Abstract
Systemic sclerosis (SSc, scleroderma) is a complex multisystem disease characterized by autoimmunity, vasculopathy, and most notably, fibrosis. Multiple lines of evidence demonstrate a variety of emerging cellular and molecular pathways which are relevant to fibrosis in SSc. The myofibroblast remains the key effector cell in SSc. Understanding the development, differentiation, and function of the myofibroblast is therefore crucial to understanding the fibrotic phenotype of SSc. Studies now show that 1) multiple cell types give rise to myofibroblasts, 2) fibroblasts and myofibroblasts are heterogeneous, and 3) that a large number of (primarily immune) cells have important influences on the transition of fibroblasts to an activated myofibroblasts. In SSc, this differentiation process involves multiple pathways, including well known signaling cascades such as TGF-β and Wnt/β-Catenin signaling, as well as epigenetic reprogramming and a number of more recently defined cellular pathways. After reviewing the major and emerging cellular and molecular mechanisms underlying SSc, this article looks to identify clinical applications where this new molecular knowledge may allow for targeted treatment and personalized medicine approaches.
Keywords: Scleroderma, systemic sclerosis, fibrosis, fibroblast, myofibroblast, skin fibrosis, mesenchymal cells, epigenetics, innate immunity, cell signaling pathways, TGF-β, personalized medicine
Introduction:
The Myofibroblast as the Key Effector Cell in SSc
Systemic sclerosis (SSc, scleroderma) is a complex multisystem disease characterized by autoimmunity, vasculopathy, and most notably, fibrosis of the skin and multiple internal organs. Fibrosis can occur both early and late in SSc, has a variable clinical progression, and generally responds poorly to therapy. Histologic studies of the skin, lungs, heart, and other organs in scleroderma demonstrate increased numbers of fibroblasts but are usually most notable for the presence of dense deposits of collagen and extracellular matrix. The fibrotic process relies on the overactivation, reprogramming, and loss of normal homeostatic properties in the fibroblast, leading to their trans-differentiation into proliferative and metabolically active myofibroblasts in peripheral tissue.
Fibrosis can occur in nearly all organs in SSc, but is noted most prominently in the skin, lung, heart, and GI tract. The primary hallmark of disease, skin fibrosis typically manifests as skin thickening and induration and can occur in either a limited (typically skin distal to the elbows and knees) or diffuse (more aggressive and widespread) pattern[1]. In patients with limited skin disease, the course of fibrosis is typically benign and does not cause significant disability, although these patients often suffer from more vascular complications. In the diffuse subset of patients, skin fibrosis tends to rapidly accelerate in the first five years of clinical disease and other organ fibrosis typically begins within this same window. There is radiographic evidence of lung fibrosis in upwards of 90% of SSc patients, and it becomes clinically significant in 25% of patients[2]. In comparison to skin fibrosis where the course typically stops worsening and improves after an initial period, lung fibrosis in SSc is typically progressive.
Even though the outcome may be straightforward, these processes are multi-factorial and likely arise from a combination of cellular, biochemical, and mechanical forces acting on the fibroblast. While the inciting events that trigger scleroderma are likely related to immune dysregulation, the activated myofibroblast remains the critical effector cell that drives the fibrotic phenotype in SSc. Therefore, understanding the influences that lead to its dysfunction are critical to understanding both the relentlessness and persistence of fibrosis in SSc, and identifying novel strategies to reverse this process.
Part 1: The Making of a Scleroderma Myofibroblast
Fibroblasts are mesenchymal-derived matrix-producing cells which are normally not highly metabolically active, but which have the ability to become activated, differentiate into myofibroblasts, and to produce matrix in response to tissue injury. Myofibroblasts regulate connective tissue remodeling by both participating in the extracellular matrix synthesis and also demonstrating cytoskeletal characteristics of contractile smooth muscle cells. Myofibroblasts contribute significantly to connective tissue remodeling by exerting traction forces and synthesizing extracellular matrix (ECM) components. They regress or die by apoptosis on wound epithelialization, but persist in fibrotic situations like SSc where they are key drivers in a persistent cycle of organ damage [3]. A number of important questions about the SSc fibroblast remain as to why it has a characteristically fibrotic phenotype. These questions include where myofibroblasts come from, how fibroblasts are activated, what keeps fibroblasts persistently activated, the specific types of fibroblasts most relevant to SSc, and the differences between fibroblasts in different organs.
Origin of SSc Myofibroblasts
While fibroblasts represent resident cells in the skin, lung, and other sites of scleroderma pathology, in SSc there is an increased number of fibroblasts and activated myofibroblasts in the fibrotic tissue. The origin of these cells has remained a source of debate and is likely multi-factorial, but this question is important as it gives clues regarding the initiating factors that lead to their recruitment.
As a heterogeneous mesenchymal cell population, fibroblasts are typically identified morphologically as spindle-shaped cells. Molecularly, a number of markers including collagens, CD90, and FSP-1 have been described as fibroblast-specific, yet none of them are entirely lineage specific and therefore tracing the origin of fibroblasts and myofibroblasts in SSc and other fibrotic disorders has proven somewhat elusive. In the skin, the origin of the fibroblast appears to be diverse with contributions from resident mesenchymal stem cells and to a lesser degree circulating fibrocytes[4].
The origin of myofibroblasts is also highly controversial, and there is a lot of evidence to support contributions from multiple cellular sources. The most well-defined pathway for myofibroblast differentiation is activation of resident fibroblasts in response to TGF-β and other mitogenic stimuli. In SSc, there is a significant inflammatory milieu which contributes directly to myofibroblast differentiation. Mouse studies suggest that the CD301+ macrophage microenvironment helps both resident fibroblasts and pre-adipocytes to become activated myofibroblasts in fibrosis [5].
A classic dogma in fibrosis, particularly in the lung, has been the epithelial to mesenchymal transition (EMT) [3], and there is now evidence in skin that epithelial cells (keratinocytes) can give rise to dermal myofibroblasts. Silencing of Friend leukemia virus integration 1(Fli-1) in keratinocytes led mice to develop dermal and esophageal fibrosis via EMT with epithelial activation, autoimmunity, and interstitial lung disease (ILD) [6].
In addition to the epithelial contributions, multiple studies in human and mouse models of SSc suggest that other cells contribute substantially to the myofibroblast pool. It is well known that endothelial cells are frequently injured in SSc, and can undergo a process whereby they become activated myofibroblasts in a process known as Endo-MT [7]. Specifically in the pulmonary tissue, cells co-expressing CD31 and CD102 indicating simultaneous mesenchymal and endothelial specific expression have been found in SSc-ILD, suggesting that EndoMT actively occurs in SSc lung [8].
Similarly, it is thought that in SSc, monocytes may be preferentially driven to differentiate towards fibrocytes. Fibrocytes are circulating cells which can hone to tissue and take on fibroblast-like functions. Monocytes from African Americans SSc patients with ILD have been shown to have low levels of caveolin-1, leading to preferential fibrocyte differentiation [9].
Moreover, adipose tissue has been found to be a significant contributor of fibroblastic and myofibroblastic cells. Recent animal work has demonstrated that adiponectin+ mature adipocytes can transdifferentiate into activated myofibroblasts [10], and that adipose precursor cells represent the most common precursor of myofibroblasts in skin fibrosis [5].
Skin Fibroblast Heterogeneity
Multiple sub-populations of fibroblastic cells with different markers and functions exist. Elegant lineage tracing studies in mice have demonstrated that skin fibroblasts arise from two distinct lineages: the first (CD26+ Sca1–) forms the upper dermis, and the second (Dlk1–, Sca1+) forms the lower dermis, including the reticular fibroblasts and the pre-adipocyte components of skin [11]. One fibroblast lineage, the CD26+ DPP4+ population, appears to be responsible for the bulk of connective tissue deposition during embryonic development, cutaneous wound healing, radiation fibrosis, and cancer stroma formation [12]. In both bleomycin-induced fibrosis and wound healing, activated myofibroblasts appear to be derived from two populations of adipocyte precursors cells: CD26+ cells and CD29High cells with both overlapping and distinct contributions to fibrosis and repair [5].
In humans, recent work using single-cell RNA sequencing has demonstrated that multiple types of resident fibroblasts with different expression profiles exist in the skin [13, 14]. These profiles match both the different dermal niches [14] as well as representing novel populations of cells of unknown function [14, 15]. Cells genetically defined by SFRP2 and FMO1 represent two major novel fibroblast populations. SFRP2 fibroblasts are small, elongated, and distributed between collagen bundles, whereas FMO1 fibroblasts are larger and distributed in both interstitial and perivascular locations [15].
