Abstract
Introduction
The purpose of this study is to evaluate splenic effects during artificial placenta (AP) support.
Methods
AP lambs (118-121d, n=14) were delivered and placed on the AP support for a goal of 10-14 days. Cannulation used right jugular drainage and umbilical vein reinfusion. Early (ETC;115-120d; n=7) and late (LTC; 125-131d; n=7) tissue controls were delivered and immediately sacrificed. Spleens were formalin fixed, H&E stained, and graded for injury, response to inflammation, and extramedullary hematopoiesis (EMH). CD68 and CD163 stains were used to assess for macrophage activation and density. Clinical variables were correlated with splenic scores. Groups were compared using Fisher’s Exact Test and descriptive statistics. P<0.05 indicated significance.
Results
Mean survival for AP lambs was 12±5d. There was no necrosis found in any of the groups. Vascular congestion and sinusoidal histiocytosis did not significantly differ between AP and control groups (p=0.72; p=0.311). There were significantly more pigmented macrophages (p=0.008), CD163 (p=<0.001), and CD68 (p=<0.001) stained cells in the AP group. ETC and LTC demonstrated more EMH than AP spleens (p=<0.001).
Conclusions
During AP support, spleens appear to develop normally and exhibit an appropriate inflammatory response. After initiation of AP support, EMH transitions away from the spleen.
Keywords: Artificial Placenta, Extracorporeal Membrane Oxygenation, Splenic Development, Splenic Injury, Premature Lambs, Premature Spleen
Introduction
Prematurity is the leading cause of death around the world in children under age 5, with nearly 1 million children dying from complications of preterm birth (1). Extremely low gestational age newborns (ELGANS), defined as <28 weeks gestation, suffer disproportionate mortality and morbidity including respiratory failure, sepsis, necrotizing enterocolitis, and retinopathy of prematurity (1). A novel solution is the Artificial Placenta (AP) which preserves fluid filled lungs, avoids mechanical ventilation (MV), and maintains fetal circulation until the lungs are sufficiently developed for gas exchange (2–4). Several organ systems such as the brain, lungs, and gastrointestinal tract, have been evaluated in premature lambs supported by the AP (3, 5, 6). However, no studies to date have evaluated the premature spleen during AP support.
The spleen is the second largest immune organ and plays many important functions. These include filtering the blood, clearing blood-borne antigens, removing injured and old cells, fighting off infection, and extramedullary hematopoiesis during development (7) (8, 9). Studies have shown that individuals who are asplenic have a higher risk of infection with encapsulated bacteria, have increased leukocytosis, and exhibit changes to red blood cell (RBC), platelet, and white blood cell (WBC) morphology (10–12). In addition, premature infants are at risk of developing systemic infections and sepsis which is a common cause of death . The spleen has many important hematologic and immunologic functions yet may not be completely developed in preterm infants (8, 13). The AP offers a promising solution to the problem of prematurity, but it is important to understand its effects on every organ, including the spleen, prior to clinical translation. Prevention of sepsis and appropriate response to inflammation is critical during the premature period, but this area has yet to be investigated during AP support. . The aim of our current study was to evaluate injury, response to inflammation, and level of EMH in the spleens of premature lambs supported by the AP. We hypothesized that there would be no significant injury and normal splenic development during AP support.
Methods
Animal Care and Treatment
All sheep used in these experiments were treated humanely and in compliance with the Guide for Care and Use of Laboratory Animals, 8th edition (14). Procedures, protocols, and details of each experiment were also approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC) (protocol 00007211).
