Abstract
Phthalates are industrial plasticizers and stabilizers commonly found in polyvinyl chloride plastic and consumer products, including food packaging, cosmetics, medical devices, and children’s toys. Di-(2-ethylhexyl) phthalate (DEHP), one of the most commonly used phthalates, exhibits endocrine-disrupting characteristics and direct exposure leads to reproductive deficits and abnormalities in anxiety-related behaviors. Importantly, increasing evidence indicates that the impacts of DEHP exposure on reproduction and social behavior persist across multiple generations. In this study, we tested the hypothesis that transgenerational DEHP exposure alters anxiety-like behavior and neural gene expression in both male and female mice. Pregnant CD-1 mice were orally dosed daily with either tocopherol-stripped corn oil or DEHP (20 or 200 μg/kg/day; 500 or 750 mg/kg/day) from gestational day 10.5 until birth to produce the F1 generation. Females from each generation were bred with untreated, unrelated CD-1 males to produce subsequent generations. Behavior and gene expression assays were performed with adult, intact F3 males and females. Transgenerational DEHP exposure increased time spent in the open arm in the elevated plus maze for adult females (750 mg/kg/day lineage), but not males. In adult females, we observed a down-regulation of mRNA expression of estrogen receptor 1 in the 200 μg/kg/day and 500 mg/kg/day treatment lineages, mineralocorticoid receptor in the 200 μg/kg/day lineage, and dopamine receptor 2 in the 20 μg/kg/day and 750 mg/kg/day lineages. In adult males, we found an up-regulation of estrogen receptor 2 in the 20 and 200 μg/kg/day lineages, and dopamine receptor 1 in the 20 μg/kg/day and 750 mg/kg/day lineages. No hippocampal gene expression modifications were observed in response to treatment. These results implicate dose-specific transgenerational effects on behavior and neural gene expression in adult male and female mice.
Keywords: Endocrine disrupting chemicals, F3 generation, plasticizers, elevated plus maze, transgenerational
1. Introduction
Phthalates are environmentally pervasive endocrine disruptors to which humans and animals are exposed daily. Phthalates, including di-(2-ethylhexyl) phthalate (DEHP), are a class of industrial plasticizers and stabilizers commonly found in polyvinyl chloride plastic products and consumer products, including food packaging, clothing, cosmetics, medical bags and tubing, and children’s toys [1, 2]. Phthalate plasticizers are non-covalently bound to plastic, and therefore can leach into the environment [2]. Normal human exposure can range from 3–30 μg/kg/day, with occupational and medical exposure occurring at higher concentrations of 0.21–2.1 mg/kg/day and estimated 8.5 mg/kg/day, respectively [1]. Exposure to DEHP mainly occurs through ingestion of contaminated food; however, exposure can also occur through dermal contact and inhalation [1]. Further, phthalate metabolites are detectable in human breast milk, cord blood, maternal and infant sera, as well as infant urine samples, implicating exposure in both the mother and the child [3, 4]. Importantly, DEHP is one of the most widely used phthalate plasticizers [1, 2] that exhibits endocrine-disrupting characteristics and is linked to reproductive and behavior abnormalities in humans and animals [5–13]. Furthermore, women have higher phthalate metabolite levels compared to men, indicating a potential sex difference in exposure and impact of phthalates on human health [14].
Developmental exposure to phthalates, including DEHP, alters anxiety-like behaviors in laboratory rodents. Oral exposure to DEHP from postnatal day (PND) 28–42 in female ICR mice at 1, 10, 50, and 200 μg/kg/day increased anxiety-like behavior in animals at approximately PND 50 [15]. Adult CD-1 mice exposed from gestational day 10.5 until birth (dams orally exposed) with 200 μg/kg/day, 500 and 750 mg/kg/day exhibited increased anxiety when assessed at 16–20 months of age [12]. Wistar rats exposed during lactation from PND1–21, and then through the drinking water from PND 21 through adulthood with 30 mg/kg/day also exhibited reduced anxiety at PND 45 and 60 [7]. Further, 6-week-old male and female mice exhibited increased anxiety-like behavior following dam oral exposure to 10 and 200 mg/kg/day DEHP from gestational day 7 until PND 21 [16].
DEHP also alters behavior in a transgenerational model of exposure. Transgenerational exposure occurs when a pregnant dam (F0) is exposed to DEHP, and the effects of this exposure are observed three (F3) or more generations after the initial exposure. Quinnies et al. showed that transgenerational DEHP exposure modified nonsocial behaviors in F3 juvenile male C57BL/6J mice [17]. Pubertal (PND 35–42) males of the 200 mg/kg/day lineage exhibited increased digging behavior and decreased self-grooming behavior. In a second study using lower doses of DEHP (5, 40, and 400 μg/kg/day), Quinnies et al. found that both male and female F3 juvenile (PND 28–32) C57BL/6J mice exhibited modified social behavior in response to transgenerational DEHP exposure [18]. Quinnies et al. also measured anxiety-like behavior in juvenile individuals (PND 25–35) in both studies, finding only a significant increase in anxiety-like behavior in the F1 generation males and females (5 and 40 μg/kg/day), and no significant change in the F3 generation male and female juvenile animals. Collectively, they found that transgenerationally exposed juvenile males exhibited more interactive social behaviors compared to age-, treatment-, and sex-matched males, whereas both males and females from the exposed lineages exhibited reduced cage-exploring behavior and no change in anxiety-like behavior in F3 juvenile animals [18]; however, the impact of transgenerational DEHP exposure on adult animals remains unknown.