The functional implications of these fibroblast subsets is not yet known, but similar studies in rheumatoid arthritis have identified that some of the fibroblast populations are intrinsically pathogenic [16]. A similar understanding in SSc would represent a significant advance.
Epigenetic Programming of SSc Fibroblasts
Multiple studies have shown an emerging role for epigenetic programming as a key determinant of fibroblast function. Epigenetic alterations such as DNA methylation and histone modification are thought to be triggered by persistent inflammation and profibrotic cytokine signaling, leading to myofibroblast reprograming [17].
SSc fibroblasts demonstrate global alterations in methylation, particularly the hypomethylation of genes including ITGA9, ADAM12, COL23A1, COL4A2 MYO1E, and the RUNX family of transcription factors [18]. Functionally, epigenetic alteration of histone demethylases such as MeCP2 and JMJD3 regulate epigenetic modifications on transcription factor promoters and determine fibroblasts’ fibrogenic potential [19, 20]. The Wnt pathway genes DKK1 and SFRP1 are also hypomethylated in both SSc fibroblasts and peripheral blood mononuclear cells (PBMCs). Treatment to induce hypermethylation effectively inhibits Wnt signaling and reduces experimental skin fibrosis [21].
Another histone modifier, the deacetylase SIRT1, attenuates fibrotic responses in skin fibroblasts due to decreased expression and function of the acetyltransferase p300 which has also shown to have a key anti-fibrotic role [22, 23].
Finally, Fli-1 is a major epigenetic regulator of fibrosis. Epigenetically silenced due to hypermethylation in SSc, Fli-1 and Klf5 modulate fibroblast function. Intriguingly, mice with a double heterozygous deficiency of Klf5 and Fli1 mimicking the epigenetic phenotype of SSc skin demonstrate fibrosis, vasculopathy of the skin and lung, B cell activation and autoantibody production [24, 25]. More recently, Fli-1 deficiency also has been shown to promote epithelial mesenchymal transition of keratinocytes [6] as well as to induce a suppression of Th1 cytokine production via Galectin-9 [26].
Part 2: Fibroblast interactions with other cells
Innate Immune cells: Monocytes, pDCs, mast cells, and neutrophils
Myeloid cells are important effector cells in SSc and appear to play a significant role in maintaining a pro-fibrotic environment [27]. Indeed, sustained signaling through toll-like receptors and other innate immune pathways can lead to either inflammation or fibrosis, influencing both immune cells as well as fibroblasts [27].
Macrophages, as a key effector cell in SSc, are skewed in an M2 direction. The release of pro-fibrotic cytokines by M2 macrophages is mediated by phosphodiesterase 4 (PDE4) and leads to altered fibroblast activation and collagen release [28]. Another key molecule in macrophages is cadherin 11, which represents both an important cell surface marker on fibroblasts as well as a regulator of TGFβ production by macrophages [29]. Lung microarray data can differentiate patients with SSc-related ILD from healthy controls on the basis of macrophage markers, as well as genes related to chemokines, collagen, and TGF-β [30].
Mast cells similarly play an important and underappreciated role in SSc. Plasminogen activator inhibitor type 1 (PAI1) attracts mast cells into the skin and upregulates ICAM1 expression on dermal fibroblasts, facilitating fibroblast - mast cell binding and directly promoting fibrosis [31].
Plasmacytoid dendritic cells (pDCs) have emerged as a new key effector cell in SSc [32–35]. These cells produce high levels of interferon as well as CXCL4. Intriguingly, depletion of pDCs prevents fibrosis in a mouse model of scleroderma and can even revert fibrosis in mice with established disease [32].
Innate Immune Pathways: TLRs, IL-6, Inflammasomes/IL-1, interferon
Innate immune cell activation is one of the hallmarks of SSc, and recent work has shown that much of the activation of myofibroblasts by innate immune cells occurs in the context of toll-like receptors (TLRs). TLR4 appears to be a key driver of fibrosis in SSc. Clinical studies have demonstrated the elevation of a TLR4-responsive gene signature in skin biopsies. Similarly, these studies have also shown that blockade of TLR4 signaling leads to myofibroblast transformation and matrix remodeling, thereby preventing and reversing experimental fibrosis [36]. The TLR4 ligand in SSc appears not to be a pathogen, but rather the alternatively spliced proteins tenascin-C and fibronectin-EDA (Fn-EDA). Exogenous tenascin-C stimulates collagen gene expression and myofibroblast transformation via TLR4 signaling, and mice lacking tenascin-C have reduced skin fibrosis [37]. Fn-EDA is an endogenous TLR4 ligand which is markedly elevated in the circulation and in lesional skin biopsies in both SSc and in experimental cutaneous fibrosis. Both genetic loss of Fn-EDA and TLR4 blockade using small molecules mitigates cutaneous fibrosis in mice [38].
In addition to TLR4, SSc patients also have high levels of skin TLR9 with elevated pathway activation, and mechanistic studies show that TLR9 dependent fibrosis is mediated through the action of endogenous TGF-β [39]. pDCs also modulate skin fibrosis via TLR8 and, to a lesser degree, TLR7 [32].
Downstream from TLRs, the pro-inflammatory cytokines IL-1 and IL-6 are thought to be key effectors of innate immune pathways in SSc. In the IL-1 pathway, SSc fibroblasts demonstrate increased expression of inflammosome signaling molecules, and mice deficient in NLR Family Pyrin Domain Containing 3 (NLRP3) and Apoptosis-Associated Speck-Like Protein Containing (ASC) are protected from bleomycin induced fibrosis [40].
IL-6 has been shown to have pro-fibrotic properties, acting on myofibroblasts via IL-6 receptor signaling [41]. In SSc fibroblasts derived from patients treated with anti-IL-6 therapy (tocilizumab), treatment altered the biological characteristics of explant dermal fibroblasts, normalizing functional properties, and reversing TGF-β-regulated molecular pathways which were present prior to treatment [42]. IL-6 also appears to play a role in macrophage polarization toward the M2 phenotype, explaining the antifibrotic effects of phosphodiesterase 4 (PDE4) inhibition [28].
Expression of both autotaxin (ATX) and IL-6 are increased at a basal level in SSc skin. With the added exacerbation of L lysophosphatidic acid (LPA), induced levels of IL-6 are disproportionately increased in fibroblasts from SSc patients. IL-6, in turn, stimulates fibroblast expression of ATX [43].
Like lupus and other autoimmune diseases, a significant subset of SSc patients have been shown to demonstrate a type 1 interferon signature [44, 45], and SSc patients additionally been shown to have genetic alterations in interferon pathways [46]. An interferon signature is present in early disease even prior to onset of skin fibrosis, and the type I interferon signature in monocytes correlates with B-cell activating factor (BAFF) expression and serum amino terminal propeptide of type III procollagen (PIIINP) levels [47]. In patient with active SSc-ILD, interferon genes in the lung are also strongly associated with disease activity/severity [30].
The Adaptive Immune system: Lymphocytes in SSc
SSc patients do not have altered numbers of lymphocytes, but do demonstrate populations of pathogenic B and T cells. Patients have increased numbers of activated B cells, and these cells show a propensity to produce both IL-6 and TGF-β, and can activate fibroblasts in vitro [48]. Moreover, SSc B cells produce large numbers of autoantibodies. Anti-centromere, anti-Scl-70 and anti-RNA polymerase III antibodies represent the most well-defined and prognostic biomarkers in SSc. In addition, there are another class of antibodies which are not clinically assayed, but which may be functionally contributing to fibrosis. These include anti-fibroblast, anti-fibrillarin, anti-PDGFR, and anti-MMP antibodies. All of these have been shown to directly play a role in clinical fibrosis, and may represent an under-appreciated humoral immune component to the pathogenesis of SSc [49]. A novel B cell population that may also play a role in SSc is the Breg, a regulatory cell subset that produces IL-10 and plays a role in preventing autoimmunity. Interesting, SSc patients have reductions in Breg cells, increased CD19 expression, impaired IL-10 production in response to TLR9, and impaired STAT3 signaling [50]. In mice with B cell-specific deficiency of IL-6 or IL-10, BAFF has been shown to regulate skin and lung fibrosis. BAFF increased B effector cells but suppresses B regulatory cells and suggest that BAFF inhibition is a potential therapeutic strategy for SSc via alteration of B cell balance [51].
