Abstract
Background
Perinatal brain injuries often impact the corticospinal system, leading to motor impairment and cerebral palsy. Although transcranial magnetic stimulation (TMS) has been widely used to study corticospinal connectivity in adults and older children, similar studies of young infants are limited.
Objectives
The objective was to establish the safety and feasibility of advanced TMS assessments of the corticospinal connectivity of young infants with perinatal brain injury.
Design
This was a pilot, cross-sectional study of 3- to 12-month-old (corrected age) infants with perinatal stroke or intracranial hemorrhage.
Methods
Six participants (2 term, 4 preterm) were assessed with stereotactic neuronavigation-guided TMS. Single-pulse TMS was applied to each hemisphere and responses were recorded simultaneously from both upper limbs. During data collection, vital signs and stress responses were measured to assess safety. Developmental motor outcomes were evaluated using the General Movements Assessment and Bayley Scales of Infant and Toddler Development (3rd edition). A clinical diagnosis of cerebral palsy was recorded, if available.
Results
No adverse events occurred during TMS testing. All sessions were well tolerated. Contralateral motor evoked responses were detected in 4 of 6 participants. Both contralateral and ipsilateral responses were observed in 2 of 6 participants.
Limitations
TMS responses were not obtained in all participants. This could be related to the location of brain injury or developmental stage of the corticospinal system controlling the wrist flexor muscle group from which responses were recorded.
Conclusions
This study provides a summary of the framework for performing novel TMS assessments in infants with perinatal brain injury. Implementing this approach to measure corticospinal connectivity in hypothesis-driven studies in young infants appears to be justified. Such studies could inform the characterization of corticospinal development and the neural mechanisms driving recovery following early interventions.
Perinatal brain injuries can occur between the 20th gestational week and the 28th postnatal day and impact typical brain development. In term babies, perinatal arterial ischemic stroke affects as many as 1 in 2300 live births1 and is the most common cause of cerebral palsy (CP),2 a disorder characterized by motor, sensory, and cognitive impairments secondary to brain lesions. Other brain injuries such as intraventricular hemorrhage and periventricular leukomalacia are common in preterm infants and can also lead to CP.3 Variations in lesion timing and location underlie lesion heterogeneity across individuals with CP, thereby creating challenges for predicting clinical outcomes and designing effective interventions. CP is typically diagnosed by the second year of life; however, as described in a recent review, infants can be identified as “high risk” for CP before 6 months corrected age (CA) using a combination of magnetic resonance imaging (MRI) and movement assessments, such as the General Movements Assessment (GMA).4 The GMA has excellent sensitivity and specificity to determine risk for developing CP and motor impairment; however, this tool has high validity only prior to 20 weeks CA.5
These recent efforts toward early identification of CP have dovetailed with mechanistic investigations of the developing brain and, in particular, the structures that contribute to sensorimotor control. The corticospinal tracts (CSTs) are the primary descending pathways involved in voluntary control of the upper limbs, with critical development occurring in the first year of life. At birth, the CSTs from both hemispheres project bilaterally to both upper limbs. With increasing voluntary movement emerging at 3 months of age, contralateral connections begin to strengthen and ipsilateral connections withdraw, producing a predominantly contralateral pattern of CST connectivity.6,7 However, following perinatal brain injury, these patterns can be disrupted, resulting in the noninjured hemisphere gaining control of the ipsilateral upper limb, while retaining control of the contralateral upper limb. Prior studies have shown that these disrupted patterns are associated with poor functional outcomes in older children with CP.8,9 Importantly, children with differing patterns of brain organization can be more responsive to behavioral or neuromodulatory interventions that are specific to a particular pattern. For example, Kuhnke et al10 found that children with congenital hemiparesis who had preserved contralateral CST connectivity demonstrated increased speed in performing tasks after constraint-induced movement therapy. This improvement was not observed in those with ipsilateral CST connectivity. These results highlight the importance of considering CST connectivity in the assessment and potential treatment of children with perinatal brain injury.
Single-pulse transcranial magnetic stimulation (TMS) is a noninvasive tool used to study the physiology of corticomotor pathways in humans. A painless pulse of electromagnetic induction excites neurons in motor cortex, resulting in a measurable electromyographic (EMG) response in peripheral muscle. TMS has been used to assess corticospinal excitability and study brain organization patterns in many pediatric populations, including children with hemiparetic CP.11–14 Studies by Eyre and colleagues represent the few published reports of TMS assessments in infants with and without neurological injury. In infants who are typically developing these studies have demonstrated CST connectivity that becomes more lateralized after approximately 6 months of age.7 Additionally, the same authors found that brain lesions altered connectivity patterns early in life such that the absence of contralateral responses following stroke was significantly associated with poor functional outcomes.15 TMS could therefore be a potential tool to noninvasively examine infant brain development and its link to motor outcomes; moreover, following early interventions, TMS could also be a useful bioindicator, or an important measure of functional change.16 However, there have been no comparative studies published in the last decade since the work of Eyre et al, and knowledge gaps remain that need to be addressed to advance our understanding of infant neuromotor development and function, especially in this young and highly neuroplastic age group.
To advance our understanding of how CST connectivity contributes to early assessment of infants at high risk for CP and other neuromotor disorders, a comprehensive TMS methodology needs to be established. This methodology will need to incorporate safety assessments and modern TMS technology to characterize CST development in the very young. We predict that these assessments, when incorporated with movement assessments, will contribute to early identification of motor impairment, and be valuable bioindicators of changes in corticomotor function following early intervention. Therefore, the purpose of this study was to evaluate the safety and feasibility of using TMS to assess CST connectivity in young infants following perinatal brain injury, and to report preliminary data on CST connectivity patterns and their relation with motor outcomes following such injury.