Adult and developmental DEHP exposure likely regulates behavior, in part, by modifying underlying neural mechanisms. In adult male CD-1 mice, prenatal DEHP exposure (200 μg/kg/day and 750 mg/kg/day) regulates gene expression and causes neuronal degeneration in the hippocampus, a brain region essential for learning and memory [19]. Prenatal exposure to DEHP decreases hippocampal NR1 and NR2B protein expression [20], two N-methyl-D-aspartate receptor (NMDAR) subunits essential for modulation of spatial memory. Lactational exposure to DEHP (10, 50, and 200 mg/kg/day) from gestational day 7 until PND 21 reduces activation of hippocampal extracellular signal-regulating protein kinases 1 and 2 (ERK1/2) by reducing phosphorylation in juvenile male and female Kunming mice [16]. ERK1/2 is essential for regulation of downstream signaling of multiple receptors, including sex steroid receptors [16]. Furthermore, this study also found that DEHP exposure reduced the expression of hippocampal androgen receptor and estrogen receptor protein in juvenile male, and juvenile and adult female mice, respectively. In adult male CD-1 mice, prenatal exposure to DEHP (500 and 750 mg/kg/day) reduced the number of hippocampal pyramidal cells in the dentate gyrus, CA1, and CA2 regions [19]. Together, these findings show that perinatal DEHP exposure can modulate gene expression and neurodegeneration in the brain.
The neural circuitry regulating anxiety-like behavior is complex. However, dysregulation of molecular machinery in the hippocampus and amygdala is implicated in changes to anxiety-like behavior [16, 21–26]. Alterations of receptor expression in the hippocampus, such as mineralocorticoid receptor, metabotropic glutamate receptor 2, and estrogen receptor 2 (ESR2), modifies anxiety-like behavior in adult Kunming and C57BL/6J mice [16, 25]. Similarly, increases in corticotrophin releasing hormone receptors in the central amygdala are correlated with altered anxiety-like behavior in PND 90 Sprague-Dawley rats [26]. Although exposure to a chemical may modify gene expression, in transgenerational models of exposure, the chemical exposure is likely modulating gene expression, in part, by regulating the epigenome, thereby altering the accessibility of specific genes [21–24]. However, no studies involving transgenerational models of phthalate exposure have investigated alterations in genetic or epigenetic expression in the hippocampus, amygdala, or other brain regions relevant to anxiety-like behavior.
It is unclear whether phthalate exposure alters adult behavior in a transgenerational manner. The effects of perinatal exposure to DEHP in other organ systems, including male and female reproductive systems, and behaviors have been observed and persist in transgenerational models of exposure [13, 17, 18, 27–29]. The only studies to investigate anxiety-like behavior in a transgenerational model of DEHP exposure found no effect; however, this was only measured in juvenile animals (PND 25–35) [17, 18]. As DEHP alters anxiety-like behavior in F1 generation juveniles and adults, we investigated if the impact of prenatal DEHP exposure on (PND 90–100) anxiety-like behavior in adult animals persists in the F3 generation. Here, we used a transgenerational model of DEHP exposure to evaluate the effects of DEHP on anxiety-like behavior and neural gene expression in adult male and female mice. First, we hypothesized that transgenerational exposure to DEHP would alter anxiety-like behavior; we examined this by testing F3 animals in the elevated plus maze. Second, we hypothesized that DEHP would modify gene expression in brain areas related to anxiety-like behaviors, including the hippocampus and amygdala. The hippocampus and amygdala were selected based on previous literature indicating their crucial role in regulating anxiety-like behavior in rodents (reviewed in [30]).
2. Methods
2.1. Animals and Housing
Adult animals were cohort siblings from a larger, published study [8, 13, 28]. Eightweek old female and male outbred CD-1 mice (Charles River, Wilmington, MA) were used to breed the F1 generation as previously published [8, 13, 28]. Animals were group housed at 25°C in conventional polystyrene cages on a 12 h light:12 h dark cycle. The mice received Teklad Rodent Diet 8604 (Envigo, Indianapolis, IN) and water ad libitum. The phytoestrogen content in the Teklad 8604 ranges from 350–650 mg/kg. All animals were used in accordance with procedures approved of by the University of Illinois Institutional Animal Care and Use Committee.
Fifty female mice (F0) at 8-weeks of age were mated with age-matched males (2 females and 1 male per cage) to produce the F1 generation. Females were monitored daily for the presence of a vaginal sperm plug. The day the vaginal sperm plug was detected was defined as gestational day (GD) 0.5. Upon presence of a vaginal sperm plug, females were individually housed and dosed with DEHP. To produce the F2 generation, the adult F1 females (> 3 months) were mated with unexposed, unrelated males following the same breeding procedure. Subsequently, the adult F2 females (> 3 months of age) were bred with unexposed, unrelated CD-1 males following the same breeding procedure mentioned above to produce the F3 generation. The maternal line of exposure was followed to produce the F3 generation.
2.2. Dosing
Pregnant F0 females were randomly assigned to one of five treatment groups. Dams were orally dosed with tocopherol-stripped corn oil (vehicle control) or 20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day of DEHP from GD 10.5 until birth of the pups (approximately GD 19.5). Oral dosing was performed by placing a pipette tip into the cheek pouch of the dam and administering the dosing solution, as previously described [8, 13]. DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). DEHP was dissolved in tocopherol-stripped corn oil (MP Biomedicals, Solon, OH) to form a stock solution and was further diluted in tocopherol-stripped corn oil to the desired concentration. A range of doses was chosen due to DEHP exhibiting a nonmonotonic dose-response curve [31]. The 20 μg/kg/day dose was chosen because it is the EPA reference dose for human exposure [1]. The 200 μg/kg/day dose was selected because it is within the range of occupational exposure and exposure via medical procedures [1]. The higher doses (500 mg/kg/day and 750 mg/kg/day) were chosen because they adversely affect reproductive function in vivo [5, 8, 13, 27, 32, 33]. The dosing window was selected because it is the critical developmental period for ovarian follicles [34], and therefore allows for the potential transmission of transgenerational effects.