Artificial Placenta Group
Pregnant ewes were anesthetized and underwent elective hysterotomies. Premature lambs (EGA 118-121d; n=14; Term=145d), whose lung development is equivalent to that of a 24 week human neonate, were delivered and either placed directly on the AP (n=7) or MV for 1 hour prior to AP support (n=7). Lambs were cannulated for ELCS using 10-14Fr cannulas (Terumo: Ann Arbor, MI) placed into the right internal jugular for drainage and umbilical vein for reinfusion. A soft 12F silicone cannula with a beveled tip was used for the reinfusion cannula in 3 lambs. An endotracheal tube (ETT) was placed and the lungs were fluid filled (amniotic fluid, Ringer’s Lactate, or perfluorodecalin [Origen: Austin, TX]), either immediately (n=7) or after 1 hour of MV (n=7). The ETT was left open to a fluid filled meniscus to allow for continued fetal breathing movements. VV-ECLS was initiated using ¼” tubing (Tygon: Lima, OH), a collapsible-tubing peristaltic pump (MC3: Ann Arbor, MI), and an oxygenator/heat exchanger (either Medos HiLite, Xenios: Heilbronn, Germany or Capiox Baby Rx, Terumo, Ann Arbor, MI; Figure 1). Two hundred units/kg/day of Epogen® was given to stimulate red blood cell production (n=6). For continuous hemodynamic monitoring and frequent arterial blood gas assessments, an arterial line (5F; Covidien-Medtronic: Minneapolis, MN) was placed into the umbilical artery for each lamb. Medication administration, total parenteral nutrition (TPN; ExactMix, Baxter Healthcare Corporation, Englewood, CO. Baxter International Inc. Supplied by the University of Michigan HomeMed- Home Infusion Pharmacy), and intravenous (IV) fluids were administered into either a triple lumen venous catheter (5 Fr; Covidien-Medtronic: Minneapolis, MN) placed into the umbilical vein (n=11) or directly into the circuit tubing (n=3). Prostaglandin E1 (Pfizer, New York, NY) (0.2mcg/kg/min) was continuously given during AP support to maintain a patent ductus arteriosus and fetal circulation. This was discontinued when lambs were transitioned to breathing air. Systemic anticoagulation using heparin sulfate (SAGENT, Schaumburg, IL) (100 U/hr, titrated to a goal activated clotting time (ACT) of 200-250 seconds) was given to prevent clotting, and solumedrol (Pfizer, New York, NY) 0.63 mg/kg was used to prevent hypocortisolemia and associated hypotension. Prophylactic IV antibiotics (piperacillin-tazobactam [Hospira Inc., Lake Forest, IL]; metronidazole [Baxter Healthcare Corporation, Deerfield, IL]), and antifungals (fluconazole [SAGENT, Schaumburg, IL]) were continuously delivered to prevent infection and sepsis. Occasionally, diazepam (2.5mg [0.7mg/kg] Hospira Inc., Lake Forest, IL) and Buprenorphine (0.1mg/kg; Parr Inc., Spring Valley, NJ) were used for sedation. During AP support ECLS settings and hemodynamics were monitored continuously. Electrolytes, ABG samples, urine output, and bowel movements were monitored every 3 hours. Lambs remained nil per os (NPO) during AP support. One of the lambs was transitioned to enteral feeds using a bottle once they were full term and weaned off the AP. In cases of volume-resistant hypotension, vasopressors [norepinephrine (Claris LifeSciences Inc., North Brunswick, NJ) or dopamine (Baxter, Deerfield, IL]) were used to maintain a MAP >40 mmHg if they were nonresponsive to fluids and blood administration. Nine of the lambs were transitioned off the AP to mechanical ventilation and three to breathing air on their own. Experiments were electively terminated after 12 days or sooner if the lamb was unstable. At the completion of each experiment, lambs were necropsied, spleens were formalin fixed, and slides were prepared using Haematoxilin and Eosin (H&E; Fisher Scientific, Pittsburgh, PA), Anti-CD68 (Abeam, Boston, MA), and Anti-CD163 stains (Abcam, Boston, MA).
Fig. 1.
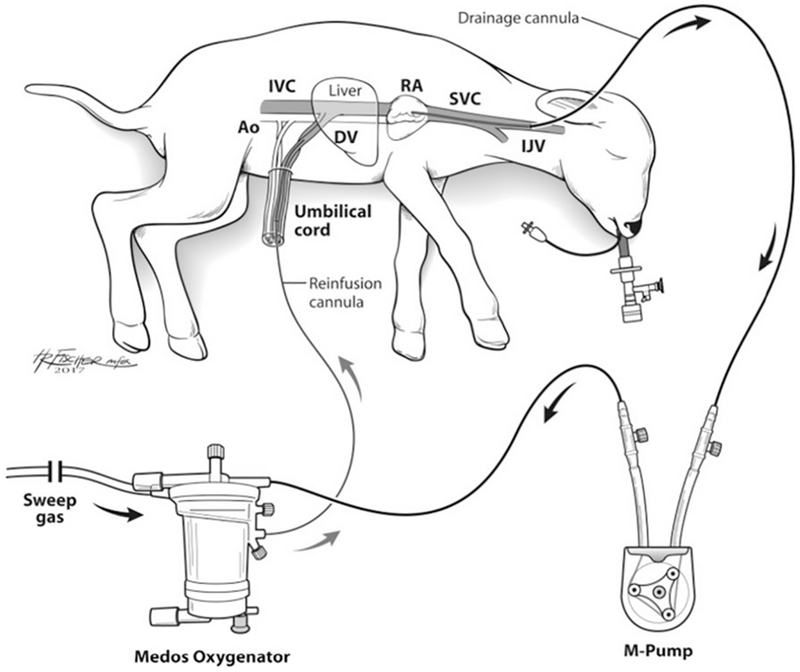
Schematic of the artificial placenta (AP) circuit utilizing VV-ELCS, collapsible-tubing roller pump (M-Pump), and oxygenator/heat exchanger in a premature lamb model. Ao: Aorta; IVC: Inferior Vena Cava; DV: Ductus Venosus; RA: Right Atrium; SVC: Superior Vena Cava; IJV: Internal Jugular Vein; VV-ECLS: venovenous extracorporeal life support.