Along with the changes in humoral immunity, T cell populations are also found to be dysregulated in SSc. Effector T cells in SSc are thought to be skewed in a Th2 pattern. CD8+CD28– T cells are increased in the blood and affected skin of SSc patients, their levels correlate with the extent of skin fibrosis, they preferentially produce IL-13, and they therefore appear to represent a pathogenic T-cell subset in SSc and likely play a critical role in the early stage of SSc skin disease [52]. Myofibroblasts can maintain the Th2 state by suppressing interferon-gamma expression of skin-infiltrating CD4(+) T cells through galectin-9 overproduction, thereby negatively regulating Th1/Th17 populations [26].
IL-13 is another dysregulated cytokine in SSc which plays a key role in driving SSc toward a Th2 phenotype, particularly CD8+ T cells which are found in the skin in early phases of SSc [53]. Thymic stromal lymphopoietin (TSLP) is an IL-7 cytokine family member which is highly produced by epithelial cells at barrier surfaces and which regulates immune cells such as dendritic cells, T cells, B cells, and granulocytes. TSLP is highly expressed in the skin of dcSSc patients and correlates with TGF-β, interferon, and IL-13 [26]. Elevated levels of TSLP have been shown in SSc patients, and these levels are associated both with vascular and fibrotic outcomes [54].
Innate lymphoid cells (ILCs) are a recently described heterogeneous group of lymphocytic cells with diverse functions [55]. While ILC diversity has not been extensively profiled in SSc as has been done in other autoimmune diseases, one study showed that CD4(+) ILC1 and NKp44(+) group 3 ILC, but not CD4(−) ILC1 or group 2 ILC, are increased in the peripheral blood of SSc patients [56], but the relevance of this finding and studies examining ILCs in relevant fibrotic SSc tissue has yet to be performed.
Vascular system: Platelets and endothelial cells
Scleroderma is a disease with prominent vascular damage, and there is speculation that vascular injury may represent the primary event leading to SSc onset. Despite the evidence of significant vascular involvement, the mechanism which ties the vascular and fibrotic features of the disease together have been somewhat elusive.
The concurrent insults of vascular injury and matrix deposition in SSc give rise to vascular damage and tissue hypoxia, which also stimulates collagen cross-linking. Hypoxia can drive TGF-β signaling and hypoxia-inducible factor (HIF) to induce mesenchymal cells to differentiate toward myofibroblasts and thus contribute to the cycle of fibrogenesis.
Platelets are a major source of TGF-β, which is secreted from alpha granules upon activation, as well as producers of other pro-fibrotic mediators such as serotonin, PDGF and CXCL4 [44]. Platelets also produce TSLP and activate endothelial cells to make TSLP which plays a role in skin fibrosis, probably in an IL-1b–dependent manner [54]. Additionally, microparticles released from activated platelets are elevated in the blood of SSc patients and express the damage-associated molecular pattern (DAMP) HMGB1, leading to autophagy and the generation of neutrophil extracellular traps (NETs) in both patients and mouse models [57].
Endothelial cell damage and dysfunction plays a major role in SSc pathogenesis, but is largely thought to be involved in the vascular impairments seen in SSc. In addition to these roles which lead to vasoconstriction and phenotypes such as Raynaud’s phenomenon, digital ulcers, and pulmonary hypertension, endothelial cells also contribute to the generation of myofibroblasts. For example, SSc endothelial cells treated with CTGF have been found to recruit, activate, and make fibroblasts more capable of invasion [58].
Vascular Pathways: Hypoxia, VEGF, PDGF, and endothelin
Vascular endothelial growth factor (VEGF) levels are elevated in SSc patients and has traditionally been thought of as solely a mediator of vasculopathy in SSc. However, recent studies have shown that transgenic mice which over-express VEGF spontaneously develop skin fibrosis and have exacerbated fibrotic responses [59]. Moreover, VEGF is able to directly induce collagen synthesis in dermal fibroblasts suggesting that this angiogenic factor may also play a vital role in fibrotic responses.
PDGR-α is produced by platelets, and functions as an important mesenchymal mitogen. It plays an important role in skin fibrosis, and is overexpressed in SSc skin. Targeting PDFR-α with the novel small molecule crenolanib attenuates skin and cardiac fibrosis in an angiotensin-induced SSc model [60].
While endothelin’s role in SSc is traditionally thought of as participating in vascular remodeling, recent work has shown that dysfunction in endothelin in SSc can also lead to a pro-fibrotic phenotype in skin and lung, possibly through focal adhesion kinase (FAK) [61, 62].
Adipose pathways: PPAR-γ and AMT
The dermal adipose depot is an embryologically unique collection of adipose cells which are dysregulated in skin fibrosis. SSc patients and multiple animal models [10, 63] demonstrate decreased dermal white adipose tissue, and cell fate mapping studies have demonstrated that adiponectin-positive progenitors are able to transition from adipocytes to myofibroblasts in the fibrotic dermis in a process now deemed adipocyte mesenchymal transition (AMT) [10].
The adipocyte precursor cells which transition to myofibroblasts create a unique subset of myofibroblasts which are specifically maintained and activated by CD301b+ macrophages [5]. Intriguingly, while most adipocytes are lost in skin fibrosis, the cells which do not die by apoptosis are maintained by lymphotoxin-β+ plasmacytoid dendritic cells via integrin signaling [33].
The chief transcription factor which mediates adipocyte biology is PPAR-γ. PPAR-γ is down-regulated in SSc skin, and down-regulated in TGF-β signaling [64]. PPAR-γ also appears to be relevant in both adipose and fibroblastic cells, as treatment of fibroblasts with PPAR-γ agonists reduces fibrosis [65, 66] and loss of PPAR-γ repression in adipocytes is able to ameliorate skin fibrosis [67].
In addition to their role in cellular fate, adipocytes are also highly secretory cells producing a variety of adipokines. Chief among these circulating factors is adiponectin, which has potent anti-fibrotic properties. Moreover, the adiponectin pathway activity is dysregulated in SSc skin biopsies. Mice lacking adiponectin have increased dermal fibrosis. Furthermore, pharmacologic treatment with adiponectin mimetic peptides and use of transgenic mice with constitutively elevated adiponectin cause expansion of dermal fat layers and protection from skin fibrosis [68].
Epithelial cells: Role of Keratinocytes in SSc
While most focus in SSc skin fibrosis has been on the dermis and infiltrating dermal cells in the pathogenesis of fibrosis, gene expression studies consistently show altered keratinocyte biology [69, 70] in SSc patients, although this has been largely under-studied. Indeed, keratinocyte abnormalities can lead to fibrotic outcomes. For example, mice with keratinocytes lacking Fli-1 spontaneously developed dermal fibrosis with epithelial activation [6].
Keratinocytes can also function as secretory cells. SSc keratinocytes promote the activation of fibroblasts in a TGF-β-independent manner, have increased NF-kB and decreased PPAR-γ expression, leading to increased cytokine production [71]. Another key secretory product of keratinocytes is PAI-1. Snail-expressing keratinocytes secrete PAI1, which functions as a chemotactic factor to increase mast cell infiltration into the skin [31].
Part 3: Key Intracellular Pathways in SSc-Associated Fibrosis
Upon activation of the fibroblast/proto-myofibroblast by many of the cellular and paracrine mechanisms described in the last section, a number of key intracellular signaling pathways become activated in the scleroderma myofibroblast. The TGF-β pathway is the most well-known and probably most significant pathway in the induction and maintenance of fibrosis, but recent work has highlighted a large number of additional pathways, many of which are aberrant activation of development pathways, which are expressed in the myofibroblast and which can likewise participate in the maintenance of pro-fibrotic conditions and production of fibrotic extracellular matrix proteins.
The TGF-β Pathway
TGF-β represents the most significant growth factor related to the fibroblast to myofibroblast transition and maintenance of pathogenic fibrosis in SSc. TGF-β and Smad signaling and their multiple roles in SSc have been extensively reviewed elsewhere [72, 73] and will therefore be addressed here only briefly.
TGF-β signaling occurs when a TGF-β homodimer interacts with two type I and two type II receptors, and ligand binding initiates the phosphorylation of the receptor which becomes able to bind and phosphorylate Smad proteins, the central modulators of canonical TGF-β signaling. Upon phosphorylation, Smads translocate to the nucleus to regulate transcription of fibrotic genes. Despite a significant experimental work, the mechanisms underlying Smad-induced fibroblast activation are incompletely understood, as both activation and inhibition of Smads can promote fibrogenesis, depending on the context [74].