2.3. Elevated Plus Maze
F3 adult males and females (PND 90–100) in all treatment group lineages were used to assess anxiety-like behavior in the elevated plus maze. Animals were placed in the center of a four-arm maze (50 cm above the ground) with two open arms (24 cm length x 5 cm width and 0.5 cm height) and two closed arms (25 cm x 5 cm x 16 cm height). All animals were tested during the light phase of their light:dark cycle, and testing was conducted in a dark room illuminated with red light. In between trials, 75% ethanol was used to clean urine and fecal boli from the maze. Animals were allowed to explore the maze freely for 10 minutes and were video recorded. The time spent and the number of entries in the open arms and closed arms were scored. Time spent in and entries into the open arms are indicative of lower levels of anxiety-like behavior. All measures were hand-scored using the video recordings, and all observers and scorers were blind to the treatment lineage and sex.
2.4. Brain Dissection and Molecular Analysis
Beginning two weeks after completion of behavior studies, all male and female mice were euthanized by CO2 asphyxiation followed by decapitation during the late light phase. The brains of all animals from each treatment group were removed and rapidly frozen indirectly in a dry ice-EtOH bath and stored at −80°C for later use. Brains were then dissected with sharpened, clean razor blades in an adult Mouse Brain Matrix (Kent Scientific, Torrington, CT) which was held at −20°C. The hippocampus and amygdala were identified by isolating a 1.0 mm slice (Harris Uni-Core, ThermoFisher™, Waltham, MA) containing a group of white matter tracts, including the corpus callosum, dorsal hippocampal commissure, and the alveus (Allen Mouse Brain Atlas, Image 78) [35]. The bilateral hippocampal 1.0 mm punches containing CA1 and dentate gyrus were taken from the most dorsal portion of the hippocampus, just lateral to the midline. For the amygdala, 1.0 mm bilateral punches containing basolateral amygdala and central amygdala were taken from the most ventral portion of the cortical layer. Both hippocampal and amygdala samples were taken from the same slice. All punches were immediately stored in RNAlater (Invitrogen, Waltham, MA) at −20°C until RNA isolation.
Total RNA was extracted using RNAqueous® Micro Kit (ThermoFisher) according to manufacturer instructions. RNA was quantified using NanoDrop™ (NanoDrop™ ND100, ThermoScientific™, Waltham, MA). RNA samples were stored at −80°C until they were synthesized to cDNA. We reverse transcribed cDNA from 90 ng (hippocampus) or 100 ng (amygdala) of RNA using ProtoScript® First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). For the hippocampus samples, we performed qPCR using 4.0 μL of cDNA, 1.0 μL of TaqMan® Gene Expression Assay (ThermoFisher), 10.0 μL TaqMan® Universal PCR Master Mix (ThermoFisher), and 5 μL of RNase-free water per gene. For the amygdala samples, after cDNA synthesis, we amplified 12.5 μL of each cDNA sample with 25 μL of TaqMan® PreAmp Master Mix and 12.5 μL of a pool of 15 TaqMan® assays and diluted each amplified sample with 1X TE Buffer (ThermoFisher) at a 1:5 dilution. Then, 250 μL of the diluted cDNA sample was combined with 195 μL of nuclease-free water and 480 μL TaqMan® Gene Expression Master Mix (ThermoFisher). To perform qPCR, 19 μL of this reaction mixture was added into each well of a 96-Well reaction plate, with each well containing 1 μL of a TaqMan® assay pre-loaded by the manufacturer. To perform qPCR analysis of both brain regions, the Applied Biosystems® 7500 RT PCR System (ThermoFisher) and Applied Biosystems® SDS Software were used according to the manufacturer protocol. Relative expression of each target gene was calculated using the reference gene, glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Expression of Gapdh did not vary between treatment groups or sexes. The genes assessed for each brain region are represented in Table 1.
Table 1.
Gene selection for the hippocampus and amygdala.
| Brain Region | Gene Name | Abbreviation |
|---|---|---|
| Both | Housekeeping gene: Glyceraldehyde 3-phosphate dehydrogenase | Gapdh |
| Hippocampus | Estrogen receptor 2 | Esr2 |
| Glucocorticoid receptor | Nr3c1 | |
| DNA methyltransferase 3a | Dnmt3a | |
| Amygdala | Estrogen receptor 1 | Esr1 |
| Estrogen receptor 2 | Esr2 | |
| Androgen receptor | Ar | |
| Corticotrophin releasing hormone receptor 1 | Crhr1 | |
| Corticotrophin releasing hormone receptor 2 | Crhr2 | |
| Glucocorticoid receptor | Nr3c1 | |
| Mineralocorticoid receptor | Nr3c2 | |
| Melanocortin receptor 4 | Mc4r | |
| Dopamine receptor D1 | Drd1 | |
| Dopamine receptor D2 | Drd2 | |
| DNA methyltransferase 1 | Dnmt1 | |
| DNA methyltransferase 3a | Dnmt3a | |
| DNA methyltransferase 3b | Dnmt3b | |
| DNA methyltransferase 3l | Dnmt3l | |
| Methyl-CpG binding protein 2 | Mecp2 |
All genes were selected based on previous literature indicating involvement in regulating behavior and/or being modulated by endocrine disrupting chemical exposure, specifically in the hippocampus or amygdala. Esr2 and Mc4r were selected as their expression in the amygdala has been shown to be modulated by other endocrine disruptors, bisphenol A and vinclozolin, and related to anxiety-like behavior [36–38]. Crhr1, Crhr2, Drd1, Drd2, Nr3c1, Esr1, Nr3c2, Ar, and Mecp2 were selected as they are involved in regulating anxiety-like behaviors [39–45]. Finally, enzymes involved in regulating the epigenome (Dnmt1, Dnmt3a, Dnmt3b, and Dnmt3l) were selected as they have been shown to be transgenerationally modified by vinclozolin in the mouse testis [46].