Tissue Control Group
Early (ETC; n=7) and Late (LTC; n=7) Tissue Controls were delivered between 105-121d and 125-131d, respectively, and immediately sacrificed.
Necropsy and Tissue Preparation
At the end of each experiment, lambs were euthanized and spleens were placed en-bloc in formalin for fixation. They were then sent to pathology for staining. Tissue sections were 3-5μm thick and were assessed by a pathologist blinded to groups.
H&E, CD 68, CD163 Stains
H&E stained splenic slides were reviewed and scored from 0-3 (0-none, 1-mild, 2-moderate, 3- severe) among 5 different categories [necrosis, congestion, sinusoidal histiocytes, pigmented macrophages, and extramedullary hematopoiesis (EMH)]. CD68 and CD163 were used to assess for macrophage activation and density and were also scored. These variables were compared between groups (AP vs ETC vs LTC). Clinical data (mean arterial pressure [MAP], white blood cell count [WBC], hemoglobin [Hb], heart rate [HR], Platelets [Pits]) among the AP group were correlated with splenic pathologic outcomes.
Statistical Analysis
Among the AP group, continuous clinical variables (MAP, HR, WBC, Hb, Plts) were measured and correlated with splenic injury scores (“none”, “mild”, “moderate”, or “severe”) among the 7 histologic categories: H&E stained necrosis, congestion, sinusoidal histiocytes, pigmented macrophages, EMH, CD163 Stain, and CD68 Stain. Correlations were measured using Spearman’s rho with accompanying p-values. When comparing groups, a Fisher’s exact test was used to determine whether the patterns differed from what would be expected by pure chance. All analyses were conducted using R software: R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org. Descriptive statistics were also used. P<0.05 indicated significance.
Results
Experimental Outcomes with AP Lambs
Mean GA for the AP group was 120±1d, with an mean birth weight of 3.2±0.6kg. ETC (n=7) and LTC (n=7) were delivered at 116±5 days and 128±2 days, respectively. Lambs supported on the AP (n=14) lived an mean of 12±5 days. Nine of these lambs were transitioned off the AP to breathing air after 1-3 weeks of AP support. Five of the AP lambs were given brief infusion of vasopressors (norepinephrine and dopamine) due to hypotension at some point during support. During AP support, lambs had an mean MAP of 50±7 mmHg (16mmHg/kg) and median (IQR) of 48.3 mmHg (10.7), HR of mean 205±15 bpm (64bmp/kg) and median 206.1 (24.1), and flows were maintained at an mean of 353±63 mL/min (110 mL/kg/min). AP lambs required 299±191 mL (94mL/kg) of pRBC transfusions to maintain Hb at a mean of 9.5±0.8 g/d and median (IQR) of 9.5 (1.1) g/dL. Mean WBC was 5.7±3 with a median (IQR) of 5.1 (2.8) and platelet was 155±79 with a median (IQR) of 122.2 (85.4) during support. Subgroup analysis of lambs who were transitioned directly to the AP compared to those who were MV for 1 hour did not affect any of the histological outcomes and were therefore excluded from this review. None of the AP lambs exhibited signs of sepsis or systemic infection.
Splenic Necrosis
There was no necrosis found in any of the spleens (AP, ETC, or LTC).
Splenic Sinusoidal Histiocytes
Decreased MAP (Median (IQR) of 52.19 (7.6) for no sinusoidal histiocytosis, 46.96 (5.0) for mild, and 41.39 (0.8) for moderate) was correlated with increased sinusoidal histiocytosis among the AP group (Spearman’s Rho= −0.684, p=.007). However, when comparing sinusoidal histiocytosis between groups, there was no significant difference seen (Fisher’s exact test p-value = 0.311).
Splenic Pigmented Macrophages
A lower Hb (median (IQR) of 9.9 (0.6) associated with mild, 9.3 (1.0) with moderate, and 8.5 (1.0) with severe) was correlated with increased presence of pigmented macrophages (Spearman’s Rho = −0.405), however this was not statistically different from zero correlation (Rho P =0.151). When comparing groups, there was no “severe level” of pigmented macrophages in any of the groups (AP, ETC, or LTC), but there were significantly more mild and moderate pigmented macrophages seen in the AP group compared to the ETC and LTC (Fisher’s Exact Test p-value = 0.008).