While it is well-established that both canonical (Smad-dependent) and non-canonical TGF-β signaling play a vital role in fibroblast function and the persistence of fibrotic phenotypes, new TGF-β mediators, such as PTP4A1, continue to be discovered. PTP4A1 is a protein tyrosine phosphatase which is highly expressed in SSc fibroblasts; it promotes TGF-β signaling through ERK activity and ultimately through SMAD3 [75].
While inhibition of TGF-β has long been an attractive therapeutic target, until recently this approach had not shown therapeutic efficacy. In a recent clinical trial, an anti-TGF-β monoclonal antibody, fresolimumab, demonstrated marked reduction of TGF-β responsive genes in patient skin and corresponding reductions in skin disease severity as measured by the Modified Rodnan Skin Score (MRSS) [76].
Developmental Pathways: Wnt, Hedgehog, Notch, and YAP/TAZ Signaling
A fundamental pathway in development, the Wnt/β-catenin pathway has been shown to play a vital role in fibrosis. For example, Wnt-3a induces β-catenin activation, stimulates fibroblast proliferation and migration, collagen gel contraction, myofibroblast differentiation, and enhances profibrotic gene expression via canonical TGF-β signaling [77]. In addition, Wnt-10b is increased in SSc skin biopsies and mice over-expressing Wnt-10b show progressive fibrosis with fibroblasts from these mice demonstrating TGF-β independent up-regulation of fibrotic gene expression [63].
Along with receptor signaling, the Wnt pathway is also regulated through hypomethylation of key pathway molecules such as DKK1 and SFRP1 [21]. Moreover, pharmacologic inhibition of Wnt has shown efficacy in mouse models [78] and topical application of Wnt inhibitors has shown changes in fibrotic gene expression in a clinical trial [79].
Other than the Wnt-β-catenin pathway, two other fundamental developmental signaling pathways which have also been implicated in fibrosis are the Hedgehog and Notch pathways. For example, sonic hedgehog (SHH) is highly expressed in SSc skin, and activation of SHH signaling induces an activated phenotype in cultured fibroblasts, differentiation of resting fibroblasts into myofibroblasts and increased collagen production. Mice with increased SHH signaling are more sensitive to dermal fibrosis [80]. Moreover, SSc patients have been found to have increased levels of Notch intracellular domain (NICD) while targeting of Notch signaling in a mouse model resulted in both prevention and regression of established experimental fibrosis [81].
The YAP/TAZ signaling pathway is another developmental pathway which has been implicated in the pathogenesis of other fibrotic diseases, and the capabilities of YAP and TAZ to regulate developmental processes due at least in part by its ability to function as a mechanical rheostat [74]. A recent study showed that therapy with dimethyl fumarate, a drug approved for multiple sclerosis, has anti-fibrotic efficacy in skin fibrosis and signals through the YAP/TAZ/Hippo pathway [82].
Beyond each of these pathways individually, there is great interest in the potential of targeting multiple fundamental signaling pathways in fibrotic disease. In the bleomycin and TGF-β adenovirus-induced models of fibrosis, combination therapy with Wnt and Hedgehog and Notch inhibitors has been shown to be both safe and to have anti-fibrotic efficacy [83].
AP-1 Transcription Factors
AP-1 transcription factors belong to the superfamily of basic- leucine zipper DNA-binding proteins. Under basal conditions cellular levels of most Fos and Jun transcription factors are low, and in some cells undetectable. In fibrosis, however, c-Jun is overexpressed in pathologic fibroblasts in SSc and other fibrotic diseases and appears to be mediated by pAkt and CD47 [84]. Fra-2, another AP-1 family transcription factor, is over-expressed in fibrosis leads to pulmonary artery hypertension with associated vascular fibrosis [84, 85]. Moreover, OX40L (TNFSF4) is up-regulated in SSc sera, has anti-fibrotic effects in the skin and lung, and appears to regulate inflammatory and myofibroblast infiltration through AP-1 [86].
JAK/STAT signaling
Another important intracellular signaling pathway which acts downstream of TGF-β is STAT3. STAT3 knockdown and pharmacological inactivation have both been shown to prevent TGF-β-induced differentiation of resting fibroblasts into myofibroblasts in vitro, and ameliorate TGF-β’s effect on collagen deposition. More specifically, fibroblast-specific knockout of STAT3 reduces bleomycin-induced skin experimental fibrosis [87]. Beyond its relevance to fibrosis, the JAK-STAT pathway has multiple roles in innate and adaptive immunity including modulation of Th1 and Th17 cells, production of proinflammatory cytokines, and cell metabolism including bioenergetics and mitochondrial function mediated by molecules such as IL-6 and oncostatin M, all of which may be relevant in SSc [88]. Along with previous genetic and functional evidence implicating STAT4 in SSc [89, 90], interest in targeting the JAK/STAT pathway is high given the recent success of JAK inhibitors in rheumatoid arthritis and other autoimmune diseases.
Integrin-Mediated Signaling
TGF-β’s downstream effects occur partially through integrin-mediated signaling. When a tissue is injured, binding of integrins along with increased mechanical forces cause release of TGF-β thus allowing it to engage its receptors.
A novel mouse model of skin fibrosis, in which mutations in fibrillin-1 matching patients with stiff skin syndrome were inserted into mice, demonstrates aggressive skin fibrosis. This fibrosis is marked by increased integrin expression and which is prevented by integrin-modulating therapy [34]. pDCs appear to play a key role in maintaining fibrotic conditions through integrin signaling [33, 34].
CTGF
Connective tissue growth factor (CTGF) is elevated in SSc serum and fibrotic skin, and has been associated with fibrosis in skin and other organs. It interacts with TGF-β to induce persistent fibrosis, is consistently up-regulated in SSc skin, and skin fibrosis is abrogated in its absence [91]. Moreover, CTFG plays a role in leading endothelial cells to transdifferentiate to become myofibroblasts [58]. Treatment with anti-CTGF antibodies also appears to be effective as an anti-fibrotic in an animal model [60].
Senescence and Metabolic Reprogramming
It has been proposed that SSc may represent an accelerated aging process. Indeed, many of the features of cellular aging including genomic instability, telomere attrition, epigenetic alterations, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication have all been suggested as potentially contributing to fibrosis in SSc[92]. Sirtuins are considered among the most promising targets for modulating aging-associated cellular and molecular processes and disease pathologies and may represent a key target for SSc-mediated fibrosis. Sirtuins promote longevity by protecting cells from oxidative, genotoxic and proteostatic stressors. SIRT levels decline during normal aging, and loss of their protective effects contributes to multiple aging-associated pathologies. Importantly, SIRT1 and SIRT3 are decreased in SSc skin and lung fibroblasts and sirtuin activity appears to regulate TGF-beta signaling[23, 93–95]. While drugs specifically targeting the sirtuins are not yet available, this does appear to represent an attractive target for blocking fibrosis in SSc.
In idiopathic pulmonary fibrosis and other fibrotic diseases, fibroblasts and macrophages have been shown to undergo a metabolic shift away from the oxidative phosphorylation to glycolysis (which is less efficient) despite adequate oxygen [96]. This type of metabolic reprogramming results in increased glucose uptake and an accumulation of TCA cycle metabolites and byproducts that act as signaling mechanisms. Evidence suggesting NAD regulating enzymes such as the PARP family are dysregulated in SSc as well as that there is mitochondrial DNA mutagenesis leading to respiratory chain dysfunction in bleomycin induced lung fibrosis [97]. This suggests that further investigation of metabolic reprogramming may be occurring in SSc cells and should be further investigated.
Mechanical Forces and Regulation of Matrix Stiffness
Mechanical resistance of the ECM, in conjunction with the action of profibrotic transforming growth factor represents an important driver of myofibroblast differentiation and persistence. Myofibroblasts only develop their characteristic contractile cytoskeletal apparatus above a certain ECM stiffness threshold [1]. Indeed, a major challenge in studying in vitro fibroblast differentiation is centered around these mechanical parameters. Fibroblasts grown in traditional cell cultures grown on plastic have high levels of stiffness compared to non-fibrotic tissue, leading to the induction of myofibroblast differentiation. Moreover, in stiff conditions, collagen cross-linking catalyzed by LOX enzymes leads to a feed-forward loop and more stiffness, while antibody-mediated inhibition of the enzyme LOXL2 suppresses the formation and progression of fibrosis [3]. In SSc lung, stiffness is modulated by a variety of factors including Netrin-1, which preferentially drive monocytic cells towards fibrocytes [98].