2.5. Statistical Analysis
Randomly selected, individual adult F3 offspring were used as the factor for EPM and qPCR analyses. Specifically, in the F2 generation, seven control dams produced 23 pups (15 female, 8 male), five 20 μg/kg/day dams produced 19 pups (11 female, 8 male), four 200 μg/kg/day dams produced 16 pups (8 female, 8 male), three 500 mg/kg/day dams produced 9 pups (9 female, 8 male), and two 750 mg/kg/day dams produced 17 pups (9 female, 8 male). All figure legends include the final n value following removal of individuals as outliers. Random individuals were selected from each treatment to equate an n=4–5 within each sex and treatment for qPCR analysi
All data were analyzed using SPSS software (SPSS Inc., Chicago, IL). Following removal of outliers using Grubbs’ test, data were analyzed for normal distribution using Shapiro-Wilk test (α=0.05). A two-way ANOVA was performed to identify statistical sex differences, as well as an interaction between sex and treatment. No significant effect of sex or interaction between sex and treatment was found, therefore, the data were then analyzed within each sex separately. Levene’s test was used to determine if the assumption of homogeneity of variances between groups was met. If normally distributed, data were then analyzed using a planned one-way analysis of variance (ANOVA). If a treatment effect was observed, we used post hoc analysis to compare treatment groups with control. Specifically, we used Dunnett’s 2-sided if equal variances were assumed or Games-Howell if equal variances were not assumed. If data were not normally distributed, the data were analyzed using the Kruskal-Wallis analysis of variance. If a main effect was found, planned comparisons using Mann-Whitney U twoindependent samples tests were used to compare DEHP-treated groups to control. Statistical significance for all analyses was assigned at P ≤ 0.05.
The following tests passed Levene’s test and were analyzed using an ANOVA: EPM total arm time (females only), hippocampal Nr3c1 expression (males and females), hippocampal Dnmt3a (females only), and hippocampal Esr2 (females only). All other data sets did not pass normality, and were therefore analyzed using non-parametric tests (Kruskal-Wallis and Mann-Whitney).
3. Results
3.1. Elevated Plus Maze (EPM)
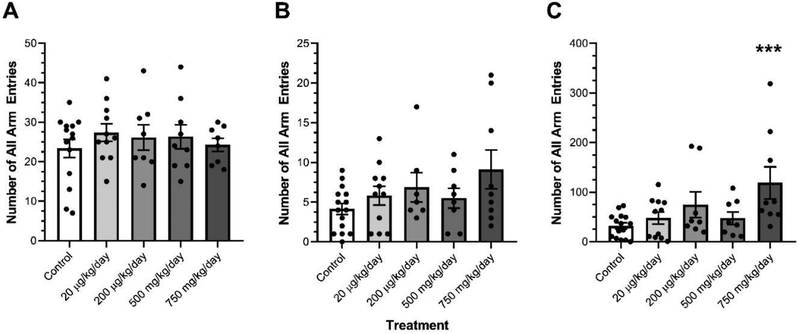
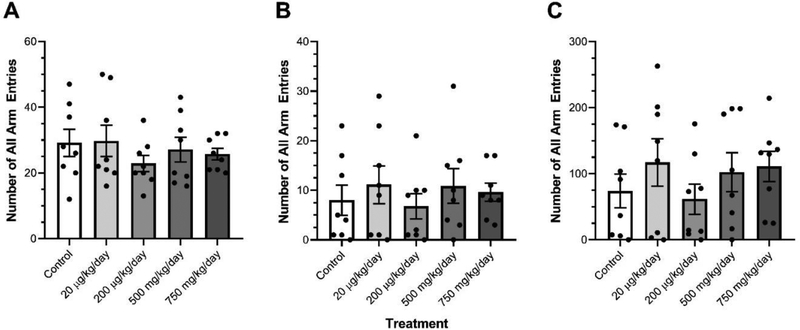
DEHP exposure did not transgenerationally affect the number of total arm entries or the number of open arm entries in females (Fig. 1A–B). Ancestral DEHP exposure significantly increased the total time spent in the open arms in the 750 mg/kg/day treatment lineage females relative to control females (Fig. 1C, p=0.001). Ancestral DEHP exposure did not affect the number of total arm entries, the number of open arm entries, or the total time spent in the open arms in any male treatment lineages relative to control males (Fig. 2A–C).
Figure 1.
The effects of ancestral exposure to DEHP on elevated plus maze performance in F3 females. The total arm entries (A), open arm entries (B), and total time in open arms (in seconds) (C) are shown for the F3 generation females. Data are representative of the means (± SEM). Control n=14, 15 and 15 respectively; 20 μg/kg/day n=11 for all; 200 μg/kg/day n=7, 8, and 8 respectively; 500 mg/kg/day n=8, 9, and 9 respectively; and 750 mg/kg/day n=9 for all analyses. ***p<0.001 (significant difference compared with the control). No asterisk indicates no significant difference compared to control.
Figure 2.
The effects of ancestral exposure to DEHP on elevated plus maze performance in F3 males. The total arm entries (A), open arm entries (B), and total males. Data are representative of the means (± SEM). n=8 for all groups and all analyses.
3.2. Gene Expression
3.2.1. Relative expression of Esr2, Nr3c1, and Dnmt3a in the hippocampus
Transgenerational DEHP exposure did not affect the relative expression of Esr2, Nr3c1, and Dnmt3a in the hippocampus in male DEHP treatment lineages relative to the male control animals (Table 2). Additionally, transgenerational DEHP exposure did not alter the relative expression of Esr2, Nr3c1, and Dnmt3a in the hippocampus in female DEHP treatment lineages relative to the female control animals (Table 2).
Table 2.