CD163 Stain
No significant correlation between hemodynamic variables and the level of CD163 staining was found among the AP group (n=14). There was, however, significantly more CD163 stained cells in the spleens of the AP group compared to both ETC and LTC (Fisher’s exact test p-value = <0.001; Figure 2).
Fig. 2.
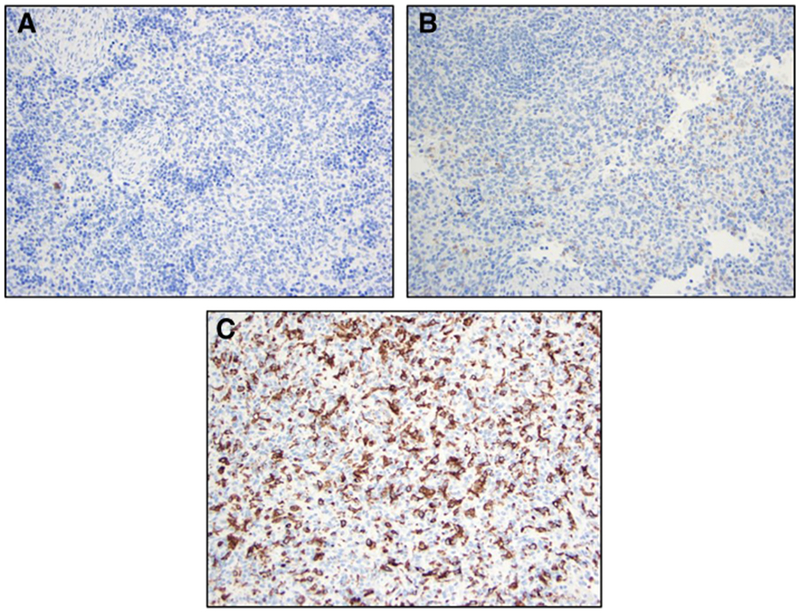
Representative histological images comparing CD163 stained spleens from tissue control lambs (ETC and LTC) and AP Lambs. (A) ETC [CD163 Stain]; (B) LTC [CD163 Stain]; (C) AP Lamb [CD163 Stain]. ETC: Early Tissue Control; LTC: Late Tissue Control; AP: Artificial Placenta.
CD68 Stain
When evaluating clinical variables, there was no significant correlation between WBC, HR, WBC, Hb, or Pits, and the level of CD68 staining within the spleens of the AP lambs. When comparing level of CD68 staining between groups, there was a significantly higher number of CD68 stained splenic cells in the AP group compared to both ETC and LTC groups (Fisher’s exact test p-value = <0.001; Figure 3).
Fig. 3.
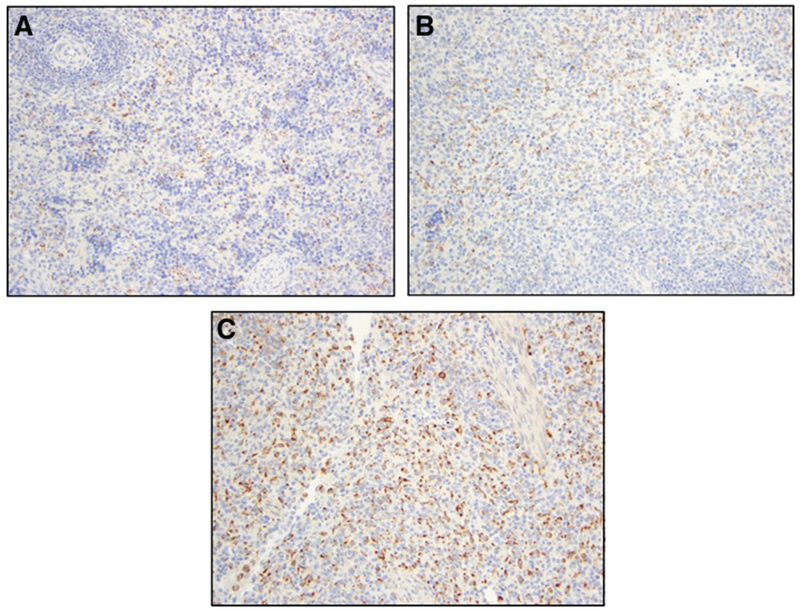
Representative histological images comparing CD68 stained spleens from tissue control lambs (ETC and LTC) and AP Lambs. (A) ETC [CD68 Stain]; (B) LTC [CD68 Stain]; (C) AP Lamb [CD68 Stain]. ETC: Early Tissue Control; LTC: Late Tissue Control; AP: Artificial Placenta.