Fibroblasts regulate matrix turnover through the expression of matrix metalloproteinases (MMPs), which degrade ECM, and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs). Consistent with increased ECM deposition in SSc patients, serum levels of TIMPs in dcSSc and limited cutaneous SSc are significantly raised compared to healthy controls[99]. This supports the hypothesis that fibroblast-regulated matrix accumulation occurs through an imbalance in turnover of the ECM and plays a role in SSc by diminishing anti-fibrotic mechanisms as has been described in other fibrotic diseases[100].
Part 4: Toward Modulation of Fibrosis in SSc
Fibrosis has traditionally been thought of as an irreversible end-stage process. The large number of animal studies in which established fibrosis can be reversed has led to the hope that anti-fibrotic therapies may ultimately be effective in SSc. Along with successful development of anti-fibrotic therapies for other fibrotic illness such as idiopathic pulmonary fibrosis [101], there is an emerging excitement about using therapies targeting the myofibroblast and the other cellular and molecular pathways outlined in this review as novel therapeutic approaches.
Interestingly, while multiple small drug trials focused on fibrotic outcomes have shown some efficacy at the gene expression level [42, 76, 79], the majority of clinical treatments for SSc are immunomodulatory drugs which primarily target the adaptive immune system. While evidence for the efficacy of agents including cyclophosphamide, mycophenolate mofetil, and autologous stem cell transplantation have been promising (particularly in lung fibrosis outcomes) [102, 103], there still remain no drugs approved to treat SSc-associated skin fibrosis. Notably, a large number of clinical trials looking at both immunomodulatory as well as anti-fibrotic therapies have failed to meet their clinical trial endpoints [104].
While some of the drug treatment failures seen have to do with lack of effective outcome measures and over-reliance on variable measures (like the MRSS), a majority of therapies which have failed and not been advanced to further stages of development have shown promise in a subset of patients [104, 105]. This situation raises the likely possibility that because SSc is a very heterogeneous disease, subsets of patients may have different underlying cellular and molecular abnormalities and therefore respond to different classes of therapy [106].
Given the exciting new understandings of SSc pathogenesis which are reviewed in this article, it is evident that there a number of novel targets for anti-fibrotic therapy and indeed many of the molecular pathways reviewed have drugs being tested in the therapeutic pipeline. While the list of new treatments has been recently reviewed elsewhere [107–110], the strategy for how to appropriately assess the efficacy of these agents should be carefully undertaken. For example, utilization of molecular biomarkers such as the intrinsic gene signature subsets [111, 112] may help identify patients who may respond better to immune-focused or anti-fibrotic focused therapies [113]. Moreover, with the advent of improved molecular diagnostics and the availability of next generation sequencing technologies, it may be worth using pre-clinical models and clinical pilot studies to identify specific molecular targets for each new therapy to identify the patients most likely to respond. While developing these approaches may be time consuming and limit the scope of patients any drug may be indicated for, using this type of personalized medicine approach could have the benefit not only of performing smaller and more focused trials, but also of better subsetting SSc patients and defining patients likely to respond to therapies and therefore leading to the approval of multiple new targeted therapies with molecularly defined indications.
Conclusion
While multiple lines of evidence have demonstrated the variety of cellular and molecular pathways which are relevant to SSc pathophysiology, the myofibroblast remains the key effector cell in SSc. Understanding its development, differentiation, and function is crucial to understand the fibrotic phenotype of SSc. Evidence now shows that 1) multiple cell types give rise to myofibroblasts, 2) fibroblasts and myofibroblasts are heterogeneous, and 3) that a large number of (primarily immune) cells have important influences on fibroblast transition to become an activated myofibroblast. In scleroderma, this process involves multiple pathways including well known signaling cascades such as TGF-β and Wnt/β-Catenin signaling, as well as the involvement of epigenetic reprogramming and a number of more recently defined cellular pathways. The identification of these pathways as profibrotic, and the impressive work demonstrating the efficacy of targeting these molecules in pre-clinical models, has opened the door to the potential development of a large number of new anti-fibrotic agents. This prospect makes the future of treating SSc much more optimistic than the traditional symptomatic management that has been employed because of the lack of approved or effective therapies to date. Development of molecular diagnostics to identify which patients have abnormalities in each of the newly identified fibrotic pathways still needs to be developed. This pursuit of personalized medicine approaches, however, is an important goal because it has the potential to lay the groundwork for more focused clinical trials and therapeutics that can target subsets of SSc patients with the molecular abnormalities that will be amenable to emerging new treatments.
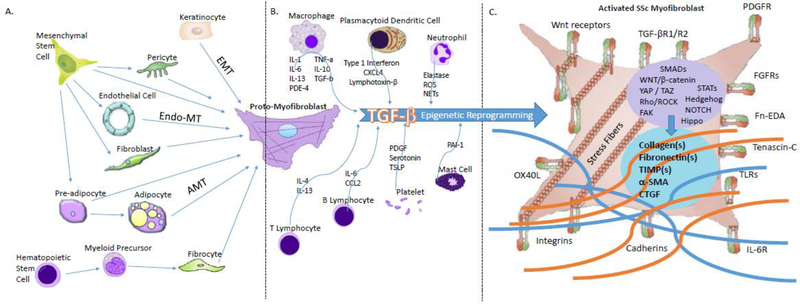
Figure 1.
Cellular source, immune interaction, and function of scleroderma myofibroblasts.
A. Cellular source of myofibroblasts. Myofibroblasts are derived from a diverse group of cells including mesenchymal cells (fibroblasts, endothelial cells, pericytes, adipocytes) and hematopoietic cells (fibrocytes).
B. Immune cell interactions with proto-myofibroblasts. Secretion of cytokines by a variety of innate and adaptive immune cells leads to TGF-β activation and epigenetic reprogramming that leads fibroblasts to become activated and develop into activated scleroderma myofibroblasts
C. The myofibroblast in scleroderma. Activated myofibroblasts express a number of cell surface receptors and undergo signal transduction that leads to collagen and matrix deposition which drive fibrosis.
Table 1.
Cells giving rise to myofibroblasts in scleroderma
| Cells Giving Rise to SSc Skin Myofibroblasts | Cell type | Other tissues described | Evidence in human SSc | References |
|---|---|---|---|---|
| Resident Fibroblasts | Mesechymal | Lung, Kidney, Liver, Heart | yes | [17, 73, 107, 110] |
| Endothelial Cells | Mesechymal | Heart, Lung | [7, 8, 58] | |
| Pericytes | Mesechymal | Vasculature | yes | [4, 114] |
| Pre-adipocytes/Adipocytes | Mesechymal | [10, 33, 63, 67] | ||
| Keratinocytes | Epithelial | Lung (epithelial cells) | [6, 31, 71] | |
| Fibrocytes (monocytes) | Hematopoeitic | Lung | yes | [4, 9, 98] |
Table 2.
Cells Involved in the Modulation of SSc Fibrosis.
| Cells Influencing Myofibroblast Phenotype | Relevant Sub-Populations | Secreted Factors and Surface Markers | Relevant Molecular Pathways | References |
|---|---|---|---|---|
| Monocytes/Macrophages | M2 macrophages | IL-13, PDE-4, Cadherin 11, MCP-1, Timp-1 | TLRs, IL-1, IL-6, Interferon | [5, 9, 25, 27–30, 41, 47, 69, 89, 115, 116] |
| Dendritic Cells | Plasmacytoid (pDCs) | TLR7/8, CXCL4 | Integrins, TLRs, Interferon | [27, 32–35, 41] |
| Mast Cells | PAI-1 | Histamine | [25, 31] | |
| Neutrophils | NETosis, inflammasome | [40, 57] | ||
| B lymphocytes | Breg, CD69+CD95+ | IL-10, BAFF, IL-6 | Autoantibody production | [48–50] |
| T lymphocytes | Th2, CD8+CD28- | TSLP, IL-13 | IL4/13 | [52, 53, 89] |
| Innate Lymphoid Cells | ILC1, ILC3 | [56] | ||
| Endothelial Cells | Fli-1 | VEGF, PDGF, endothelin, complement | [7, 8, 25, 54, 117] | |
| Platelets | HIF, serotonin, PDGF, CXCL4 | Hypoxia, coagulation | [35, 44, 54, 57] | |
| Adipocytes | Adiponectin, FABP4 | PPAR-gamma | [10, 64, 67, 77] | |
| Keratinocytes | Fli-1, Snail | [6, 31, 71] |
Acknowledgments:
The author has no potential conflicts of interest to declare, and has read the journal’s policy on disclosure of potential conflicts of interest, and has read the journal’s authorship agreement, reviewed and approved the manuscript. A special thank you goes to Emily Wu, Neil Korman, and Anya Niazov for editorial support and preparation of the manuscript.