Summary of the effect of ancestral DEHP exposure on hippocampal and amygdala gene expression in F3 males and females.
| Males | Females | |
|---|---|---|
| Gene Name | Hippocampus | |
| Esr2 | NC | NC |
| Nr3c1 | NC | NC |
| Dnmt3a | NC | NC |
| Gene Name | Amygdala | |
| Esr1 | NC |
Down-regulated (200 μg/kg/day- and 500 mg/kg/day-treatment lineages) |
| Esr2 |
Up-regulated (20 μg/kg/day- and 200 μg/kg/day-treatment lineages) |
NC |
| Ar | NC | NC |
| Nr3c1 | NC | NC |
| Nr3c2 | NC |
Down-regulated (200 μg/kg/day-treatment lineage) |
| Crhr1 | NC | NC |
| Crhr2 | NC | NC |
| Mc4r | NC | NC |
| Drd1 |
Up-regulated (20 μg/kg/day- and 750 mg/kg/day-treatment lineages) |
NC |
| Drd2 | NC |
Down-regulated (20 μg/kg/day- and 750 mg/kg/day-treatment lineages) |
| Dnmt1 | NC | NC |
| Dnmt3a | NC | NC |
| Dnmt3b | NC | NC |
| Dnmt3l | NC | NC |
| Mecp2 | NC | NC |
3.2.2. Relative expression of genes in the amygdala
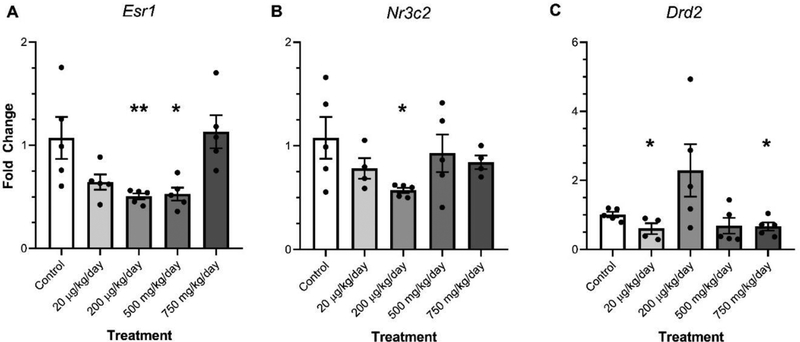
Transgenerational DEHP exposure resulted in increases and decreases in the relative expression of specific genes in the amygdala of both female and males. In females, transgenerational DEHP significantly decreased the expression of Esr1 in the amygdala (200 μg/kg/day and 500 mg/kg/day) relative to control (Fig. 3A, p≤0.05). Additionally, transgenerational DEHP exposure significantly decreased the expression of Nr3c2 in the amygdala of 200 μg/kg/day females relative to control females (Fig. 3B, p≤0.05). Transgenerational DEHP significantly decreased the expression of Drd2 in the amygdala of the 750 mg/kg/day treatment lineage females (p≤0.05) and significantly decreased the expression of Drd2 in the amygdala of the 20 μg/kg/day (p≤0.050) treatment lineage females relative to control (Fig. 3C).
Figure 3.
The effect of ancestral DEHP exposure on gene expression in the amygdala of F3 females. The relative expression of Estrogen receptor 1 (Esr1) (A), Mineralocorticoid receptor (Nr3c2) (B), and dopamine receptor D2 (Drd2) (C) are shown for the F3 generation females. Data are representative of the means (± SEM). Control n=5, 4, and 4 respectively; 20 μg/kg/day n=5 for all; 200 μg/kg/day n=5, 5, and 4 respectively; 500 mg/kg/day n=5 for all; and 750 mg/kg/day n=5, 4, and 5 respectively for all analyses. **p<0.01, *p≤0.05 (significant difference compared with the control).
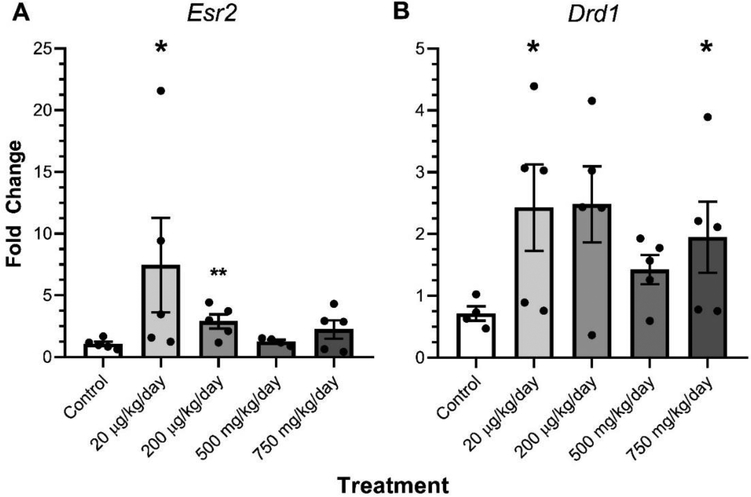
In males, transgenerational DEHP exposure significantly increased the expression of the Esr2 in the amygdala (20 μg/kg/day and 200 μg/kg/day) relative to control (Fig. 4A, p≤0.05). Additionally, transgenerational DEHP exposure significantly increased the expression of the Drd1 (20 μg/kg/day and 750 mg/kg/day) relative to control males (Fig. 4B, p≤0.05). Out of the other 15 genes analyzed for relative expression changes, no other genes exhibited any significant changes in the DEHP treatment lineages relative to their respective female and male controls (Table 2).
Figure 4.
The effect of ancestral DEHP exposure on gene expression in the amygdala of F3 males. The relative expression of Estrogen receptor 2 (Esr2) (A) and dopamine receptor D1 (Drd1) (B) are shown for the F3 generation females. Data are representative of the means (± SEM). Control n=5; 20 μg/kg/day n=5; 200 μg/kg/day n=5 for all; 500 mg/kg/day n=4 and 5 respectively; and 750 mg/kg/day n=5 for all analyses. **p=0.016, *p≤0.05 (significant difference compared with the control).
