Abstract
Background and Purpose
Cerebral palsy (CP) is characterized by decreased passive joint range-of-motion and impaired walking, resulting in progressive loss of function. Typical gait training interventions for children with CP appear insufficient to mitigate these effects. The purpose of this case report is to describe the use of a new treadmill-based gait training intervention using active correction with functional electrical stimulation (FES) in 2 adolescents with CP.
Case Description
Two participants with CP (13-year-old girls, Gross Motor Function Classification System [GMFCS] level II and III) trained by walking on a treadmill, with FES assistance, for 30 minutes, 3 times per week, for 12 weeks. The intervention used a feedback control system to detect all 7 phases of gait in real time and triggered FES to the appropriate muscle groups (up to 5 bilaterally) based on the detected gait phase. Joint kinematics, step width, stride length, walking endurance, peak oxygen uptake (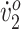 ), and oxygen (O2) cost of walking were evaluated preintervention and postintervention.
), and oxygen (O2) cost of walking were evaluated preintervention and postintervention.
Outcomes
Both participants showed improved knee and ankle angles and step width relative to children who are typically developing, and both exhibited increased stride length. One participant (GMFCS III) improved peak 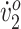 and walking endurance but not O2 cost of walking at her original self-selected walking speed. The other participant (GMFCS II) improved O2 cost of walking but not peak
and walking endurance but not O2 cost of walking at her original self-selected walking speed. The other participant (GMFCS II) improved O2 cost of walking but not peak 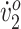 or walking endurance. These differences are partly explained by differences in gait type, functional abilities, and initial fitness levels. Most improvements persisted at follow-up, indicating short-term neurotherapeutic effects.
or walking endurance. These differences are partly explained by differences in gait type, functional abilities, and initial fitness levels. Most improvements persisted at follow-up, indicating short-term neurotherapeutic effects.
Discussion
Most improvements persisted at follow-up, suggesting short-term neurotherapeutic effects. This case series demonstrates the promising utility of FES-assisted gait-training interventions, tailored to target individual gait deviations, in improving walking performance.
Cerebral palsy (CP) is a movement disorder caused by brain injury during development. Movement pathologies typically worsen over time, resulting in progressive loss of function, decreased quality of life, and increased risk of mortality and morbidity. Gait training interventions, such as body-weight–supported treadmill training, have shown some success in improving gait in individuals with CP.1 However, interventions that provide physiologically based corrections to reinforce movements, such as functional electrical stimulation (FES), might produce longer-lasting effects. We hypothesize that a physiologically based FES walking intervention can be more effective than usual training methods in mitigating functional decline in children with CP.
FES-assisted walking interventions have shown immediate (neuroprosthetic) effects in children with CP, including improved spatiotemporal gait parameters2,3 and improved gait kinematics3–6 and kinetics.6 Most research on FES in individuals with CP has focused on stimulating the ankle dorsiflexors, improving foot clearance in the swing phase and initial contact while FES is applied.5,7–10 Positive neuroprosthetic results have also been shown in the application of FES to the hip muscles,2 hamstring,11 plantarflexor,5 and quadriceps femoris muscles.7 Unfortunately, these interventions have shown only secondary lasting improvements. For example, in a randomized controlled trial on 32 children with CP (mean age 10 years 8 months, GMFCS level I or II), Pool et al3 showed increased ankle angle at initial contact and increased maximum dorsiflexion in swing, but follow-up improvements were limited to mobility and balance scores and reduced gastrocnemius spasticity. Similarly, in a case series involving 14 children/adolescents with CP (mean [SD] age = 13.1 [3.56] years; GMFCS level I or II), Damiano et al12 showed increased maximum ankle dorsiflexion while FES was applied to the ankle to correct for foot drop. However, they reported that barefoot ankle motion returned to baseline values at follow-up.