Funding statement: Dr. Korman is supported by NIH/NIAMS award K08AR070285-03
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, Varga J: Systemic sclerosis. Nat Rev Dis Primers 2015, 1:15002. [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld SR, Castelino FV: Evaluation and management approaches for scleroderma lung disease. Ther Adv Respir Dis 2017, 11:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G: Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 2012, 180:1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebmeier S, Horsley V: Origin of fibrosing cells in systemic sclerosis. Curr Opin Rheumatol 2015, 27:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, Lopez-Giraldez F, Dash BC, Munoz-Rojas AR, Aultman KD, Zwick RK, Lei V, et al. : Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, Asano Y, Sugawara K, Yamashita T, Nakamura K, Saigusa R, Ichimura Y, Toyama T, Taniguchi T, Akamata K, et al. : Epithelial Fli1 deficiency drives systemic autoimmunity and fibrosis: Possible roles in scleroderma. J Exp Med 2017, 214:1129–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, Ibba-Manneschi L, Matucci-Cerinic M: Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis 2017, 76:924–934. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza FA, Piera-Velazquez S, Farber JL, Feghali-Bostwick C, Jimenez SA: Endothelial Cells Expressing Endothelial and Mesenchymal Cell Gene Products in Lung Tissue From Patients With Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol 2016, 68:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese C, Perry B, Heywood J, Bonner M, Visconti RP, Lee R, Hatfield CM, Silver RM, Hoffman S, Tourkina E: Caveolin-1 deficiency may predispose African Americans to systemic sclerosis-related interstitial lung disease. Arthritis Rheumatol 2014, 66:1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J: Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 2015, 67:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM: Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, et al. : Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348:aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu MS, Moore AL, Longaker MT: A Fibroblast Is Not a Fibroblast Is Not a Fibroblast. J Invest Dermatol 2018, 138:729–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philippeos C, Telerman SB, Oules B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, Watt FM: Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J Invest Dermatol 2018, 138:811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabib T, Morse C, Wang T, Chen W, Lafyatis R: SFRP2/DPP4 and FMO1/LSP1 Define Major Fibroblast Populations in Human Skin. J Invest Dermatol 2018, 138:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, Nguyen HN, Noss EH, Turner JD, Earp BE, et al. : Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun 2018, 9:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyftaki-Venieri DA, Abraham DJ, Ponticos M: Insights into myofibroblasts and their activation in scleroderma: opportunities for therapy? Curr Opin Rheumatol 2018, 30:581–587. [DOI] [PubMed] [Google Scholar]
- 18.Altorok N, Tsou PS, Coit P, Khanna D, Sawalha AH: Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann Rheum Dis 2015, 74:1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Tsou PS, Khanna D, Sawalha AH: Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis 2018, 77:1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergmann C, Brandt A, Merlevede B, Hallenberger L, Dees C, Wohlfahrt T, Potter S, Zhang Y, Chen CW, Mallano T, et al. : The histone demethylase Jumonji domain-containing protein 3 (JMJD3) regulates fibroblast activation in systemic sclerosis. Ann Rheum Dis 2018, 77:150–158. [DOI] [PubMed] [Google Scholar]
- 21.Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, Lin NY, Beyer C, Distler O, Schett G, Distler JH: The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann Rheum Dis 2014, 73:1232–1239. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh AK, Bhattacharyya S, Lafyatis R, Farina G, Yu J, Thimmapaya B, Wei J, Varga J: p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-beta: epigenetic feed-forward amplification of fibrosis. J Invest Dermatol 2013, 133:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Ghosh AK, Chu H, Fang F, Hinchcliff ME, Wang J, Marangoni RG, Varga J: The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor beta Signaling. Arthritis Rheumatol 2015, 67:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I, Nakamura K, Yamashita T, Saigusa R, Akamata K, et al. : Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun 2014, 5:5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi T, Asano Y, Akamata K, Noda S, Takahashi T, Ichimura Y, Toyama T, Trojanowska M, Sato S: Fibrosis, vascular activation, and immune abnormalities resembling systemic sclerosis in bleomycin-treated Fli-1-haploinsufficient mice. Arthritis Rheumatol 2015, 67:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saigusa R, Asano Y, Nakamura K, Hirabayashi M, Miura S, Yamashita T, Taniguchi T, Ichimura Y, Takahashi T, Yoshizaki A, et al. : Systemic Sclerosis Dermal Fibroblasts Suppress Th1 Cytokine Production via Galectin-9 Overproduction due to Fli1 Deficiency. J Invest Dermatol 2017, 137:1850–1859. [DOI] [PubMed] [Google Scholar]
- 27.Chia JJ, Lu TT: Update on macrophages and innate immunity in scleroderma. Curr Opin Rheumatol 2015, 27:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier C, Ramming A, Bergmann C, Weinkam R, Kittan N, Schett G, Distler JHW, Beyer C: Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis 2017, 76:1133–1141. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Pedroza M, Lafyatis R, George AT, Mayes MD, Assassi S, Tan FK, Brenner MB, Agarwal SK: Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheumatol 2014, 66:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, Parra ER, Spira A, Simms R, Capellozzi VL, Lafyatis R: Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol 2014, 66:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pincha N, Hajam EY, Badarinath K, Batta SPR, Masudi T, Dey R, Andreasen P, Kawakami T, Samuel R, George R, et al. : PAI1 mediates fibroblast-mast cell interactions in skin fibrosis. J Clin Invest 2018, 128:1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ah Kioon MD, Tripodo C, Fernandez D, Kirou KA, Spiera RF, Crow MK, Gordon JK, Barrat FJ: Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chia JJ, Zhu T, Chyou S, Dasoveanu DC, Carballo C, Tian S, Magro CM, Rodeo S, Spiera RF, Ruddle NH, et al. : Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. J Clin Invest 2016, 126:4331–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, Dietz HC: Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 2013, 503:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, Farina GA, Stifano G, Mathes AL, Cossu M, et al. : Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014, 370:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Wang W, Qin W, Cheng K, Coulup S, Chavez S, Jiang S, Raparia K, De Almeida LMV, Stehlik C, et al. : TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, Lafyatis R, Lee J, Hinchcliff M, Feghali-Bostwick C, et al. : Tenascin-C drives persistence of organ fibrosis. Nat Commun 2016, 7:11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J: FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med 2014, 6:232ra250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang F, Marangoni RG, Zhou X, Yang Y, Ye B, Shangguang A, Qin W, Wang W, Bhattacharyya S, Wei J, et al. : Toll-like Receptor 9 Signaling Is Augmented in Systemic Sclerosis and Elicits Transforming Growth Factor beta-Dependent Fibroblast Activation. Arthritis Rheumatol 2016, 68:1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD: The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 2011, 63:3563–3574. [DOI] [PubMed] [Google Scholar]
- 41.Laurent P, Sisirak V, Lazaro E, Richez C, Duffau P, Blanco P, Truchetet ME, Contin-Bordes C: Innate Immunity in Systemic Sclerosis Fibrosis: Recent Advances. Front Immunol 2018, 9:1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denton CP, Ong VH, Xu S, Chen-Harris H, Modrusan Z, Lafyatis R, Khanna D, Jahreis A, Siegel J, Sornasse T: Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis 2018, 77:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castelino FV, Bain G, Pace VA, Black KE, George L, Probst CK, Goulet L, Lafyatis R, Tager AM: An Autotaxin/Lysophosphatidic Acid/Interleukin-6 Amplification Loop Drives Scleroderma Fibrosis. Arthritis Rheumatol 2016, 68:2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherlinger M, Guillotin V, Truchetet ME, Contin-Bordes C, Sisirak V, Duffau P, Lazaro E, Richez C, Blanco P: Systemic lupus erythematosus and systemic sclerosis: All roads lead to platelets. Autoimmun Rev 2018, 17:625–635. [DOI] [PubMed] [Google Scholar]