4. Discussion
We tested the hypotheses that DEHP exposure transgenerationally alters anxiety like behavior and gene expression in the hippocampus and amygdala. We found that ancestral DEHP exposure had a sex-specific impact on anxiety-like behavior in the F3 generation; it decreased anxiety in high-dose lineage adult females but not in adult males in any treatment lineage. Further, we found that genes relevant to anxiety (Esr1, Esr2, Nr3c2, Drd1, and Drd2) were either up- or down-regulated in the amygdala in a dose- and sex-specific manner. Importantly, we not only observed changes in amygdala gene expression in our high dose lineages, but also in our lineages treated with environmentally relevant doses. We found no significant effect of DEHP exposure on gene expression in the hippocampus of either males or females, suggesting a brain region-specific effect of DEHP on our target genes. All findings from this study are summarized in Table 2.
The change in anxiety-like behavior we observed was only present in the highest dose-lineage in the females; therefore, the environmental relevance of this dose must be considered when interpreting the results. Further, this behavior alteration likely does not indicate a therapeutic effect, but a maladaptive anxiety-like behavior or hyperactivity. Additionally, we did not observe any behavioral changes in our males, which could be due to differences in circulating endogenous hormones (e.g. sex steroid hormones, corticosterone), differential disruption of these hormones in response to ancestral DEHP exposure, or differences in responsiveness of the brain to these circulating hormones. We also found no sexually dimorphic behavior between control males and females, consistent with previous work showing no sex differences in EPM performance between 12-week-old male and female CD-1 mice [47].
Previous studies by Quinnies et al. reported no effect on anxiety-like behavior in a transgenerational model of DEHP exposure; however, our study found that DEHP decreases anxiety-like behavior in our 750 mg/kg/day dose lineage F3 females. When we were completing our experiments, Quinnies et al. published that transgenerational DEHP exposure (200 mg/kg/day dose lineage) does not modify performance in the EPM in F3 generation juvenile male and female mice [17]. A second study released in 2017 from Quinnies et al. using lower doses (5, 40, 400 μg/kg/day dose lineages) found that juvenile F1 male and female mice spent more time in the closed arms of the EPM relative to F1 controls [18]. However, they did not observe any effect of DEHP lineage on anxiety-like behavior changes in F3 juveniles in males or females, whereas we did observe changes in F3 adult females. It is possible that neural and hormonal changes that occur during the pubertal period account for the differences observed in the two studies. Further, we did not assess the same doses and our window of exposure was GD 10.5 until birth, whereas Quinnies et al. used GD 7–14 for their first study and GD 0 until PND 10 in their second study.
No change (NC). Estrogen receptor 1 (Esr1). Estrogen receptor 2 (Esr2). Androgen receptor (Ar). Glucocorticoid receptor (Nr3c1). Mineralocorticoid receptor (Nr3c2). Corticotrophin releasing hormone receptor 1 (Crhr1). Corticotrophin releasing hormone receptor 2 (Crhr2). Melanocortin receptor (Mc4r). Dopamine receptor D1 (Drd1). Dopamine receptor D2 (Drd2). DNA methyltransferase 1 (Dnmt1). DNA methyltransferase 3a (Dnmt3a). DNA methyltransferase 3b (Dnmt3b). DNA methyltransferase 3l (Dnmt3l). Methyl-CpG binding protein 2 (Mecp2).
Studies with either prenatal or perinatal exposure to DEHP found that direct exposure increases anxiety-like behavior in adult animals [7, 16, 48]. Importantly, other studies using transgenerational models of EDC exposure have found that the phenotype observed in one generation may be different or opposite of other generations [13, 17, 18, 28]. These differing phenotypes are likely due to the mechanisms by which each generation is exposed to the compound. Alterations in behavior and gene expression in the F1 generation (i.e. prenatal or perinatal exposure) are due, in part, to direct actions of the compound on the developing brain, whereas alterations in the F2 generation are due to modifications in the F1 germ cell genome and/or epigenome [49]. However, transgenerational effects observed in the F3 and subsequent generations are due to the meiotic transmission of changes in the genome or epigenome from the F2 generation [49].
We also observed changes in expression of genes in the amygdala shown to be crucial for regulating anxiety-like behaviors in rodents, including estrogen receptors, dopamine receptors, and mineralocorticoid receptors. For example, animals with Esr1 knocked out in the amygdala made more entries into and spent more time in the light chamber compared to the control animals in a light:dark box task, behavior that indicates decreased anxiety [50]. Conversely, Esr2 knockout female mice, but not males, exhibited decreased time spent in the open arms on the elevated plus maze, indicating increased anxiety-like behavior [51]. Injection of the D1 receptor agonist SKF38393 into the basolateral amygdala of rats resulted in an anxiogenic effect in the EPM [52]. Results from D2 manipulation studies are variable, with anxiogenic or anxiolytic effects observed depending on the behavior test, injection site, dose, and chemical used [41]. Additionally, a single postnatal exposure to different types of endocrine disruptors, including dibutyl phthalate (DBP) and DEHP, modifies Drd1 and Drd2 mRNA expression in the midbrain of male rats [53]. Furthermore, overexpression of mineralocorticoid receptor in the basolateral amygdala of male rats increases anxietylike behavior in the EPM and the open field test [43]. Collectively, these studies show that the genes altered in our model (Esr1, Esr2, Drd1, Drd2, and Nr3c2) are also involved in regulating anxiety-like behaviors, implicating potential neural targets responsible for altered behavior.