Methods
Participants
Data were collected at the University of Minnesota and University of Minnesota Masonic Children's Hospital from August 1, 2016 to May 1, 2018. We studied 6 infants between 3 and 12 months CA at a single time point. Diagnoses included unilateral or bilateral perinatal intracranial hemorrhage, or stroke confirmed by cranial ultrasound and/or MRI. Infants with genetic disorders, neoplasms, disorders of cellular migration and proliferation, traumatic brain injury, MRI-incompatible indwelling medical devices, surgical procedures that constrained spontaneous movements, uncontrolled seizures, or other neurological disorders were excluded (n = 74). Participants were recruited from local neonatal intensive care units and neonatal follow-up clinics in the Minnesota Twin Cities metropolitan area, as well as through online sources (eg, Children's Hemiplegia and Stroke Association website). The University of Minnesota Medical Director screened infants for eligibility prior to enrollment. Parent(s)/guardian(s) listed on the child's birth record provided informed consent for their child to be enrolled in the study. All procedures were approved by the Institutional Review Board, the Clinical and Translational Science Institute, the Center for Magnetic Resonance Research, and the Minnesota Discovery Research and Innovation Economy steering committee at the University of Minnesota, and by Fairview Health Services. Additionally, we received a preapproved investigational device exemption from the Food and Drug Administration to research TMS in this population.
Study Design
This was a cross-sectional, pilot study consisting of 2 visits. During the first visit, a research MRI was completed. During the second visit, TMS and behavioral assessments were completed. TMS assessments were completed within the University of Minnesota Neuromodulation Laboratory for 5 participants; 1 TMS assessment was completed in the University of Minnesota Masonic Children's Hospital Neonatal Intensive Care Unit for a participant who had yet to be discharged and met the eligibility criteria to enroll. Of the 3 total GMA assessments performed, video for the GMA was either obtained at home by caregivers (n = 1) or during 1 of the in-person visits at University of Minnesota research facilities (n = 2). Parents/caregivers had the option to provide an MRI previously performed in a clinical facility (eg, a Neonatal Intensive Care Unit), in which case only TMS and movement assessments were conducted. Our sample included 6 participants to establish the safety and feasibility of our TMS methods and to present preliminary data on CST connectivity patterns.
MRI
MRI scanning provided data of individual participant neuroanatomy to guide stereotactic neuronavigation (see TMS Assessment) and to confirm the primary diagnosis of perinatal brain injury. Scans completed at our MRI research facility used a 3-T Siemens Prisma scanner (Siemens, Erlanger, Germany) and a nonsedative protocol to scan participants during natural sleep.17 One investigator accompanied the participant in the scanner room throughout the scanning procedure to monitor the participant for signs of wakefulness. A T1-weighted structural image was obtained using a 3-dimensional magnetization-prepared rapid-acquisition gradient echo sequence (0.8-mm3 isotropic resolution; repetition time = 2400 milliseconds; echo time = 2.22 milliseconds; inversion time = 1000 milliseconds; flip angle = 8°; acquisition time = 6 minutes, 38 seconds). Scans completed at other facilities (n = 3) used a Siemens Skyra scanner (3-dimensional sagittal T1-weighted; 0.86 × 0.86 × 4-mm resolution; repetition time = 240 milliseconds; echo time = 2.46 milliseconds) or a GE Discovery MR750w (GE Healthcare, Chicago, IL, USA) scanner (3-dimensional axial T1-weighted; 0.45 × 0.45 × 1.2-mm resolution; repetition time = 8.62 milliseconds; echo time = 3.28 milliseconds). If a contraindication to MRI but not TMS was present as defined by our imaging facility (eg, supplemental oxygen per nasal cannula), a cranial ultrasound was used to confirm a diagnosis of perinatal brain injury.
TMS Assessment
Individual T1-weighted structural image data were reconstructed as a 3-dimensional representation of the brain using a frameless stereotactic neuronavigation system (Brainsight; Rogue Research, Montreal, Quebec, Canada), and used to guide the TMS coil location during assessment (Fig. 1). MRI was contraindicated (criteria listed above) in 1 participant, therefore the participant's head and brain were registered and scaled to a Brainsight template. A lightweight head tracker was attached to the forehead of each participant. The head was then registered with the Brainsight system using predefined anatomical landmarks (nasion, tip of nose, left and right preauricular notch). The location of the primary motor cortex, used as the initial TMS testing location, was visually identified and labeled in Brainsight.
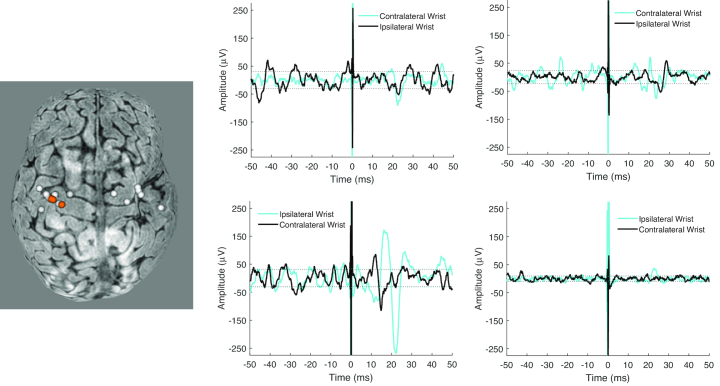
Figure 1.
Left, Brain reconstruction from Brainsight frameless stereotactic neuronavigation system of 1 participant and corresponding stimulation sites (spheres) in the left hemisphere. Orange spheres are locations where motor evoked potentials were elicited. Right, Representative electromyography traces from 1 participant receiving transcranial magnetic stimulation to the left hemisphere. Stimulation intensity was 85% machine stimulator output. Responses are mainly observed in the contralateral (right) wrist; bilateral responses are seen in the left column. Traces show 50 milliseconds before and after stimulation (time = 0 milliseconds). Dotted lines for each trace are 120% of variability in prestimulus activity, used to determine potential responses.
Stainless-steel surface EMG electrodes (101085; Natus Neurology Inc, Pleasanton, CA, USA) were attached bilaterally over wrist flexors on the anterior proximal surface of the forearm to record EMG activity elicited by TMS. This muscle group was chosen based on our pilot EMG testing of differing upper limb muscle groups in infants who are typically developing. We found that reliable EMG signals were challenging to obtain from distal muscle groups (eg, intrinsic hand muscles), whereas proximal muscle groups (eg, biceps brachii) resulted in cardiac artifacts when recording from bilateral muscle groups.18 Movement-related increases in EMG activity were confirmed by engaging the participant in reaching and grasping tasks. TMS was delivered using a MagStim 2002 stimulator and 70-mm diameter figure-of-8 coil (The MagStim Company Ltd, Whitland, Carmarthenshire, United Kingdom). The coil was placed directly on the scalp, with the handle positioned posterior-laterally 45 degrees to the midsagittal plane. Participant positioning was tailored for participant age and caregiver comfort; younger (3–5 months) participants were held in a caregiver's lap, whereas older participants sat or stood independently. Toys and games were used to engage children throughout testing. We began testing the left hemisphere at 50% to 60% maximum stimulator output. Next, we increased the intensity by 5% to 10% and collected 2–3 trials at each intensity based on participants’ responses and tolerance. If tolerated, the maximum stimulator output was increased up to 90% to detect a motor evoked potential (MEP). To facilitate potential responses, an investigator or caregiver engaged with participants, through play and positioning, to facilitate muscle contraction of the wrist flexors during stimulation. This process was repeated for the right hemisphere. EMG data were collected and saved for later analysis using a custom-written LabView (National Instruments, Austin, TX, USA) program, or a portable Cadwell EMG system (Cadwell Industries, Inc, Kennewick, WA, USA) when recorded in the Neonatal Intensive Care Unit (n = 1).