Splenic Vascular Congestion
Evaluation of hemodynamic variables compared to moderate and severe congestion in the AP group demonstrated no significant increase in congestion in relation to changes in the MAP, HR, WBC, Hb, or Pits. There was slightly more congestion/erythroblastic islands found in controls, but not statistically significant (p=0.072).
Splenic Extramedullary Hematopoiesis (EMH)
Clinical variables among the AP group were not associated with splenic EMH outcomes. It was found that the ETC and LTC groups had significantly more EMH compared to the AP group (Fisher’s exact test p-value = <0.001; Figure 4).
Fig. 4.
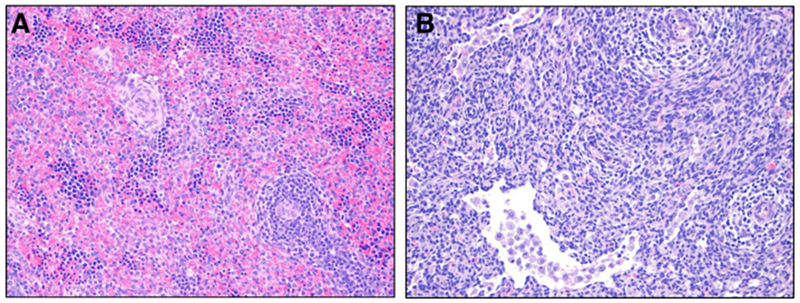
Representative histological images comparing H&E stained spleens from ETC and AP lambs. (A) ETC [H&E Stain]: this is a representative tissue control image with congestion/erythropoietic islands (score 3), EMH (score 3). and no necrosis, sinusoidal histiocytosis or pigmented macrophages (score 0); (B) AP [H&E Stain]: this is a representative image from AP lambs with sinusoidal histiocytosis (score 2), and no necrosis, congestion, pigmented macrophages, or EMH (score 0). ETC: Early Tissue Control; LTC: Late Tissue Control; AP: Artificial Placenta; EMH: Extramedullary Hematopoiesis.
Gestational Age
There were no significant correlations between the 5 histologic categories and gestational age for any of the groups.
Discussion
Premature infants suffer from high rates of morbidity and mortality (1, 15). The AP is a promising alternative in the management of ELGANS and we have previously demonstrated ongoing development and organ protection in the lung, brain, and gastrointestinal tract (5, 16, 17). We must also investigate its effects on other organ systems such as the spleen because sepsis is a major problem in prematurity and the spleen plays an important role in fighting infection, filtering the blood, and EMH, especially in premature infants (7, 9, 11). The current study demonstrated that during AP support splenic development continued, necrosis was absent, and congestion/erythroblastic islands were slightly less than those of controls but not significantly different. It was also found that AP lambs mounted appropriate inflammatory macrophage response to long term support and did not develop sepsis. In addition, EMH transitioned away from the spleen after delivery.
In human neonates and animal models splenic development plays important roles in immunity, hematologic filtering, and hematopoiesis (7, 12, 13, 18) (19, 20). In the ovine fetal model, splenic cellular distribution and maturation occurs between day 25 and birth. Initially mesodermal cellular formation occurs in the dorsal mesogastrium of the fetal spleen (day 25), then develops within the omentum (day 27), splenic parenchymal lymphocytes (day 45), and differentiation of T cell variants (day 55) (19). Between day 77-89 through birth, ovine spleens undergo cellular differentiation with MHCII and MHC I antigens, periarterial lymphatic sheaths (PALS), and periarteriolar T lymphocytes develop into the splenic follicles (19). Leukocytes, macrophages, and B cells migrate and distribute within the spleen from 40-134d in ovine gestation (term=145-150d) (20). Studies have shown that CD68 and CD163 can be used as a marker of macrophage presence and distribution. Specifically, CD163 stains mark a macrophage receptor for bacteria and macrophage cellular markers can be stained with CD68 (21). Our studies found an increase in CD163 and CD68 stained splenic macrophages during AP support compared to both ETC and LTC (Rho P=<0.001). This suggests premature lambs mount an appropriate response to inflammation during long term AP support.