- 45.Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, et al. : Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011, 70:2029–2036. [DOI] [PubMed] [Google Scholar]
- 46.Sharif R, Mayes MD, Tan FK, Gorlova OY, Hummers LK, Shah AA, Furst DE, Khanna D, Martin J, Bossini-Castillo L, et al. : IRF5 polymorphism predicts prognosis in patients with systemic sclerosis. Ann Rheum Dis 2012, 71:1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brkic Z, van Bon L, Cossu M, van Helden-Meeuwsen CG, Vonk MC, Knaapen H, van den Berg W, Dalm VA, Van Daele PL, Severino A, et al. : The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann Rheum Dis 2016, 75:1567–1573. [DOI] [PubMed] [Google Scholar]
- 48.Dumoitier N, Chaigne B, Regent A, Lofek S, Mhibik M, Dorfmuller P, Terrier B, London J, Berezne A, Tamas N, et al. : Scleroderma Peripheral B Lymphocytes Secrete Interleukin-6 and Transforming Growth Factor beta and Activate Fibroblasts. Arthritis Rheumatol 2017, 69:1078–1089. [DOI] [PubMed] [Google Scholar]
- 49.Choi MY, Fritzler MJ: Progress in understanding the diagnostic and pathogenic role of autoantibodies associated with systemic sclerosis. Curr Opin Rheumatol 2016, 28:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari CG, Bogdanos DP, Sakkas LI: Breg Cells Are Numerically Decreased and Functionally Impaired in Patients With Systemic Sclerosis. Arthritis Rheumatol 2016, 68:494–504. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita T, Kobayashi T, Mizumaki K, Kano M, Sawada T, Tennichi M, Okamura A, Hamaguchi Y, Iwakura Y, Hasegawa M, et al. : BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv 2018, 4:eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Larregina AT, Domsic RT, Stolz DB, Medsger TA Jr., Lafyatis R, Fuschiotti P: Skin-Resident Effector Memory CD8(+)CD28(−) T Cells Exhibit a Profibrotic Phenotype in Patients with Systemic Sclerosis. J Invest Dermatol 2017, 137:1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA Jr.: Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 2013, 65:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Truchetet ME, Demoures B, Eduardo Guimaraes J, Bertrand A, Laurent P, Jolivel V, Douchet I, Jacquemin C, Khoryati L, Duffau P, et al. : Platelets Induce Thymic Stromal Lymphopoietin Production by Endothelial Cells: Contribution to Fibrosis in Human Systemic Sclerosis. Arthritis Rheumatol 2016, 68:2784–2794. [DOI] [PubMed] [Google Scholar]
- 55.Klose CS, Artis D: Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016, 17:765–774. [DOI] [PubMed] [Google Scholar]
- 56.Roan F, Stoklasek TA, Whalen E, Molitor JA, Bluestone JA, Buckner JH, Ziegler SF: CD4+ Group 1 Innate Lymphoid Cells (ILC) Form a Functionally Distinct ILC Subset That Is Increased in Systemic Sclerosis. J Immunol 2016, 196:2051–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maugeri N, Capobianco A, Rovere-Querini P, Ramirez GA, Tombetti E, Valle PD, Monno A, D’Alberti V, Gasparri AM, Franchini S, et al. : Platelet microparticles sustain autophagyassociated activation of neutrophils in systemic sclerosis. Sci Transl Med 2018, 10. [DOI] [PubMed] [Google Scholar]
- 58.Serrati S, Chilla A, Laurenzana A, Margheri F, Giannoni E, Magnelli L, Chiarugi P, Dotor J, Feijoo E, Bazzichi L, et al. : Systemic sclerosis endothelial cells recruit and activate dermal fibroblasts by induction of a connective tissue growth factor (CCN2)/transforming growth factor beta-dependent mesenchymal-to-mesenchymal transition. Arthritis Rheum 2013, 65:258–269. [DOI] [PubMed] [Google Scholar]
- 59.Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, Distler JH, Distler O: Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014, 73:1880–1887. [DOI] [PubMed] [Google Scholar]
- 60.Makino K, Makino T, Stawski L, Lipson KE, Leask A, Trojanowska M: Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res Ther 2017, 19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akashi K, Saegusa J, Sendo S, Nishimura K, Okano T, Yagi K, Yanagisawa M, Emoto N, Morinobu A: Knockout of endothelin type B receptor signaling attenuates bleomycin-induced skin sclerosis in mice. Arthritis Res Ther 2016, 18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagares D, Busnadiego O, Garcia-Fernandez RA, Lamas S, Rodriguez-Pascual F: Adenoviral gene transfer of endothelin-1 in the lung induces pulmonary fibrosis through the activation of focal adhesion kinase. Am J Respir Cell Mol Biol 2012, 47:834–842. [DOI] [PubMed] [Google Scholar]
- 63.Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, Gottardi CJ, MacDougald OA, Varga J: Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum 2011, 63:1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C, Varga J: PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One 2010, 5:e13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei J, Zhu H, Komura K, Lord G, Tomcik M, Wang W, Doniparthi S, Tamaki Z, Hinchcliff M, Distler JH, Varga J: A synthetic PPAR-gamma agonist triterpenoid ameliorates experimental fibrosis: PPAR-gamma-independent suppression of fibrotic responses. Ann Rheum Dis 2014, 73:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruzehaji N, Frantz C, Ponsoye M, Avouac J, Pezet S, Guilbert T, Luccarini JM, Broqua P, Junien JL, Allanore Y: Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann Rheum Dis 2016, 75:2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korman B, Marangoni RG, Lord G, Olefsky J, Tourtellotte W, Varga J: Adipocyte-specific Repression of PPAR-gamma by NCoR Contributes to Scleroderma Skin Fibrosis. Arthritis Res Ther 2018, 20:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marangoni RG, Masui Y, Fang F, Korman B, Lord G, Lee J, Lakota K, Wei J, Scherer PE, Otvos L, et al. : Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci Rep 2017, 7:4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taroni JN, Greene CS, Martyanov V, Wood TA, Christmann RB, Farber HW, Lafyatis RA, Denton CP, Hinchcliff ME, Pioli PA, et al. : A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med 2017, 9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taroni JN, Martyanov V, Mahoney JM, Whitfield ML: A Functional Genomic Meta-Analysis of Clinical Trials in Systemic Sclerosis: Toward Precision Medicine and Combination Therapy. J Invest Dermatol 2017, 137:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCoy SS, Reed TJ, Berthier CC, Tsou PS, Liu J, Gudjonsson JE, Khanna D, Kahlenberg JM: Scleroderma keratinocytes promote fibroblast activation independent of transforming growth factor beta. Rheumatology (Oxford) 2017, 56:1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lafyatis R: Transforming growth factor beta--at the centre of systemic sclerosis. Nat Rev Rheumatol 2014, 10:706–719. [DOI] [PubMed] [Google Scholar]
- 73.Kendall RT, Feghali-Bostwick CA: Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 2014, 5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piersma B, Bank RA, Boersema M: Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med (Lausanne) 2015, 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacchetti C, Bai Y, Stanford SM, Di Benedetto P, Cipriani P, Santelli E, Piera-Velazquez S, Chernitskiy V, Kiosses WB, Ceponis A, et al. : PTP4A1 promotes TGFbeta signaling and fibrosis in systemic sclerosis. Nat Commun 2017, 8:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, Nakerakanti S, York M, Farina G, Whitfield ML, et al. : Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015, 125:2795–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, Gottardi CJ, Atit R, Whitfield ML, Varga J: Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum 2012, 64:2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beyer C, Reichert H, Akan H, Mallano T, Schramm A, Dees C, Palumbo-Zerr K, Lin NY, Distler A, Gelse K, et al. : Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann Rheum Dis 2013, 72:1255–1258. [DOI] [PubMed] [Google Scholar]
- 79.Lafyatis R, Mantero JC, Gordon J, Kishore N, Carns M, Dittrich H, Spiera R, Simms RW, Varga J: Inhibition of beta-Catenin Signaling in the Skin Rescues Cutaneous Adipogenesis in Systemic Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial of C-82. J Invest Dermatol 2017, 137:2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horn A, Palumbo K, Cordazzo C, Dees C, Akhmetshina A, Tomcik M, Zerr P, Avouac J, Gusinde J, Zwerina J, et al. : Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum 2012, 64:2724–2733. [DOI] [PubMed] [Google Scholar]