Safety
To monitor participant safety during and after TMS testing, and at the beginning and end of the session, we measured vital signs of heart rate, respiratory rate, and blood pressure, which were compared with age-typical values.19 Throughout the testing session we monitored and documented the following as they occurred: (1) the ongoing “infant state” (quiet sleep, active sleep, drowsy, quiet awake, active awake, crying/fussy); and (2) discrete stress responses (skin coloration, yawning, stretching, sneezing, coughing, crying). To determine any after effects following TMS, we contacted parents/caregivers 24 hours after data collection to document whether any adverse events occurred, and if any changes in behavior were noted. Additional safety protocols during movement assessments were not introduced because adverse events were not revealed nor predicted from the assessments.
Motor Outcomes and Risk of CP
The GMA was used to identify participants in our sample at risk of CP. General movements are age-specific, endogenously generated, spontaneous infant motor activities. Between 9 and 20 weeks CA, predominant general movements are termed fidgety movements—a pattern of continuous, small-amplitude movements of the neck, trunk, and limbs in all directions.20 Video recordings, 3 to 5 minutes long, were acquired with the participant in a calm, awake state, during which interaction from caregivers and researchers was minimized. Videos were coded and scored by an advanced-certificated GMA investigator, who was blinded to medical history, imaging, and outcome data. In this study, fidgety movements were classified as normal if present (intermittent or continual), aberrant if abnormal (exaggerated with respect to speed and amplitude), sporadic (interspersed with long pauses), or absent.20 For participants older than 20 weeks CA at time of assessment, we used the motor subscales of Bayley Scales of Infant and Toddler Development—III (Bayley-III) to assess motor development and estimate developmental equivalent age.21 The Bayley-III was administered by a trained pediatric physical therapist on the same day as TMS assessment. For all participants in our sample, we also examined medical records to determine if a diagnosis of CP was made by 12 months CA.
Data Analysis
Ages are presented as gestational age at birth and CA at time of assessment. Participant state data were summarized as the number of 5-minute epochs in each “infant state” during TMS assessment. Discrete stress responses were summarized as the number of participants exhibiting a particular stress response and the total number of each stress response. EMG data were analyzed separately for each hemisphere. MEPs in wrist flexors were defined as those occurring as identifiable biphasic waveforms within 50 ms following TMS, when waveform amplitude exceeded 120% of the SD of prestimulus activity. In efforts to characterize CST connectivity, we evaluated contralateral and ipsilateral wrist flexor responses following stimulation of each hemisphere. For each participant, we then compared motor outcomes, including available GMA, Bayley-III, and CP diagnosis, with CST connectivity pattern.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Clinical and Motor Outcomes
Table 1 summarizes clinical characteristics of the sample. Radiological (n = 5 MRI; n = 1 cranial ultrasound) summaries of brain injuries for each participant are shown in Figure 2. Overall, 4 of the 6 participants had a clinical diagnosis of CP by age 12 months. Based on the location of their brain injuries, the CST was likely affected in these 4 participants, whereas the CST was likely not affected in the 2 participants without a clinical CP diagnosis. The GMA was performed on 3 participants between 13 and 18 weeks CA. Two of these participants had absent fidgety movements and were subsequently diagnosed with CP at 10 to 12 months CA; the participant with fidgety movements did not have a diagnosis of CP. We administered the Bayley-III motor subscales for the 3 other participants older than 20 weeks CA at enrollment, thus precluding use of the GMA. All 3 of these participants revealed poor motor development based on their CA, and 2 had a diagnosis of CP by 12 months CA.
Table 1.
Infant Clinical Characteristicsa
| Infant | GA, wk | CA at TMS Assessment | Neuroradiology Findings | GMA Findings | Clinical Motor Outcomes |
|---|---|---|---|---|---|
| 1 | 31 | 13 wk | Bilateral parieto-occipital cystic PVL | Absent fidgety movements | Spastic QCP |
| 2b | 36 | 12 mo | Left parietal encephalomalacic cleft, similar but milder cleft on right parietal vertex; hemosiderin deposits in left temporal, posterior left frontal, and left parietal lobes | Fidgety movements | No observable motor impairments, no CP diagnosis |
| 3 | 26 | 18 wk | Bilateral cystic PVL, ex vacuo dilatation of the lateral and third ventricles; left thinning of parietal and occipital lobes | Absent fidgety movements | Spastic QCP |
| 4 | 22 | 9 mo | Bilateral cerebellar hemorrhages, ex vacuo dilatation of the fourth ventricle | Too old at exam | Gross motor delays, DEA = 3 mo 2 d; no CP diagnosis |
| 5 | 39 | 10 mo | Extensive bilateral ischemia in the right parietal, occipital, temporal, and posterior frontal lobes; BG and Thal; and in left parietal, posterior temporal, posterior occipital, and frontal lobes | Too old at exam | HCP, left upper and lower limb impairment, DEA = 4 mo |
| 6 | 41 | 12 mo | Gliosis and encephalomalacia along the right paracentral region, corona radiata, centrum semiovale, and PLIC | Too old at exam | HCP, left upper limb impairment, DEA = 10 mo |
a BG = basal ganglia; CA = corrected age; CP = cerebral palsy; DEA = developmental equivalent age from Bayley-III fine motor subscale; GA = gestational age; GMA = General Movements Assessment; HCP = hemiplegic cerebral palsy; PLIC = posterior limb of internal capsule; PVL = periventricular leukomalacia; QCP = quadriplegic cerebral palsy; Thal = thalamus; TMS = transcranial magnetic stimulation.
b Infant had prior GMA at age CA 13 wk. Centrally acting medications: Infant 3: amlodipine, clonidine, valium, gabapentin, melatonin; Infant 5: phenobarbital.