Thrombocytopenia is a common finding in the neonatal intensive care unit (NICU), with extremely low birth weight (ELBW) infants at highest risk. A study evaluating 284 ELBW infants found that thrombocytopenia was found at a rate twice that of term infants in the NICU, and infants <800g were at the greatest risk (22). Sometimes thrombocytopenia can be associated with idiopathic thrombocytopenic purpura (ITP) and increased splenic histiocytosis (23–25). ITP has been studied in patients on ECMO indicating thrombocytopenia is not related to the duration of ECMO, but rather the severity of illness of the patient and the starting platelet level during ECMO initiation (26). ITP and macrophage response to inflammation were studied in our experiments. Thrombocytopenia did not develop in any AP lambs, and there was no association between WBC, HR, MAP, Hb, or Plts in relation to CD163 or CD68 stained splenic macrophages, indicating appropriate response to inflammation, but no signs of ITP.
Splenic vascular congestion related to erythroblastic islands are common in fetal spleen development (27). Erythroblastic islands contain specialized macrophages that enable the development and differentiation of erythroblasts (27, 28). Erythroblastic islands are composed of erythroblasts that develop and differentiate into mature red blood cells (erythropoiesis) at a rate up to 1010 RBC per hour (29). In our studies, AP lambs received 200 units/kg/day of Epogen® to stimulate red blood cell production. Despite this medication, there were still higher levels of congestion/erythroblastic islands in the ETC and LTC groups compared to AP lambs, but this difference was not significant (Rho p =0.72). This shows that erythroblastic islands may decrease after birth but are overall similar to that of spleens in utero.
Neonatal sepsis is a common problem among premature infants (30). Infants treated with ECLS can develop a systemic inflammatory response (SIRS) with leukocyte activation, multiorgan dysfunction, and cytokine activation (‘cytokine storm’). A study evaluating pigs supported on ECMO found that increased inflammatory cytokines, mast cell degranulation, and increased TNF-alpha and IL-8 were associated with ECMO support (31). Appropriate distribution and response to inflammatory changes is important in the developing fetus to prevent death from sepsis. In our experiments, none of the AP lambs developed sepsis or signs of infection. Furthermore, none of the experiments demonstrated splenic necrosis. The association found between decreasing MAPs and mild to moderate sinusoidal histiocytosis, and increased CD68/CD163 cellular changes in the AP group may be explained by the expected inflammatory response seen with long term ECLS support and response to increase in cellular debris and sequestration.
Macrophages play an important role in regulating erythropoiesis, cellular differentiation, and breakdown of cellular debris of aged cells. It is common to find increased macrophages and pigmented macrophages in the spleen during fetal development and in preterm infants (29, 32). Pigmented splenic macrophages can be caused by melanin, hemosiderin, ceroid/lipofuscin accumulation from the breakdown of cellular debris from aged or damaged cells (33). Macrophages phagocytize melanosomes and synthesize their own, both of which lead to a pigmented appearance (33, 34). The role of pigmented cells may be related to cytoprotective functions or enhanced phagocytic properties; however, is not completely understood (33). In our experiments, there was no correlation between WBC, Hb, HR, MAP, or Plts and the number of pigmented macrophages in the AP group, but there were significantly more pigmented macrophages seen in the AP group (p=0.008). This suggests that increased debris and cellular breakdown may be present in premature lambs with long term AP support. This is an expected finding and suggests that AP spleens responded appropriately to removal of splenic debris.
In addition to important immunologic functions, the spleen also plays a role in erythropoiesis and EMH (13). The spleen is one of the primary sites for EMH during fetal development, and contains myeloid precursors, erythroid precursors, and megakaryocytes. Splenic EMH can occur in relation to hemolysis, anemia, and systemic infection, but also as a primary site during fetal development (35). During fetal growth, active EMH occurs in the yolk sac and transfers to the fetal spleen and liver prior to migration to the bone marrow. Around 4-5 months gestation for human fetuses, EMH begins to shift to the bone marrow after birth and as a result the spleen usually shrinks in relation to shift of blood cell stores (10, 11, 18, 27). In our studies, there was significantly more EMH found in both control groups. This suggests that EMH transitions away from the spleen after delivery as seen in the literature.
Limitations of the current study include a small sample size. Another limitation is that splenic volume and weights were not measured during support or necropsy. Future studies should evaluate splenic size and changes in blood flow, as well as organ development and splenic injury after lambs have been transitioned off the AP and sent to the farm for up to one year of life.
Conclusion
The AP has great potential to improve mortality and morbidity in extremely premature infants. As the technology becomes closer to clinical applicability its effects on development of premature organ systems is vital. The current study demonstrated that there was an appropriate inflammatory response, and no sepsis during AP support. In addition, EMH transitioned away from the spleen after delivery, and congestion/erythroblastic islands decrease in frequency but are still similar to those in utero. Overall, this study gives an updated assessment of the effects of the AP on the developing premature spleen.
