therapy. 2011;11(8):1055–1077. [DOI] [PubMed] [Google Scholar]
- 127.Callegari A, Bollini S, Iop L, et al. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials. 2007;28(36):5449–5461. [DOI] [PubMed] [Google Scholar]
- 128.Simpson D, Liu H, Fan T-HM, Nerem R, Dudley SC, Jr. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem cells (Dayton, Ohio). 2007;25(9):2350–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM Trial): Clinical Feasibility Study. The Annals of Thoracic Surgery. 2008;85(3):901–908. [DOI] [PubMed] [Google Scholar]
- 130.Kawamura M, Miyagawa S, Miki K, et al. Feasibility, Safety, and Therapeutic Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets in a Porcine Ischemic Cardiomyopathy Model. Circulation. 2012;126(11_suppl_1):S29–S37. [DOI] [PubMed] [Google Scholar]
- 131.Xiong Q, Ye L, Zhang P, et al. Functional consequences of human induced pluripotent stem cell therapy: myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation. 2013;127(9):997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang RM, Christman KL. Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Advanced Drug Delivery Reviews. 2016;96:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59(8):751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ott HC, Matthiesen TS, Goh S-K, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature Medicine. 2008;14:213. [DOI] [PubMed] [Google Scholar]
- 136.Mewhort HEM, Turnbull JD, Satriano A, et al. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. The Journal of Heart and Lung Transplantation. 2016;35(5):661–670. [DOI] [PubMed] [Google Scholar]
- 137.Turner NA. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). Journal of Molecular and Cellular Cardiology. 2016;94:189–200. [DOI] [PubMed] [Google Scholar]
- 138.Fedak P Epicardial Infarct Repair Using CorMatrix®-ECM: Clinical Feasibility Study (EIR). https://clinicaltrials.gov/ct2/show/NCT02887768. Published 2017. Accessed December 16, 2018.
- 139.Aziyo Biologics I. Obtain Additional Information on Use of CorMatrix ECM (Extracellular Matrix) (RECON). https://clinicaltrials.gov/ct2/show/study/NCT02073331. Published 2018. Accessed December 16, 2018.
- 140.Ventrix I A Study of VentriGel in Post-MI Patients. https://clinicaltrials.gov/ct2/show/NCT02305602?term=NCT02305602&rank=1. Published 2018. Accessed December 16, 2018.
- 141.Cleland JGF, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. European Journal of Heart Failure. 2005;8(1):105–110. [DOI] [PubMed] [Google Scholar]
- 142.Mansour S, Vanderheyden M, De Bruyne B, et al. Intracoronary delivery of hematopoietic bone marrow stem cells and luminal loss of the infarct-related artery in patients with recent myocardial infarction. J Am Coll Cardiol. 2006;47(8):1727–1730. [DOI] [PubMed] [Google Scholar]
- 143.Schächinger V, Erbs S, Elsässer A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–2783. [DOI] [PubMed] [Google Scholar]
- 144.Marelli D, Desrosiers C, el-Alfy M, Kao RL, Chiu RC. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1992;1(6):383–390. [DOI] [PubMed] [Google Scholar]
- 145.Chen S-l, Fang W-w, Qian J, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chinese medical journal. 2004;117(10):1443–1448. [PubMed] [Google Scholar]
- 146.Koh GY, Klug MG, Soonpaa MH, Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. The Journal of Clinical Investigation. 1993;92(3):1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Povsic TJ, Henry TD, Traverse JH, et al. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34+ Cell Administration in Patients With Refractory Angina. JACC: Cardiovascular Interventions. 2016;9(15):1576–1585. [DOI] [PubMed] [Google Scholar]
- 148.Beltrami AP, Barlucchi L, Torella D, et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell. 2003;114(6):763–776. [DOI] [PubMed] [Google Scholar]
- 149.Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. European Heart Journal. 2015;36(30):2011–2017. [DOI] [PubMed] [Google Scholar]
- 150.Skelton RJP, Brady B, Khoja S, et al. CD13 and ROR2 Permit Isolation of Highly Enriched Cardiac Mesoderm from Differentiating Human Embryonic Stem Cells. Stem cell reports. 2016;6(1):95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skelton RJP, Kamp TJ, Elliott DA, Ardehali R. Biomarkers of Human Pluripotent Stem Cell-Derived Cardiac Lineages. Trends Mol Med. 2017;23(7):651–668. [DOI] [PubMed] [Google Scholar]
- 152.Skelton RJ, Costa M, Anderson DJ, et al. SIRPA, VCAM1 and CD34 identify discrete lineages during early human cardiovascular development. Stem Cell Res. 2014;13(1):172–179. [DOI] [PubMed] [Google Scholar]
- 153.Fernandes S, Chong JJH, Paige SL, et al. Comparison of Human Embryonic Stem Cell-Derived Cardiomyocytes, Cardiovascular Progenitors, and Bone Marrow Mononuclear Cells for Cardiac Repair. Stem Cell Reports. 2015;5(5):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Riegler J, Tiburcy M, Ebert A, et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circulation research. 2015;117(8):720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ye L, Chang Y-H, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell stem cell. 2014;15(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. [DOI] [PubMed] [Google Scholar]
- 158.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.The Lancet Editors. Expression of concern: the SCIPIO trial. Lancet. 2014;383(9925):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282(5391):1145. [DOI] [PubMed] [Google Scholar]
- 161.Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–391. [DOI] [PubMed] [Google Scholar]
- 162.Almeida SO, Skelton RJ, Adigopula S, Ardehali R. Arrhythmia in stem cell transplantation. Card Electrophysiol Clin. 2015;7(2):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345(6199):1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. [DOI] [PubMed] [Google Scholar]