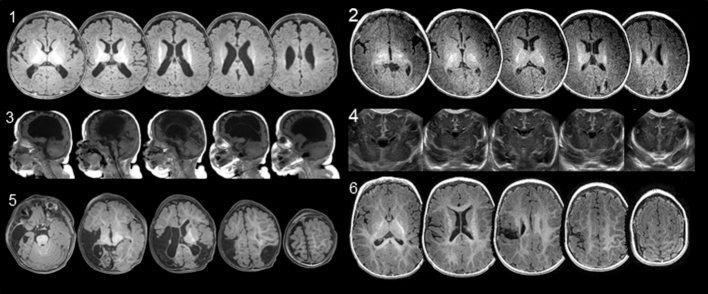
Figure 2.
Neuroimaging showing extent of lesion location in each participant. Images are multiple magnetic resonance imaging slices. Axial views are ordered inferior to superior, and sagittal views are ordered left to right. Infant 4 shows coronal views from cranial ultrasound. All axial images are in radiological orientation (top of image is anterior, left side of image is right side of the head). Participants’ corrected age at time of imaging for image sets 1–6 are 3 months, 3 months, 4 months, 0 weeks, 10 months, and 8 months, respectively.
TMS Assessments
Table 2 indicates motor outcomes, the number of pulses delivered, and responses observed for each participant. Participants received 14 to 37 pulses, and MEP amplitudes ranged from 50 to 200 µV. In 2 of the 5 participants with bilateral brain injury, MEPs were not observed in the contralateral or ipsilateral wrist flexors after stimulation of either hemisphere. Among the other 3 participants with bilateral injury, we observed varied response patterns consisting of contralateral and ipsilateral MEPs, or contralateral MEPs only. In the 1 participant with right unilateral injury, we observed contralateral and ipsilateral MEPs after stimulation of the left hemisphere, and no MEPs after stimulation of the right hemisphere. Figure 1 shows an example of the head reconstruction used in stereotactic neuronavigation and representative EMG traces from the participant who had contralateral and ipsilateral responses..
Table 2.
TMS Summary and Motor Outcomea
| Infant | Unilateral (U) or Bilateral (B) Injury | TMS Pulses Delivered | MSO, % | Contralateral Response Detected | Ipsilateral Response Detected | Motor Outcome | ||
|---|---|---|---|---|---|---|---|---|
| LH | RH | LH | RH | |||||
| 1 | B | 22 | 85 | Yes | Yes | No | No | Spastic QCP |
| 2 | B | 20 | 85 | No | Yes | No | No | No CP diagnosis |
| 3 | B | 14 | 80 | No | Yes | No | Yes | Spastic QCP |
| 4 | B | 29 | 85 | No | No | No | No | DEA = 3 mo 2 d |
| 5 | B | 37 | 90 | No | No | No | No | Left HCP, DEA = 4 mo |
| 6 | U | 21 | 85 | Yes | No | Yes | No | Left HCP, DEA = 10 mo |
a CP = cerebral palsy; DEA = developmental equivalent age from Bayley-III fine motor subscale; HCP = hemiplegic cerebral palsy; LH = left hemisphere stimulation; MSO = maximum stimulator output; QCP = quadriplegic cerebral palsy; RH = right hemisphere stimulation; TMS = transcranial magnetic stimulation.
Safety Evaluation
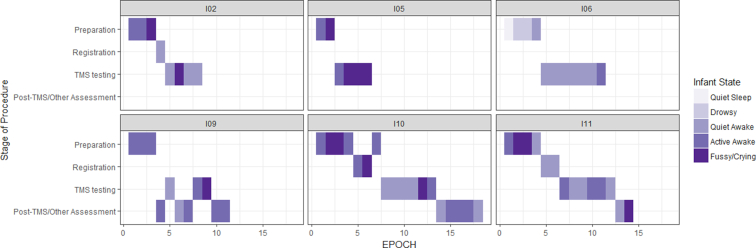
No serious adverse events occurred during any of the MRI, GMA, or TMS sessions. All participants who were evaluated with TMS completed their session. Quantitative summaries of safety assessments during TMS are shown in Table 3. Participants were primarily in quiet or active awake states during testing (80% of all testing time). The most common stress responses, if they occurred, were coughing, crying, and face redness. As shown by the frequency maps in Figure 3, further analysis revealed time spent in a crying or fussy state occurred primarily during preparation (eg, attaching EMG electrodes). Caregivers did not report any concerns for the well-being of their child during testing. Follow-up phone calls to caregivers within 24 hours after testing, confirmed no adverse events had occurred and there were no additional parental concerns.
Table 3.
Safety Assessment of Infant State and Stress Response
| Infant State (proportion in each state) | |||||||
|---|---|---|---|---|---|---|---|
| Infant | Quiet Sleep | Active Sleep | Drowsy | Quiet Awake | Active Awake | Fussy/Crying | Total Epochsa Measured |
| 1 | 0 | 0 | 0 | 0.5 | 0.25 | 0.25 | 8 |
| 2 | 0 | 0 | 0 | 0 | 0.33 | 0.67 | 6 |
| 3 | 0 | 0 | 0.22 | 0.78 | 0 | 0 | 9 |
| 4 | 0 | 0 | 0 | 0.18 | 0.73 | 0.09 | 11 |
| 5 | 0 | 0 | 0 | 0.29 | 0.42 | 0.29 | 14 |
| 6 | 0 | 0 | 0 | 0.5 | 0.33 | 0.17 | 12 |
| Total epochs in each state | 0 | 0 | 2 | 23 | 22 | 13 | 60 |
| Stress Response (no. of responses) | |||||||
|---|---|---|---|---|---|---|---|
| Infant | Skin Color | Yawn | Stretch | Hiccup/Cough | Sneeze | Cries | Individual Total |
| 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| 2 | 1 | 0 | 0 | 0 | 0 | 2 | 3 |
| 3 | 0 | 2 | 0 | 1 | 0 | 0 | 3 |
| 4 | 2 | 0 | 0 | 0 | 1 | 1 | 4 |
| 5 | 1 | 0 | 0 | 3 | 0 | 3 | 7 |
| 6 | 1 | 0 | 0 | 4 | 0 | 3 | 8 |
| Total per category | 5 | 3 | 0 | 9 | 1 | 9 | 27 |
a Epochs are 5-min spans when infant state was documented.