Acknowledgements:
The authors would like to thank: Cindy Cook and Marie Cornell for assistance with experiment management, ECLS laboratory students for the chronic care of the animals, and Unit for Laboratory Animal Medicine (ULAM) at University of Michigan, especially Wendy Rosebury-Smith, Kathy Toy, and Dr. Hoenerhoff, for preparation of the slides.
Funding: This work was supported by the National Institutes of Health NIH 1R01HD073475-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Type: Research Paper/Therapeutic Potential
Level of Evidence: N/A
Conflicts of Interest: None
References:
- 1.Beck S, Wojdyla D, Say L, al. e The Worldwide Incidence of Preterm Birth: A Systematic Review of Maternal Mortality and Morbidity. Bull World Health Organ. 2010;88(1):31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryner B, Gray B, Perkins E, Davis R, Hoffman H, Barks J, Owens G, Bocks M, Rojas-Pena A, Hirschl R, Bartlett R, Mychaliska G. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. J Pediatr Surg. 2015;50(1):44–9. doi: 10.1016/j.jpedsurg.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlin MA, Werner NL, Church JT, al. e An Artificial Placenta Protects against Lung Injury and Promotes Continued Lung Development in Extremely Premature Lambs Podium Presentation by J Church at American Society for Artificial Internal Organs (ASAIO). June 22, 2017; Chicago, IL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray BW, El-Sabbagh A, Zakem SJ, Koch KL, Rojas-Pena A, Owens GE, Bocks ML, Rabah R, Bartlett RH, Mychaliska GB. Development of an artificial placenta V: 70 h veno-venous extracorporeal life support after ventilatory failure in premature lambs. Journal of pediatric surgery. 2013;48(1):145–53. Epub 2013/01/22. doi: 10.1016/j.jpedsurg.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church JT, Werner NL, Coughlin MA, al. e Effects of an Artificial Placenta on Brain Development and Injury in Premature Lambs. Journal of Pediatric Surgery. 2017;ACCEPTED FOR PUBLICATION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLeod JS, Church JT, Yerramilli PY, al. e Gastrointestinal Mucosal Development and Injury in Premature Lambs Supported by the Artificial Placenta. Journal of Pediatric Surgery. 2017;MANUSCRIPT ACCEPTED FOR PUBLICATION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesta MF. Normal structure, function, and histology of the spleen. Toxicologic pathology. 2006;34(5):455–65. Epub 2006/10/28. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 8.Kim KY, Choi JW, Sohn YM, Chung KS. A prospective study of development of splenic reticuloendothelial function in premature and term infants. Yonsei medical journal. 1980;21(2):110–5. Epub 1980/01/01. doi: 10.3349/ymj.1980.21.2.110. [DOI] [PubMed] [Google Scholar]
- 9.Cofre F, Cofre J. Children with asplenia or hyposplenia: Preventing overwhelming post splenectomy infection. Revista chilena de infectologia : organo oficial de la Sociedad Chilena de Infectologia. 2014;31(1):66–72. Epub 2014/04/18. doi: 10.4067/s0716-10182014000100010. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. Journal of blood medicine. 2010;1:13–9. Epub 2010/01/01. doi: 10.2147/jbm.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosby WH. Hematopoiesis in the human spleen. Archives of internal medicine. 1983;143(7):1321–2. Epub 1983/07/01. [PubMed] [Google Scholar]
- 12.Burn SF, Boot MJ, de Angelis C, Doohan R, Arques CG, Torres M, Hill RE. The dynamics of spleen morphogenesis. Developmental biology. 2008;318(2):303–11. Epub 2008/05/03. doi: 10.1016/j.ydbio.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Jones JF. Development of the spleen. Lymphology. 1983;16(2):83–9. Epub 1983/06/01. [PubMed] [Google Scholar]
- 14.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th Edition. Washington (DC): National Academies Press (US) 2011. [PubMed] [Google Scholar]