These studies focused on the use of FES on the tibialis anterior muscle as a neuroprosthesis in activities of daily living, for 8 weeks and 3 months, respectively. In contrast, Hakansson et al13 showed significant functional improvements, including increased walking speed, after 12 weeks of FES treadmill training (3 days a week for approximately 1 hour per day) for 11 patients with stroke. Similarly, Sabut et al14 showed that the combination of FES and conventional physical therapy for 12 weeks (6 days per week, approximately 1 hour per day) could lead to significant motor recovery in patients with stroke. Therefore, we hypothesize that adapting the task-specific training protocol in Hakansson et al to train children with CP will produce significant neurotherapeutic improvements.
The heterogeneity of gait pathologies in children with CP requires the use of customized stimulation protocols. Zahradka15 reported neuroprosthetic improvements from tailored FES in 6 individuals with CP during treadmill walking. Using a novel gait-phase detection system, combinations of 5 muscle groups (glutei maximi, quadriceps femores, hamstrings, dorsiflexors, and plantarflexors) were stimulated as needed in each gait phase. The individualized stimulation protocols used in this study resulted in patient-specific improvements such as decreased ankle dorsiflexion at initial contact and improved hip and knee extension during stance. Our outcomes should be interpreted keeping in mind our methods for determining FES stimulation protocols: protocols—3 physical therapists reviewed side, front, and back views of each participant walking and indicated to which muscles and during which phases they deemed stimulation necessary to induce a more typical functional gait pattern. Only by consensus were the recommended muscle/phase combinations added to the participant's FES protocol. We understand that this methodology differs from a more repeatable stimulation protocol based on a generic physiological gait, that is, one that matches typical muscle activation timing. However, Zahradka15 has shown that for patients with CP, such physical therapist–derived protocols are more effective than those derived from generic gait patterns in producing neurotherapeutic improvements in gait deviations.
The purpose of this case series was to describe functional carryover of walking performance improvements in 2 adolescent patients with CP after training for 12 weeks using an FES treadmill training protocol similar to that of Hakansson et al,13 combined with the gait-phase detection system and methodology from Zahradka.15
History and Examination
Two 13-year-old female participants with spastic diplegic CP participated in this case series (Tab. 1). Procedures were approved by Shriners Hospitals for Children, Philadelphia (Western Institutional Review Board), and the University of Delaware. Consent and assent were obtained prior to participation. Initial findings are presented in Table 2. These 2 participants were a sample of convenience from a larger study investigating the immediate effects of FES on walking gait deviations. They agreed to participate in a 12-week treadmill training intervention with FES applied to up to 5 muscle groups.
Table 1.
Participant Informationa
| Characteristics | Participant 1 | Participant 2 |
|---|---|---|
| Height, m | 1.31 | 1.44 |
| Weight, kg | 32.13 | 42.53 |
| GMFCS level | III | II |
| GMFM section D | 27 | 37 |
| GMFM section E | 23 | 63 |
| SCALE (left/right) | 1/1 | 3/4 |
| Assistive devices | Rollator | None |
a GMFCS = Gross Motor Function Classification System; GMFM = Gross Motor Function Measure; SCALE = Selective Control Assessment of the Lower Extremity.23
Table 2.
Metabolic, Spatiotemporal, Walking, and Endurance Outcomesa
| Participant 1 | Participant 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Pre | Post | % Change | Follow-up | % Change | Pre | Post | % Change | Follow-up | % Change |
Peak 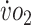 , mL/kg/min , mL/kg/min |
23.7 | 37.6 | 58.6 | 36.7 | 54.9 | 36.1 | 35.5 | −1.66 | 35.8 | −0.83 |
| O2 cost of walking, mL/kg/m | 0.46 | 0.45 | −2.17 | 0.53 | 15.2 | 0.24 | 0.14 | −41.7 | 0.15 | −37.5 |
| Step width, mm | 86 | 111 | 29.1 | 106 | 23.3 | 172 | 141 | −18.0 | 126 | −26.7 |
| Stride length, mm | 648 | 970 | 49.7 | 945 | 45.8 | 819 | 949 | 15.9 | 915 | 11.7 |
| Walking speed,b m/s | 0.54 | 0.81 | 50.0 | 0.82 | 51.9 | 1.68 | 1.60 | −4.76 | 1.70 | 1.19 |
| Walking distance, m | 224 | 281 | 25.4 | 302 | 34.8 | 555 | 567 | 2.14 | 559 | 0.66 |
| Self-selected walking speed, m/s | 0.45 | N/A | N/A | N/A | N/A | 0.80 | N/A | N/A | N/A | N/A |
a
% Change is relative to pretraining. Pre = pretraining; Post = posttraining; 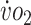 = oxygen uptake.