Figure 3.
Frequency of infant state during different stages of the TMS protocol (preparation, Brainsight registration, TMS testing, postassessment/break). Overall, infants were in an active or quiet awake state during TMS testing, and fussy/crying states were most common during preparatory activities. The numbers above each panel correspond to the Infant number in Tables 1-3. TMS = transcranial magnetic stimulation.
Relationship Between Brain Lesion, TMS, and Motor Outcomes
Participants with CP diagnosis
The 2 participants who had bilateral periventricular white matter injuries (Infants 1 and 3) did not have fidgety movements, and were diagnosed with quadriplegic CP. We measured contralateral MEPs from stimulation of both hemispheres in Infant 1. We also measured contralateral and ipsilateral MEPs following stimulation of only the right hemisphere in Infant 3. Two participants also had a diagnosis of hemiparetic CP (Infants 5 and 6). Infant 5 had extensive asymmetric bilateral parenchymal ischemia (right hemisphere more affected than left). We did not measure any MEPs following stimulation of either hemisphere in Infant 5. Infant 6 had right precentral and postcentral gyrus encephalomalacia. We measured contralateral and ipsilateral MEPs (ie, in the more- and less-affected wrist flexors) following stimulation of the left, less-affected hemisphere in Infant 6.
Participants without CP diagnosis
Infant 2 showed asymmetric bilateral hemorrhage (left hemisphere more affected than right), normal fidgety movements following the GMA, and at 12 months CA was not diagnosed with CP. We measured contralateral MEPs in left wrist flexors from stimulation of only the right, less-affected hemisphere in Infant 2. Infant 4 had bilateral cerebellar hemorrhages and gross motor delays but did not have a diagnosis of CP. We did not detect any MEPs from stimulation of either hemisphere in Infant 4.
Discussion
In this pilot study, we incorporated objective safety assessments, modern TMS technology consisting of stereotactic neuronavigation, and focal 70-mm figure-of-8 coils. To the best of our knowledge, we are the first group in the United States to perform CST excitability assessments using advanced noninvasive stimulation methodology in infants with perinatal brain injury, while incorporating the GMA as a risk assessment of CP, when age appropriate. In doing so we demonstrate the potential application of TMS to characterize connectivity of motor pathways early in life. From a safety perspective, single-pulse TMS assessments appear safe and feasible to perform as early as 12 weeks of age. In our sample, no serious adverse events occurred, although we acknowledge larger sample sizes will be required to rigorously assess safety. The present pilot study supports the notion that risk mitigation plans associated with infant TMS are similar to those for older children with CP as described in recently published guidelines.22 Although infants with unmanaged seizures were excluded from this study, we did include those who had experienced seizures in the past but were currently seizure-free, either with or without antiepileptic medications. The safety of single-pulse TMS in primarily older pediatric populations has been confirmed by systematic reviews.23–25 In the work of Eyre et al7,15 and Santiago-Rodriguez et al,26 no adverse events in infants were reported; however, no formal safety assessments or analyses were reported. Our safety assessments indicated that participants were primarily in quiet or active awake states, and that stress responses were more frequent during preparation (ie, attaching EMG electrodes and registering the neuronavigation system) than during stimulation periods. Crying and fussiness during TMS assessment that tended to persist throughout the testing session could be explained by the unfamiliar environment or some need unrelated to the study (eg, tiredness, hunger). Rest and snack breaks were provided to address these needs, and parents did not report any concern for unusual behavior during the testing session. We conclude that involving the parent/caregiver during the procedure was important for infant tolerability. Involvement of parents in this way allows them to help position and comfort the infant as needed. Our protocol provides a family-oriented method for TMS cortical excitability testing in a nonclinical setting that can be employed for future studies.
Single-pulse TMS is a noninvasive tool that has been used to assess neurophysiology in healthy adults and children,27,28 as well as adults and children with neurological and psychiatric conditions.29–33 Furthermore, measures of corticospinal excitability have been demonstrated as key bioindicators to monitor neuroplastic changes following behavioral11,34 and neuromodulatory interventions.35,36 Our data indicate that measuring evoked responses from the distal musculature of infants is feasible, confirming similar reports of MEPs being recorded from infants.7,15,26 We consider an important aim for this and future investigations is to determine the link between CST connectivity early after injury and the risk of CP. Overall, we did not observe a consistent pattern between presence or absence of MEPs and CP diagnosis: MEPs were found in 3 of 4 (75%) participants with a CP diagnosis, and in 1 of 2 (50%) participants without a CP diagnosis. One individual finding is worth noting. In the participant with unilateral right brain injury (Infant 6), we observed bilateral (ie, contralateral and ipsilateral) MEPs when the noninjured left hemisphere was stimulated, and no MEPs when the injured right hemisphere was stimulated. This pattern of CST circuitry is consistent with the motor outcome of left hemiplegic CP and the findings of Eyre et al regarding infants with unilateral brain injury.15 Bilateral responses are less prevalent after 6 months of age in infants who are typically developing, but persist in infants with brain injuries.7,15 Bilateral responses present at 12 months that continue throughout life, as seen in some older children with CP, are consistent with poor motor outcomes.37–39 For the 2 participants in whom we did not detect any MEPs (Infants 4 and 5), both had developmental delays based on the Bayley-III, but only 1 had a diagnosis of CP. Overall, we expected to observe the presence of MEPs, particularly for Infant 4 with no identified injury to the corticospinal system. Reasons for lack of MEP response could be related to the target muscle for recording EMG, small head volume, and overall development of the CST (these factors are addressed in Study Limitations and Challenges below). Complementary neuroimaging measures, such as diffusion-weighted tractography and resting-state functional connectivity, now commonly used in infants,17 could be implemented in future work to help address CST connectivity in such cases where MEPs are not obtained.
Overall, our results illustrate the heterogeneous profiles of both lesion presentation and subsequent CST connectivity in this population. Heterogeneity related to lesion characteristics and timing of injury is common following perinatal brain injury, and likely underlies the variability of response to standardized treatments. As shown in older children with CP, CST connectivity could represent a bioindicator that can be used to stratify samples to examine treatment effects across subgroups.13,40 Based on the TMS assessment we described in our protocol,18 similar approaches can be translated to infants in the first year of life. We anticipate that CST connectivity can ultimately be a factor that will guide individualized approaches to rehabilitation, which includes both intensive motor training and neuromodulation.