- 15.Bryner BS, Mychaliska GB. ECLS for preemies: the artificial placenta. Semin Perinatol. 2014;38(2):122–9. doi: 10.1053/j.semperi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Church JT, Coughlin MA, Perkins EM, Hoffman HR, Barks JD, Rabah R, Bentley JK, Hershenson MB, Bartlett RH, Mychaliska GB. The artificial placenta: Continued lung development during extracorporeal support in a preterm lamb model. Journal of pediatric surgery. 2018. Epub 2018/07/02. doi: 10.1016/j.jpedsurg.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod JS, Church JT, Yerramilli P, Coughlin MA, Perkins EM, Rabah R, Bartlett RH, Rojas-Pena A, Greenson JK, Perrone EE, Mychaliska GB. Gastrointestinal mucosal development and injury in premature lambs supported by the artificial placenta. Journal of pediatric surgery. 2018;53(6):1240–5. Epub 2018/04/02. doi: 10.1016/j.jpedsurg.2018.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzanji J, Emery JL. Changes in the spleen related to birth. Journal of anatomy. 1979;129(Pt 4):819–22. Epub 1979/12/01. [PMC free article] [PubMed] [Google Scholar]
- 19.Maddox JF, Mackay CR, Brandon MR. Ontogeny of ovine lymphocytes. II. An immunohistological study on the development of T lymphocytes in the sheep fetal spleen. Immunology. 1987;62(1):107–12. Epub 1987/09/01. [PMC free article] [PubMed] [Google Scholar]
- 20.Press CM, Hein WR, Landsverk T. Ontogeny of leucocyte populations in the spleen of fetal lambs with emphasis on the early prominence of B cells. Immunology. 1993;80(4):598–604. Epub 1993/12/01. [PMC free article] [PubMed] [Google Scholar]
- 21.Ogembo JG, Milner DA Jr., Mansfield KG, Rodig SJ, Murphy GF, Kutok JL, Pinkus GS, Fingeroth JD. SIRPalpha/CD172a and FHOD1 are unique markers of littoral cells, a recently evolved major cell population of red pulp of human spleen. Journal of immunology (Baltimore, Md : 1950). 2012;188(9):4496–505. Epub 2012/04/12. doi: 10.4049/jimmunol.1103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, Kiehn TI, Ainsworth S. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. Journal of perinatology : official journal of the California Perinatal Association. 2006;26(6):348–53. Epub 2006/04/28. doi: 10.1038/sj.jp.7211509. [DOI] [PubMed] [Google Scholar]
- 23.Czernobilsky B, Freedman HH, Frumin AM. Foamy histiocytes in spleens removed for chronic idiopathic thrombocytopenic purpura. Blood. 1962;19:99–108. Epub 1962/01/01. [PubMed] [Google Scholar]
- 24.Landing BH, Strauss L, Crocker AC, Braunstein H, Henley WL, Will JR, Sanders M. Thrombocytopenic purpura with histiocytosis of the spleen. The New England journal of medicine. 1961;265:572–7. Epub 1961/09/21. doi: 10.1056/nejm196109212651203. [DOI] [PubMed] [Google Scholar]
- 25.Summerell JM, Gibbs WN. Splenic histiocytosis associated with thrombocytopenia. Acta haematologica. 1972;48(1):34–8. Epub 1972/01/01. doi: 10.1159/000208436. [DOI] [PubMed] [Google Scholar]
- 26.Abrams D, Baldwin MR, Champion M, Agerstrand C, Eisenberger A, Bacchetta M, Brodie D. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive care medicine. 2016;42(5):844–52. Epub 2016/03/24. doi: 10.1007/s00134-016-4312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Veterinary pathology. 2012;49(3):508–23. Epub 2012/01/21. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- 28.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–8. Epub 2008/07/25. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Frontiers in physiology. 2014;5:9 Epub 2014/02/14. doi: 10.3389/fphys.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama. 2015;314(10):1039–51. Epub 2015/09/09. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mc IRB, Timpa JG, Kurundkar AR, Holt DW, Kelly DR, Hartman YE, Neel ML, Karnatak RK, Schelonka RL, Anantharamaiah GM, Killingsworth CR, Maheshwari A. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Laboratory investigation; a journal of technical methods and pathology. 2010;90(1):128–39. Epub 2009/11/11. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Variend S, Drummond A, Coombs R. Splenic macrophages in preterm infants: a necropsy study. Journal of clinical pathology. 1996;49(5):391–4. Epub 1996/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franken L, Klein M, Spasova M, Elsukova A, Wiedwald U, Welz M, Knolle P, Farle M, Limmer A, Kurts C. Splenic red pulp macrophages are intrinsically superparamagnetic and contaminate magnetic cell isolates. Scientific reports. 2015;5:12940 Epub 2015/08/12. doi: 10.1038/srep12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallone A, Guida G, Maida I, Cicero R. Spleen and liver pigmented macrophages of Rana esculenta L. A new melanogenic system? Pigment cell research. 2002;15(1):32–40. Epub 2002/02/12. [DOI] [PubMed] [Google Scholar]
- 35.Suttie AW. Histopathology of the spleen. Toxicologic pathology. 2006;34(5):466–503. Epub 2006/10/28. doi: 10.1080/01926230600867750. [DOI] [PubMed] [Google Scholar]