= oxygen uptake.
b Walking speed was determined from the first minute of walking during the 6-Minute Walk Test.
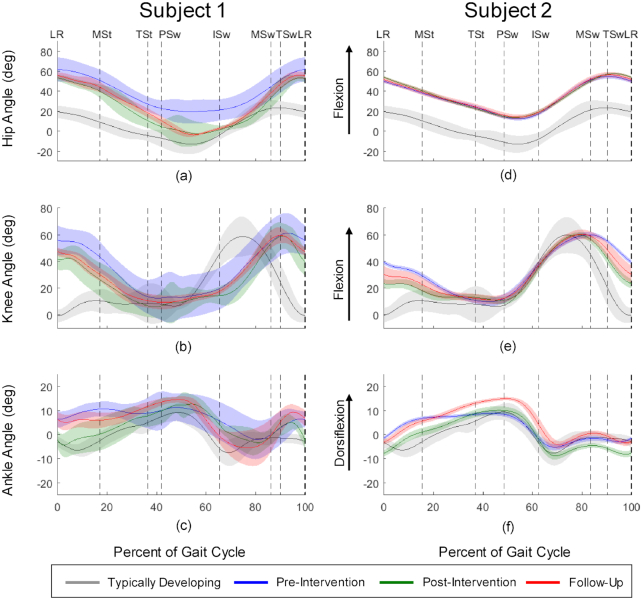
Participant 1 had a scissoring crouch gait and used a rollator for ambulation. She walked with short step width (0.086 m) and stride length (0.648 m) relative to typically developing healthy children walking on treadmills (0.105 m and 0.979 m, respectively).16 Her peak oxygen uptake (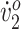 ) (23.7 mL/kg/min) was considered “very poor” compared with normative data.17 Her O2 cost of walking (0.46 mL/kg/min) was high compared with typical walking values for children (0.17 mL/kg/min).18 Her self-selected walking speed (SSWS) was 0.45 m/s. Her initial gait analysis revealed increased hip flexion throughout gait (Fig. 1a), increased knee flexion from midswing (MSw) through midstance (MSt) (Fig. 1b), and increased ankle dorsiflexion throughout most of the gait cycle (Fig. 1c).
) (23.7 mL/kg/min) was considered “very poor” compared with normative data.17 Her O2 cost of walking (0.46 mL/kg/min) was high compared with typical walking values for children (0.17 mL/kg/min).18 Her self-selected walking speed (SSWS) was 0.45 m/s. Her initial gait analysis revealed increased hip flexion throughout gait (Fig. 1a), increased knee flexion from midswing (MSw) through midstance (MSt) (Fig. 1b), and increased ankle dorsiflexion throughout most of the gait cycle (Fig. 1c).
Figure 1.
Pretraining and posttraining hip, knee, and ankle angles for Participant 1 and Participant 2 (left leg). Gait-phase transitions are indicated by dashed vertical lines and are determined by each participant's individual gait pattern. Each transition is labeled with the gait phase transitioned into: loading response (LR); midstance (MSt); terminal stance (TSt); preswing (PSw); initial swing (ISw); midswing (MSw); and terminal swing (TSw). The horizontal axes on each graph represent the percentage of the gait cycle. The shaded areas around each trace represent 1 SD. Positive angles represent flexion (dorsiflexion for ankle angle), and negative angles represent extension (plantarflexion for ankle angle).