Our work has important implications for future research and clinical practice relating to the early detection, diagnosis, and treatment of CP. The GMA is a highly sensitive tool used to detect risk for CP4; and it correctly predicted the 2 participants with CP diagnosis and 1 participant without CP diagnosis in our study. Advanced MRI assessments, such as diffusion tensor imaging performed within 3 months CA, can also be predictive of future motor impairment.41–43 Within the first 12 months of life, combining the GMA and MRI findings with CST patterns of connectivity derived from TMS assessments can provide complementary information that is crucial for identifying infants with different trajectories of atypical development. Opportunities for early intervention provided by this approach could therefore result in greatly improved clinical outcomes.
Measuring corticospinal excitability could also contribute toward our understanding of how the infant brain responds to early interventions. Currently, few available methods can characterize neurophysiological changes in infants, and thus early intervention studies alone might lack the ability to build a mechanistic understanding of proposed treatment effects. The strength of establishing TMS assessments in the infant population is the ability to provide a neural marker of change, namely CST excitability, which can be monitored before and after treatments. In adults with stroke, TMS has been used to show the effects of rehabilitation interventions such as constraint-induced movement therapy on motor cortex plasticity.44,45 A recently published study protocol proposed the use of TMS as a physiological tool to examine connectivity following intensive walking rehabilitation following perinatal brain injury. This could be the first study to examine changes in CST excitability using TMS in this population.46 Characterizing brain circuitry can provide insight into effective rehabilitation interventions that promote motor recovery. For instance, older children with CP with both contralateral and ipsilateral CST projections experience motor improvements following bimanual training,40 whereas children with only ipsilateral CST projections show less improvement in movement speed after constraint-based upper-limb training, compared with children with contralateral CST projections.10 Future early rehabilitation interventions could help reveal similar approaches where intervention is guided by CST connectivity in an infant population.10,47 Finally, TMS of the motor cortex can provide an insight into targets for adjuvant noninvasive neuromodulatory treatments. In previous work, we have demonstrated that the motor “hotspot” coordinates as determined by TMS are not the same as the anatomical location of motor cortex based on standardized EEG coordinates.48 In adults with stroke, the motor hotspot of the cortex, or scalp location of the coil where greatest excitability is found, is used as an individualized target for noninvasive stimulation designed to enhance neuroplasticity in the motor cortex. Incorporating stereotactic neuronavigation in the present study to determine the optimal motor cortex stimulation site in young infants is expected to be a key contributor to the identification of targets for neuromodulatory interventions.
Study Limitations and Challenges
There are several limitations to address in this exploratory work. Clearly, we are unable to draw significant conclusions regarding the relationship between CST connectivity and risk of CP in this small and heterogeneous sample. For example, the location of brain injury in 2 participants likely did not affect the CST. We plan to capture additional TMS data in the 9- to 20-week age range in a future cohort specifically with CST injury to link developing brain structure and function with spontaneous motor activity after brain injury in young infants. Considering the study overall, we emphasize that our results support the safety and feasibility of TMS assessments in infants with perinatal brain injury. In regard to measuring MEPs in infants in the 3- to 12-month CA age range, we observed MEPs in at least 1 wrist flexor in 4 of 6 participants. Eyre et al found MEPs in 50% of infants at 24 months of age but it is unknown what percentage of that sample had MEPs at earlier ages.15
The challenge of recording MEPs in infants can be related to factors such as: (1) suboptimal target muscle, (2) small head anatomy, and (3) immature CST development and excitability. First, our choice to measure from wrist flexor muscles was based on initial pilot data showing cardiac artifacts during bilateral biceps EMG.18 Although Eyre et al reported MEPs from intrinsic hand muscles in neonates,15 we found the long finger flexors located in the proximal forearm of young infants provided a practical location for recording EMG. Because there is no consensus on the optimal muscle group for infant TMS, additional recordings from other muscle groups intrinsic and extrinsic to the hand could provide more opportunities to measure MEPs and allow for thorough characterization of CST connectivity. Second, a smaller head volume can impact the distribution of TMS-induced electric fields because these fields are proportional to head size.49 Therefore, the peak electric field is likely smaller in the infant head compared with an adult head, which could decrease the likelihood of eliciting a MEP. Advances in computational modeling of electric fields produced by magnetic stimulation have begun to identify the influence of individual variability of stimulation effects related to differences in brain anatomy in adults.50,51 These advanced approaches would have value for the continued study of noninvasive brain stimulation in infants, and guiding more targeted stimulation methods. Finally, developmental changes in brain connectivity and excitability in the first year of life can influence the ability to obtain MEPs. A recent EMG study in infants who are typically developing revealed that the period between 9 and 25 weeks of age, which coincides with the appearance of fidgety movements, is associated with a significant increase in corticomuscular coherence of wrist flexors.52 The reverse relationship, that is, a reduction (or no change) in distal muscle coherence, could be related to the absence of fidgety movements, thereby explaining the variability and lack of response observed in some infants. Because of the overall decreased excitability and smaller head volume in infants, activating motor pathways using TMS could then require higher stimulation intensities (ie, >90%), as also reported by Eyre et al, levels we did not test in this pilot work.
Conclusion
Early detection of motor impairment is pivotal in working toward our goal of providing effective motor interventions within the first year of life, thereby capitalizing on the neuroplastic potential of the young developing brain. Developing and applying TMS protocols in combination with neuroimaging and behavioral assessments for infants as represented in this study, is expected to help build a greater understanding of how the young brain recovers. Despite the heterogeneity and small sample size of the present study, we provide here a methodological foundation for the integration of novel and individualized early assessments in order to guide interventions and optimize motor outcomes.
Contributor Information
Samuel T Nemanich, Department of Rehabilitation Medicine, University of Minnesota, MMC 388, 420 Delaware St SE, Minneapolis, MN 55455 (USA). Address all correspondence to Dr Nemanich at: nemanich@umn.edu.
Chao-Ying Chen, Department of Rehabilitation Sciences, Hong Kong Polytechnic University, Kowloon, Hong Kong.
Mo Chen, Department of Psychiatry and Behavioral Sciences, University of Minnesota.
Elizabeth Zorn, Department of Pediatrics, University of Minnesota.