- 167.Wang LT, Ting CH, Yen ML, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Behfar A, Faustino RS, Arrell DK, Dzeja PP, Perez-Terzic C, Terzic A. Guided stem cell cardiopoiesis: discovery and translation. J Mol Cell Cardiol. 2008;45(4):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mathur A, Fernández-Avilés F, Dimmeler S, et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur Heart J. 2017;38(39):2930–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jeevanantham V, Afzal MR, Zuba-Surma EK, Dawn B. Clinical trials of cardiac repair with adult bone marrow-derived cells. Methods in molecular biology (Clifton, NJ). 2013;1036:179–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nowbar AN, Mielewczik M, Karavassilis M, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ (Clinical research ed). 2014;348:g2688–g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell stem cell. 2011;8(4):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Malliaras K, Zhang Y, Seinfeld J, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO molecular medicine. 2013;5(2):191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quijada P, Salunga HT, Hariharan N, et al. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circulation research. 2015;117(8):695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hatzistergos KE, Takeuchi LM, Saur D, et al. cKit+ cardiac progenitors of neural crest origin. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(42):13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9(6):527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gomes SA, Rangel EB, Premer C, et al. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110(8):2834–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Engel JL, Ardehali R. Direct Cardiac Reprogramming: Progress and Promise. Stem Cells International. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ieda M, Fu J-D, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):12667–12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Song K, Nam Y-J, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Inagawa K, Miyamoto K, Yamakawa H, et al. Induction of Cardiomyocyte-Like Cells in Infarct Hearts by Gene Transfer of Gata4, Mef2c, and Tbx5. Circulation Research. 2012;111(9):1147–1156. [DOI] [PubMed] [Google Scholar]
- 187.Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004—an overview. The Journal of Gene Medicine. 2004;6(6):597–602. [DOI] [PubMed] [Google Scholar]
- 188.Mathison M, Singh VP, Chiuchiolo MJ, et al. In situ reprogramming to transdifferentiate fibroblasts into cardiomyocytes using adenoviral vectors: Implications for clinical myocardial regeneration. The Journal of Thoracic and Cardiovascular Surgery. 2017;153(2):329–339.e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wold WSM, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Current gene therapy. 2013;13(6):421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Anne KZaDAM. Immune Responses to Adeno-Associated Virus Vectors. Current Gene Therapy. 2005;5(3):323–331. [DOI] [PubMed] [Google Scholar]
- 191.Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Molecular therapy Methods & clinical development. 2017;8:87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yoo SY, Jeong S-N, Kang J-I, Lee S-W. Chimeric Adeno-Associated Virus-Mediated Cardiovascular Reprogramming for Ischemic Heart Disease. ACS Omega. 2018;3(5):5918–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakanishi M, Otsu M. Development of Sendai Virus Vectors and their Potential Applications in Gene Therapy and Regenerative Medicine. Current Gene Therapy. 2012;12(5):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miyamoto K, Akiyama M, Tamura F, et al. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell. 2018;22(1):91–103.e105. [DOI] [PubMed] [Google Scholar]
- 195.Engel JL, Ardehali R. Sendai virus based direct cardiac reprogramming: what lies ahead? Stem Cell Investig. 2018;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang H, Cao N, Spencer CI, et al. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Reports. 2014;6(5):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fu Y, Huang C, Xu X, et al. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Research. 2015;25(9):1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jayawardena TM, Finch EA, Zhang L, et al. MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function. Circ Res. 2015;116(3):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dong D-l Yang B-f. Role of microRNAs in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharmaceutica Sinica B. 2011;1(1):1–7. [Google Scholar]
- 200.Qu X, Song X, Yuan W, et al. Expression signature of lncRNAs and their potential roles in cardiac fibrosis of post-infarct mice. Bioscience reports. 2016;36(3):e00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. [DOI] [PubMed] [Google Scholar]
- 202.van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang Y, Qi Y, Du JQ, Zhang DF. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets. 2014;18(12):1355–1365. [DOI] [PubMed] [Google Scholar]
- 204.Micheletti R, Plaisance I, Abraham BJ, et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Science translational medicine. 2017;9(395):eaai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qu X, Du Y, Shu Y, et al. MIAT Is a Pro-fibrotic Long Non-coding RNA Governing Cardiac Fibrosis in Post-infarct Myocardium. Scientific reports. 2017;7:42657–42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Felisbino MB, McKinsey TA. Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation. JACC Basic to translational science. 2018;3(5):704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nural-Guvener HF, Zakharova L, Nimlos J, Popovic S, Mastroeni D, Gaballa MA. HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 2014;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoon S, Eom GH. HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med J. 2016;52(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Duan Q, McMahon S, Anand P, et al. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Science Translational Medicine. 2017;9(390). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bolli R, Chugh A, Loughran J, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy – Authors’ reply. The Lancet. 2012;379(9819):891–892. [DOI] [PubMed] [Google Scholar]