- 81.Dees C, Zerr P, Tomcik M, Beyer C, Horn A, Akhmetshina A, Palumbo K, Reich N, Zwerina J, Sticherling M, et al. : Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum 2011, 63:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toyama T, Looney AP, Baker BM, Stawski L, Haines P, Simms R, Szymaniak AD, Varelas X, Trojanowska M: Therapeutic Targeting of TAZ and YAP by Dimethyl Fumarate in Systemic Sclerosis Fibrosis. J Invest Dermatol 2018, 138:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Distler A, Lang V, Del Vecchio T, Huang J, Zhang Y, Beyer C, Lin NY, Palumbo-Zerr K, Distler O, Schett G, Distler JH: Combined inhibition of morphogen pathways demonstrates additive antifibrotic effects and improved tolerability. Ann Rheum Dis 2014, 73:1264–1268. [DOI] [PubMed] [Google Scholar]
- 84.Wernig G, Chen SY, Cui L, Van Neste C, Tsai JM, Kambham N, Vogel H, Natkunam Y, Gilliland DG, Nolan G, Weissman IL: Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci U S A 2017, 114:4757–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maurer B, Reich N, Juengel A, Kriegsmann J, Gay RE, Schett G, Michel BA, Gay S, Distler JH, Distler O: Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis 2012, 71:1382–1387. [DOI] [PubMed] [Google Scholar]
- 86.Elhai M, Avouac J, Hoffmann-Vold AM, Ruzehaji N, Amiar O, Ruiz B, Brahiti H, Ponsoye M, Frechet M, Burgevin A, et al. : OX40L blockade protects against inflammation-driven fibrosis. Proc Natl Acad Sci U S A 2016, 113:E3901–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakraborty D, Sumova B, Mallano T, Chen CW, Distler A, Bergmann C, Ludolph I, Horch RE, Gelse K, Ramming A, et al. : Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 2017, 8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGarry T, Orr C, Wade S, Biniecka M, Wade S, Gallagher L, Low C, Veale DJ, Fearon U: JAK/STAT Blockade Alters Synovial Bioenergetics, Mitochondrial Function, and Proinflammatory Mediators in Rheumatoid Arthritis. Arthritis Rheumatol 2018, 70:1959–1970. [DOI] [PubMed] [Google Scholar]
- 89.Avouac J, Furnrohr BG, Tomcik M, Palumbo K, Zerr P, Horn A, Dees C, Akhmetshina A, Beyer C, Distler O, et al. : Inactivation of the transcription factor STAT-4 prevents inflammation-driven fibrosis in animal models of systemic sclerosis. Arthritis Rheum 2011, 63:800–809. [DOI] [PubMed] [Google Scholar]
- 90.Gourh P, Agarwal SK, Divecha D, Assassi S, Paz G, Arora-Singh RK, Reveille JD, Shete S, Mayes MD, Arnett FC, Tan FK: Polymorphisms in TBX21 and STAT4 increase the risk of systemic sclerosis: evidence of possible gene-gene interaction and alterations in Th1/Th2 cytokines. Arthritis Rheum 2009, 60:3794–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Makino K, Makino T, Stawski L, Mantero JC, Lafyatis R, Simms R, Trojanowska M: Blockade of PDGF Receptors by Crenolanib Has Therapeutic Effect in Patient Fibroblasts and in Preclinical Models of Systemic Sclerosis. J Invest Dermatol 2017, 137:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luckhardt TR, Thannickal VJ: Systemic sclerosis-associated fibrosis: an accelerated aging phenotype? Curr Opin Rheumatol 2015, 27:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akamata K, Wei J, Bhattacharyya M, Cheresh P, Bonner MY, Arbiser JL, Raparia K, Gupta MP, Kamp DW, Varga J: SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 2016, 7:69321–69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wyman AE, Noor Z, Fishelevich R, Lockatell V, Shah NG, Todd NW, Atamas SP: Sirtuin 7 is decreased in pulmonary fibrosis and regulates the fibrotic phenotype of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2017, 312:L945–L958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, Schett G, Distler JH: Sirt1 regulates canonical TGF-beta signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis 2016, 75:226–233. [DOI] [PubMed] [Google Scholar]
- 96.Zank DC, Bueno M, Mora AL, Rojas M: Idiopathic Pulmonary Fibrosis: Aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front Med (Lausanne) 2018, 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Potter S, Chen CW, Liang R, Gelse K, Ludolph I, Horch RE, Distler O, Schett G, Distler JHW, Dees C: Poly(ADP-ribose) polymerase-1 regulates fibroblast activation in systemic sclerosis. Ann Rheum Dis 2018, 77:744–751. [DOI] [PubMed] [Google Scholar]
- 98.Sun H, Zhu Y, Pan H, Chen X, Balestrini JL, Lam TT, Kanyo JE, Eichmann A, Gulati M, Fares WH, et al. : Netrin-1 Regulates Fibrocyte Accumulation in the Decellularized Fibrotic Sclerodermatous Lung Microenvironment and in Bleomycin-Induced Pulmonary Fibrosis. Arthritis Rheumatol 2016, 68:1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young-Min SA, Beeton C, Laughton R, Plumpton T, Bartram S, Murphy G, Black C, Cawston TE: Serum TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis, primary Raynaud’s phenomenon, and in normal controls. Ann Rheum Dis 2001, 60:846–851. [PMC free article] [PubMed] [Google Scholar]
- 100.Giannandrea M, Parks WC: Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 2014, 7:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raghu G, Selman M: Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med 2015, 191:252–254. [DOI] [PubMed] [Google Scholar]
- 102.Volkmann ER, Tashkin DP, Li N, Roth MD, Khanna D, Hoffmann-Vold AM, Kim G, Goldin J, Clements PJ, Furst DE, Elashoff RM: Mycophenolate Mofetil Versus Placebo for Systemic Sclerosis-Related Interstitial Lung Disease: An Analysis of Scleroderma Lung Studies I and II. Arthritis Rheumatol 2017, 69:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, Mayes MD, Nash RA, Crofford LJ, Eggleston B, et al. : Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med 2018, 378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung L, Denton CP, Distler O, Furst DE, Khanna D, Merkel PA, Scleroderma Clinical Trials C: Clinical trial design in scleroderma: where are we and where do we go next? Clin Exp Rheumatol 2012, 30:S97–102. [PubMed] [Google Scholar]
- 105.Khanna D, Berrocal VJ, Giannini EH, Seibold JR, Merkel PA, Mayes MD, Baron M, Clements PJ, Steen V, Assassi S, et al. : The American College of Rheumatology Provisional Composite Response Index for Clinical Trials in Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol 2016, 68:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martyanov V, Whitfield ML: Molecular stratification and precision medicine in systemic sclerosis from genomic and proteomic data. Curr Opin Rheumatol 2016, 28:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schulz JN, Plomann M, Sengle G, Gullberg D, Krieg T, Eckes B: New developments on skin fibrosis - Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol 2018, 68–69:522–532. [DOI] [PubMed] [Google Scholar]
- 108.Distler O, Cozzio A: Systemic sclerosis and localized scleroderma--current concepts and novel targets for therapy. Semin Immunopathol 2016, 38:87–95. [DOI] [PubMed] [Google Scholar]
- 109.Baron M: Targeted Therapy in Systemic Sclerosis. Rambam Maimonides Med J 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Caam A, Vonk M, van den Hoogen F, van Lent P, van der Kraan P: Unraveling SSc Pathophysiology; The Myofibroblast. Front Immunol 2018, 9:2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lofgren S, Hinchcliff M, Carns M, Wood T, Aren K, Arroyo E, Cheung P, Kuo A, Valenzuela A, Haemel A, et al. : Integrated, multicohort analysis of systemic sclerosis identifies robust transcriptional signature of disease severity. JCI Insight 2016, 1:e89073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hinchcliff M, Huang CC, Wood TA, Matthew Mahoney J, Martyanov V, Bhattacharyya S, Tamaki Z, Lee J, Carns M, Podlusky S, et al. : Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol 2013, 133:1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castelino FV, Varga J: Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy. Curr Opin Rheumatol 2014, 26:607–614. [DOI] [PubMed] [Google Scholar]
- 114.Cipriani P, Di Benedetto P, Ruscitti P, Liakouli V, Berardicurti O, Carubbi F, Ciccia F, Guggino G, Zazzeroni F, Alesse E, et al. : Perivascular Cells in Diffuse Cutaneous Systemic Sclerosis Overexpress Activated ADAM12 and Are Involved in Myofibroblast Transdifferentiation and Development of Fibrosis. J Rheumatol 2016, 43:1340–1349. [DOI] [PubMed] [Google Scholar]
- 115.Christmann RB, Mathes A, Affandi AJ, Padilla C, Nazari B, Bujor AM, Stifano G, Lafyatis R: Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor beta. Arthritis Rheum 2013, 65:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X, Mayes MD, Tan FK, Wu M, Reveille JD, Harper BE, Draeger HT, Gonzalez EB, Assassi S: Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum 2013, 65:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsou PS, Wren JD, Amin MA, Schiopu E, Fox DA, Khanna D, Sawalha AH: Histone Deacetylase 5 Is Overexpressed in Scleroderma Endothelial Cells and Impairs Angiogenesis via Repression of Proangiogenic Factors. Arthritis Rheumatol 2016, 68:2975–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]