Most of the observed changes in gene expression were seen at doses different than those in which we observed the changes in behavior. This discrepancy suggests that the majority of the observed gene changes are not related to the behavioral changes we measured. Importantly, endocrine disrupting chemicals (EDCs), including DEHP, have been shown to exhibit both low-dose and non-monotonic (i.e. non-linear) dose effects (reviewed in [54]), possibly by different mechanisms of action at each dose. EDCs mimic endogenous hormones, and therefore at low doses can act by binding to hormone receptors similarly to endogenous ligands [54–56]. Although transgenerational exposure to phthalates has been shown to have both low-dose and non-monotonic effects, the mechanism for these effects is still largely understudied [13, 28].
Several strengths to our study, as well as areas of improvement in future studies, must be noted. We used a range of doses that includes human-relevant exposures. Further, we were able to use both sexes to show the sex-dependent impact of DEHP exposure on behavior and the brain. Finally, to our knowledge, we are the first to identify transgenerational impacts of DEHP on gene expression in the brain. Notably, we did not stage the females for estrous cyclicity, which could have contributed to the high variability [57]. Further, it is possible that there are other behaviors not investigated in our study that are altered, as we were limited to only performing EPM observations.
5. Conclusion
Overall, we found that transgenerational exposure to DEHP decreased anxiety-like behavior in females, but not males, and modified gene expression in the amygdala, but not the hippocampus, of both male and female mice. Our data are among the first to indicate that DEHP is capable of transgenerationally modulating gene expression in the brain, future work should investigate the mechanisms underlying the observed effects in transgenerational DEHP exposure models.
Highlights.
DEHP decreased anxiety in the high-dose lineage in F3 females, but not males
DEHP down-regulated Esr1, Nr3c2, and Drd2 expression in the F3 female amygdala
DEHP up-regulated Esr2 and Drd1 expression in the F3 male amygdala
DEHP did not alter gene expression in the hippocampus of F3 males and females
Acknowledgments
We would like to thank The Interdisciplinary Environmental Toxicology Program (NIH T32 ES007326) for training support of JW, SR, and CC. We would also like to thank the Interdisciplinary Environmental Toxicology Program (KMH) and the Billie A. Field Fellowship in Reproductive Biology (KMH, SR, and CC) at the University of Illinois at Urbana-Champaign for fellowship support
Funding
This work was supported by the National Institutes of Health (NIH P01 ES 022848 and NIH T32 ES007326) and Environmental Protection Agency (EPA RD83 543401) to JAF. This work was also supported by funding from the Department of Comparative Biosciences at the College of Veterinary Medicine at the University of Illinois at Urbana-Champaign to MMM.
Abbreviations
- DEHP
Di-(2-ethylhexyl) phthalate
- NMDA
N-methyl-D-aspartate
- EPM
Elevated plus maze
- ERK1/2
Extracellular signal-regulating protein kinases 1 and 2
- GD
Gestational Day
- PND
Postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors have no conflicts of interest to declare with respect to the research, authorship, and/or publication of this research.
References
- 1.ATSDR, Toxicological Profile for Di(2-ethylhexyl) phthalate, U.S.D.o.H.a.H. Services, Editor. 2002. [Google Scholar]
- 2.Heudorf U, Mersch-Sundermann V, and Angerer J, Phthalates: toxicology and exposure. Int J Hyg Environ Health, 2007. 210(5): p. 623–34. [DOI] [PubMed] [Google Scholar]
- 3.Lin S, Ku HY, Su PH, Chen JW, Huang PC, Angerer J, and Wang SL, Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere, 2011. 82(7): p. 947–55. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, Somerville SE, and Kucklick JR, Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere, 2018. 193: p. 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svechnikova I, Svechnikov K, and Soder O, The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol, 2007. 194(3): p. 603–9. [DOI] [PubMed] [Google Scholar]
- 6.Culty M, Thuillier R, Li W, Wang Y, Martinez-Arguelles DB, Benjamin CG, Triantafilou KM, Zirkin BR, and Papadopoulos V, In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol Reprod, 2008. 78(6): p. 1018–28. [DOI] [PubMed] [Google Scholar]
- 7.Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, Reynoso R, Scacchi P, Moguilevsky JA, and Cutrera R, Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav, 2013. 63(5): p. 692–9. [DOI] [PubMed] [Google Scholar]
- 8.Niermann S, Rattan S, Brehm E, and Flaws JA, Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol, 2015. 53: p. 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, Monfort N, Ventura R, Sunyer J, and Vrijheid M, Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health, 2015. 218(6): p. 550–8. [DOI] [PubMed] [Google Scholar]
- 10.Pocar P, Fiandanese N, Berrini A, Secchi C, and Borromeo V, Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol Appl Pharmacol, 2017. 322: p. 113–121. [DOI] [PubMed] [Google Scholar]
- 11.Messerlian C, Bellinger D, Minguez-Alarcon L, Romano ME, Ford JB, Williams PL, Calafat AM, Hauser R, and Braun JM, Paternal and maternal preconception urinary phthalate metabolite concentrations and child behavior. Environ Res, 2017. 158: p. 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barakat R, Lin PC, Park CJ, Best-Popescu C, Bakry HH, Abosalem ME, Abdelaleem NM, Flaws JA, and Ko C, Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice. Toxicol Sci, 2018. 164(2): p. 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rattan S, Brehm E, Gao L, Niermann S, and Flaws JA, Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod, 2018. 98(1): p. 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, and Webster TF, Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health, 2008. 7: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Xu X, and Zhu Q, Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere, 2016. 144: p. 1771–9. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Yang Y, Wang R, Wang Y, Ruan Q, and Lu Y, Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere, 2015. 124: p. 22–31. [DOI] [PubMed] [Google Scholar]