Bryon Mueller, Department of Psychiatry and Behavioral Sciences, University of Minnesota.
Colleen Peyton, Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Jed T Elison, Department of Pediatrics; and Institute of Child Development, College of Education and Human Development, University of Minnesota.
James Stinear, Department of Exercise Sciences, University of Auckland, Auckland, New Zealand.
Raghu Rao, Department of Pediatrics, University of Minnesota.
Michael Georgieff, Department of Pediatrics, University of Minnesota.
Jeremiah Menk, School of Public Health, Division of Biostatistics, University of Minnesota.
Kyle Rudser, School of Public Health, Division of Biostatistics, University of Minnesota.
Bernadette Gillick, Department of Rehabilitation Medicine, University of Minnesota.
Author Contributions and Acknowledgments
Concept/idea/research design: C-Y. Chen, M. Chen, B. Mueller, C. Peyton, J.T. Elison, J. Stinear, R. Rao, M. Georgieff, J. Menk, K. Rudser, B. Gillick
Writing: S.T. Nemanich, C-Y. Chen, M. Chen, E. Zorn, B. Mueller, C. Peyton, J.T. Elison, J. Stinear, R. Rao, M. Georgieff, J. Menk, K. Rudser, B. Gillick
Data collection: S.T. Nemanich, C-Y. Chen, M. Chen, B. Mueller, J.T. Elison, B. Gillick
Data analysis: S.T. Nemanich, C-Y. Chen, M. Chen, E. Zorn, B. Mueller, C. Peyton, J. Stinear, R. Rao, J. Menk, K. Rudser, B. Gillick
Project management: S.T. Nemanich, C-Y. Chen, B. Gillick
Fund procurement: C-Y. Chen, B. Gillick
Providing participants: E. Zorn, M. Georgieff, B. Gillick
Providing facilities/equipment: M. Chen, J.T. Elison, M. Georgieff, B. Gillick
Providing institutional liaisons: M. Georgieff, B. Gillick
Consultation (including review of manuscript before submitting): S.T. Nemanich, C-Y. Chen, M. Chen, E. Zorn, B. Mueller, C. Peyton, J.T. Elison, J. Stinear, R. Rao, J. Menk, K. Rudser, B. Gillick
The authors thank Jesse Kowalski, Tanjila Nawshin, and Ellen Sutter for assistance with data collection; the Neonatal Intensive Care Unit team/occupational therapists at the University of Minnesota Masonic Children's Hospital, and Drs Suresh Kotagal and Joline Brandenburg at the Mayo Clinic in Rochester, Minnesota, for assistance with recruitment. Finally, we appreciate the dedication and involvement of families participating in our research.
Ethics Approval
All procedures were approved by the Institutional Review Board, the Clinical and Translational Science Institute, the Center for Magnetic Resonance Research, and the Minnesota Discovery Research and Innovation Economy steering committee at the University of Minnesota, and by Fairview Health Services. Additionally, we received a preapproved investigational device exemption from the Food and Drug Administration to research TMS in this population.
Funding
This study was funded by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institutes of Child Health and Development K01 Award (HD078484-01A1), the Cerebral Palsy Alliance Research Foundation, the University of Minnesota's Discovery, Research, and Innovation Economy (MnDRIVE) initiative, the University of Minnesota Academic Health Center, the University of Minnesota Center for Magnetic Resonance Research (P41 EB015894 and 1S10OD017974-01), and the University of Minnesota Clinical and Translational Science Institute (National Center for Advancing Translational Sciences of the National Institutes of Health-UL1TR002494).
Disclosures and Presentations
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
This article is adapted in part from a poster abstract presented at the Joint Meeting of the New York City Neuromodulation Conference & NANS Summer Series, August 24–26, 2018, and from an oral presentation made at the Australian Academy of Cerebral Palsy and Developmental Medicine, March 21–24, 2018; the Australasian Cerebral Palsy Alliance, March 23, 2018, Auckland, New Zealand; and the American Academy of Cerebral Palsy and Developmental Medicine, October 9–14, 2018, Cincinnati, Ohio.
References
- 1. Kirton A, Deveber G. Life after perinatal stroke. Stroke. 2013;44:3265–3271. [DOI] [PubMed] [Google Scholar]
- 2. Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014;51:760–768. [DOI] [PubMed] [Google Scholar]
- 3. Rogers B, Msall M, Owens Tet al. Cystic periventricular leukomalacia and type of cerebral palsy in preterm infants. J Pediatr. 1994;125:S1–S8. [DOI] [PubMed] [Google Scholar]
- 4. Novak I, Morgan C, Adde Let al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol. 2008;50:254–266. [DOI] [PubMed] [Google Scholar]
- 6. Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. [DOI] [PubMed] [Google Scholar]
- 7. Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. [DOI] [PubMed] [Google Scholar]
- 8. Holmstrom L, Vollmer B, Tedroff Ket al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52:145–152. [DOI] [PubMed] [Google Scholar]
- 9. Islam M, Nordstrand L, Holmstrom L, Kits A, Forssberg H, Eliasson AC. Is outcome of constraint-induced movement therapy in unilateral cerebral palsy dependent on corticomotor projection pattern and brain lesion characteristics?. Dev Med Child Neurol. 2014;56:252–258. [DOI] [PubMed] [Google Scholar]
- 10. Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50:898–903. [DOI] [PubMed] [Google Scholar]
- 11. Friel KM, Kuo HC, Fuller Jet al. Skilled bimanual training drives motor cortex plasticity in children with unilateral cerebral palsy. Neurorehabil Neural Repair. 2016;30:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juenger H, Kumar V, Grodd W, Staudt M, Krageloh-Mann I. Preserved crossed corticospinal tract and hand function despite extensive brain maldevelopment. Pediatr Neurol. 2009;41:388–389. [DOI] [PubMed] [Google Scholar]
- 13. Gillick B, Rich T, Nemanich Set al. Transcranial direct current stimulation and constraint-induced therapy in cerebral palsy: a randomized, blinded, sham-controlled clinical trial. Eur J Paediatr Neurol. 2018;22:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121:1922–1929. [DOI] [PubMed] [Google Scholar]
- 15. Eyre JA, Smith M, Dabydeen Let al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system?. Ann Neurol. 2007;62:493–503. [DOI] [PubMed] [Google Scholar]
- 16. Raiten D, Combs GF Jr. Biomarkers and bioindicators: providing clarity in the face of complexity. Directions in Nutritional Assessment. 2015;29:39–44. [Google Scholar]