- 17.Quinnies KM, Doyle TJ, Kim KH, and Rissman EF, Transgenerational Effects of Di-(2-Ethylhexyl) Phthalate (DEHP) on Stress Hormones and Behavior. Endocrinology, 2015. 156(9): p. 3077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinnies KM, Harris EP, Snyder RW, Sumner SS, and Rissman EF, Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One, 2017. 12(2): p. e0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barakat R, Lin PC, Park CJ, Best-Popescu C, Bakery HH, Abosalum ME, Abdelaleem NM, Flaws JA, and Ko C, Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice. Toxicol Sci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Y, Yang Y, Xu X, and Hu Y, Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm Behav, 2015. 71: p. 41–8. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero-Bosagna C, Settles M, Lucker B, and Skinner MK, Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One, 2010. 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner MK, Anway MD, Savenkova MI, Gore AC, and Crews D, Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One, 2008. 3(11): p. e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck D, Sadler-Riggleman I, and Skinner MK, Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ Epigenet, 2017. 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gore AC, Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol, 2008. 29(3): p. 358–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasca C, Bigio B, Zelli D, Nicoletti F, and McEwen BS, Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry, 2015. 20(6): p. 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunton PJ, Donadio MV, and Russell JA, Sex differences in prenatally programmed anxiety behaviour in rats: differential corticotropin-releasing hormone receptor mRNA expression in the amygdaloid complex. Stress, 2011. 14(6): p. 634–43. [DOI] [PubMed] [Google Scholar]
- 27.Doyle TJ, Bowman JL, Windell VL, McLean DJ, and Kim KH, Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod, 2013. 88(5): p. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brehm E, Rattan S, Gao L, and Flaws JA, Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 2018. 159(2): p. 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C, Gao L, and Flaws JA, Exposure to an Environmentally Relevant Phthalate Mixture Causes Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 2017. 158(6): p. 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoon GG and Tye KM, Resolving the neural circuits of anxiety. Nat Neurosci, 2015. 18(10): p. 1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, and Taylor JA, Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol, 2012. 34(4): p. 614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis BJ, Maronpot RR, and Heindel JJ, Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicology and Applied Pharmacology, 1994. 128: p. 216–223. [DOI] [PubMed] [Google Scholar]
- 33.Hannon PR, Peretz J, and Flaws JA, Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod, 2014. 90(6): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirshfield AN, Development of Follicles in the Mammalian Ovary. 1991. 124: p. 43–101. [DOI] [PubMed] [Google Scholar]
- 35.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA and Jones AR, Genome-wide atlas of gene expression in the adult mouse brain. Nature, 2007. 445(7124): p. 168–76. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, and Patisaul HB, Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci, 2013. 133(1): p. 157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, and Gore AC, Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One, 2012. 7(9): p. e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaki S and Okuyama S, Involvement of melanocortin-4 receptor in anxiety and depression. Peptides, 2005. 26(10): p. 1952–64. [DOI] [PubMed] [Google Scholar]
- 39.Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, and Hill MN, Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci, 2015. 35(9): p. 3879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel SE and Rainnie DG, Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology, 2016. 41(1): p. 103–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarrindast M-R and Khakpai F, The Modulatory Role of Dopamine in Anxiety-like Behavior. Archives of Iranian Medicine, 2015. 18(9): p. 591–603. [PubMed] [Google Scholar]
- 42.Weiser MJ, Foradori CD, and Handa RJ, Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res, 2010. 1336: p. 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra R, Ferguson D, and Sapolsky RM, Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry, 2009. 66(7): p. 686–90. [DOI] [PubMed] [Google Scholar]
- 44.Weiser MJ, Goel N, Sandau US, Bale TL, and Handa RJ, Androgen regulation of corticotropin-releasing hormone receptor 2 (CRHR2) mRNA expression and receptor binding in the rat brain. Exp Neurol, 2008. 214(1): p. 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betz A, Jayatilaka S, Joshi J, Ramanan S, DeBartolo D, Pylypiw H, and Franke E, Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats-Alterations in amygdalar MeCP2, ERK1/2 and Erα. Neuroendocrinology Letters, 2013. 34(5): p. 347–358. [PubMed] [Google Scholar]
- 46.Anway MD, Rekow SS, and Skinner MK, Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics, 2008. 91(1): p. 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aujnarain AB, Luo OD, Taylor N, Lai JKY, and Foster JA, Effects of exercise and enrichment on behaviour in CD-1 mice. Behav Brain Res, 2018. 342: p. 43–50. [DOI] [PubMed] [Google Scholar]
- 48.Wang DC, Chen TJ, Lin ML, Jhong YC, and Chen SC, Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm Behav, 2014. 66(4): p. 674–84. [DOI] [PubMed] [Google Scholar]
- 49.Rissman EF and Adli M, Minireview: transgenerational epigenetic inheritance: focus on endocrine disrupting compounds. Endocrinology, 2014. 155(8): p. 2770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, and Agmo A, The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res, 2010. 210(2): p. 211–20. [DOI] [PubMed] [Google Scholar]
- 51.Wojciech K, Dupont S, Krust A, Chambon P, and Chapman PF, Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. PNAS, 2001. 98(21): p. 12276–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bananej M, Karimi-Sori A, Zarrindast MR, and Ahmadi S, D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J Psychopharmacol, 2012. 26(4): p. 564–74. [DOI] [PubMed] [Google Scholar]
- 53.Ishido M, Morita M, Oka S, and Masuo Y, Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul Pept, 2005. 126(1–2): p. 145–53. [DOI] [PubMed] [Google Scholar]
- 54.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, and Myers JP, Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev, 2012. 33(3): p. 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta C, Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med, 2000. 224(2): p. 61–8. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DM, Activity of environmentally relevant low doses of endocrine disruptors and the bisphenol A controversy: initial results confirmed. Proc Soc Exp Biol Med, 2000. 224(2): p. 57–60. [DOI] [PubMed] [Google Scholar]
- 57.D’Souza D and Sadananda M, Estrous Cycle Phase-Dependent Changes in Anxiety- and Depression-Like Profiles in the Late Adolescent Wistar-Kyoto Rat. Ann Neurosci, 2017. 24(3): p. 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]