- 17. Howell BR, Styner MA, Gao Wet al. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage. 2019;185:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen CY, Georgieff M, Elison Jet al. Understanding brain reorganization in infants with perinatal stroke through neuroexcitability and neuroimaging. Pediatr Phys Ther. 2017;29:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleming S, Thompson M, Stevens Ret al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Einspieler C, Prechtl HF, Bos AF, Ferrari F, Cioni G. Prechtl's Method on the Qualitative Assessment of General Movements in Preterm, Term, and Young Infants. London: Mac Keith Press; 2004. [DOI] [PubMed] [Google Scholar]
- 21. Albers CA, Grieve AJ. Test review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoeduc Assess. 2007;25:180–190. [Google Scholar]
- 22. Gillick BT, Gordon AM, Feyma Tet al. Non-invasive brain stimulation in children with unilateral cerebral palsy: a protocol and risk mitigation guide. Front Pediatr. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen CH, Kluger BM, Buard I. Safety of transcranial magnetic stimulation in children: a systematic review of the literature. Pediatr Neurol. 2017;68:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frye RE, Rotenberg A, Ousley M, Pascual-Leone A. Transcranial magnetic stimulation in child neurology: current and future directions. J Child Neurol. 2008;23:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santiago-Rodriguez E, Leon-Castillo C, Harmony T, Fernandez-Bouzas A, Garcia-Gomar ML. Motor potentials by magnetic stimulation in periventricular leukomalacia. Pediatr Neurol. 2009;40:282–288. [DOI] [PubMed] [Google Scholar]
- 27. Garvey MA, Kaczynski KJ, Becker DA, Bartko JJ. Subjective reactions of children to single-pulse transcranial magnetic stimulation. J Child Neurol. 2001;16:891–894. [DOI] [PubMed] [Google Scholar]
- 28. Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114:1662–1670. [DOI] [PubMed] [Google Scholar]
- 29. Barlow KM, Seeger TA, Gilbert DL. Transcranial magnetic stimulation neurophysiology of pediatric traumatic brain injury. In: Kirton A, Gilbert D, eds. Pediatric Brain Stimulation. Oxford: Academic Press; 2016:345–374. [Google Scholar]
- 30. Limburg K, Jung NH, Mall V, Gilbert DL. Assessing normal developmental neurobiology with brain stimulation. In: Kirton A, Gilbert D, eds. Pediatric Brain Stimulation. Oxford: Academic Press; 2016:23–43. [Google Scholar]
- 31. Gilbert DL. TMS Applications in ADHD and developmental disordersIn: Kirton A, Gilbert D, eds. Pediatric Brain Stimulation. Oxford: Academic Press; 2016:153–182. [Google Scholar]
- 32. Staudt M, Gilbert DL. TMS mapping of motor development after perinatal brain injury. In: Kirton A, Gilbert D, eds. Pediatric Brain Stimulation. Oxford: Academic Press; 2016:183–194. [Google Scholar]
- 33. Stinear CM, Petoe MA, Byblow WD. Primary motor cortex excitability during recovery after stroke: implications for neuromodulation. Brain Stimul. 2015;8:1183–1190. [DOI] [PubMed] [Google Scholar]
- 34. Boake C, Noser EA, Ro Tet al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. [DOI] [PubMed] [Google Scholar]
- 35. Cassidy JM, Chu H, Anderson DCet al. A comparison of primed low-frequency repetitive transcranial magnetic stimulation treatments in chronic stroke. Brain Stimul. 2015;8:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398–410. [DOI] [PubMed] [Google Scholar]
- 37. Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. [DOI] [PubMed] [Google Scholar]
- 38. Staudt M. Reorganization after pre- and perinatal brain lesions. J Anat. 2010;217:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mackey A, Stinear C, Stott S, Byblow WD. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Front Neurol. 2014;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smorenburg AR, Gordon AM, Kuo HCet al. Does corticospinal tract connectivity influence the response to intensive bimanual therapy in children with unilateral cerebral palsy?. Neurorehabil Neural Repair. 2017;31:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Aa NE, Leemans A, Northington FJet al. Does diffusion tensor imaging-based tractography at 3 months of age contribute to the prediction of motor outcome after perinatal arterial ischemic stroke?. Stroke. 2011;42:3410–3414. [DOI] [PubMed] [Google Scholar]
- 42. van der Aa NE, Verhage CH, Groenendaal Fet al. Neonatal neuroimaging predicts recruitment of contralesional corticospinal tracts following perinatal brain injury. Dev Med Child Neurol. 2013;55:707–712. [DOI] [PubMed] [Google Scholar]
- 43. Massaro AN. MRI for neurodevelopmental prognostication in the high-risk term infant. Semin Perinatol. 2015;39:159–167. [DOI] [PubMed] [Google Scholar]
- 44. Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liepert J, Miltner WH, Bauder Het al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. [DOI] [PubMed] [Google Scholar]
- 46. Hurd C, Livingstone D, Brunton Ket al. Early intensive leg training to enhance walking in children with perinatal stroke: protocol for a randomized controlled trial. Phys Ther. 2017;97:818–825. [DOI] [PubMed] [Google Scholar]
- 47. Boyd RN, Ziviani J, Sakzewski Let al. REACH: study protocol of a randomised trial of rehabilitation very early in congenital hemiplegia. BMJ Open. 2017;7:e017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rich TL, Menk JS, Rudser KDet al. Determining electrode placement for transcranial direct current stimulation: a comparison of EEG- versus TMS-guided methods. Clin EEG Neurosci. 2017;48:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weissman JD, Epstein CM, Davey KR. Magnetic brain stimulation and brain size: relevance to animal studies. Electroencephalogr Clin Neurophysiol. 1992;85:215–219. [DOI] [PubMed] [Google Scholar]
- 50. Opitz A, Paulus W, Will S, Antunes A, Thielscher A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015;109:140–150. [DOI] [PubMed] [Google Scholar]
- 51. Thielscher A, Opitz A, Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;54:234–243. [DOI] [PubMed] [Google Scholar]
- 52. Ritterband-Rosenbaum A, Herskind A, Li Xet al. A critical period of corticomuscular and EMG-EMG coherence detection in healthy infants aged 9-25 weeks. J Physiol. 2017;595:2699–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]