Participant 2 had a jump gait and did not use an assistive device. Her step width (0.172 m) was wide and stride length (0.819 m) was short relative to children who are typically developing.16 Her initial peak 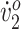 (36.1 mL/kg/min) was in the “good” range for typically developing children.17 Her O2 cost of walking (0.24 mL/kg/min) indicated a high level of fitness relative to most children with diplegic CP.18 Her SSWS was 0.8 m/s. Her baseline gait analysis showed increased hip flexion throughout the gait cycle (Fig. 2d), increased knee flexion from MSw through terminal stance (TSt) (Fig. 2e), and nearly normal ankle dorsiflexion (Fig. 2f). Participant 2 also presented with mild asthma.
(36.1 mL/kg/min) was in the “good” range for typically developing children.17 Her O2 cost of walking (0.24 mL/kg/min) indicated a high level of fitness relative to most children with diplegic CP.18 Her SSWS was 0.8 m/s. Her baseline gait analysis showed increased hip flexion throughout the gait cycle (Fig. 2d), increased knee flexion from MSw through terminal stance (TSt) (Fig. 2e), and nearly normal ankle dorsiflexion (Fig. 2f). Participant 2 also presented with mild asthma.
Figure 2.
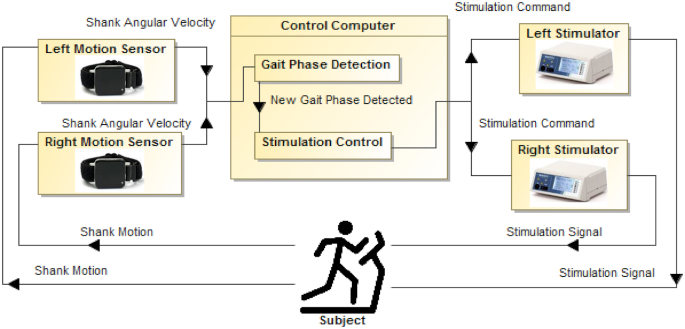
Block diagram of the cerebral palsy functional electrical stimulation gait training system. The participant walks on a treadmill with motion sensors attached to each shank. The motion sensors send shank angular velocity to the control computer. A gait-phase detection algorithm (eAppendix) detects gait-phase transitions based on shank angular velocity. Upon gait-phase transition, a stimulation control algorithm sends stimulation commands (indicating current amplitude and pulse duration) to left and right stimulators based on the participant's stimulation protocol (eAppendix).
Neither participant used orthotic devices.
Intervention
CP FES Gait Training System
The FES system used in this study detected all 7 phases of gait (loading response [LR], MSt, TSt, preswing (PSw), initial swing (ISw), MSw, and terminal swing (TSw)) and stimulated up to 5 muscle groups (glutei maximi, quadriceps femores, hamstrings, dorsiflexors, and plantarflexors) for each leg during each gait phase (eAppendix A, available at https://academic.oup.com/ptj). Stimulation amplitude and pulse duration were independently set for each muscle group and gait phase to tailor stimulation to each participant's gait deviations. Individualized stimulation protocols can be found in eAppendix B (available at https://academic.oup.com/ptj). Reliability has been reported for both the gait-phase detection system and the FES system.15,19
The FES system (Fig. 3) consisted of 2 wireless inertial measurement unit sensors (Opal; APDM, Portland, OR, USA), also referred to as motion sensors, 2 stimulators (Hasomed RehaStim GmbH, Magdeburg, Germany), and a personal computer (Alienware; Dell, Round Rock, TX, USA) running custom LabVIEW (National Instruments, Austin, TX, USA) software. The motion sensors were placed on both shanks and sampled at 128 Hz. Each sensor contained a 3-axis gyroscope, magnetometer, and accelerometer. The accelerometer and magnetometer were disabled during training. The motion sensors streamed angular velocity to the computer, and a gait-phase detection algorithm (eAppendix C, available at https://academic.oup.com/ptj) in LabVIEW detected gait-phase transitions defined by peaks, valleys, and zero-crossings of shank angular velocity (Fig. 1). For each gait phase, the software sent stimulation commands (pulse duration and current amplitude) to the stimulator for the appropriate muscles based on the participant's individualized stimulation protocol.
Figure 3.
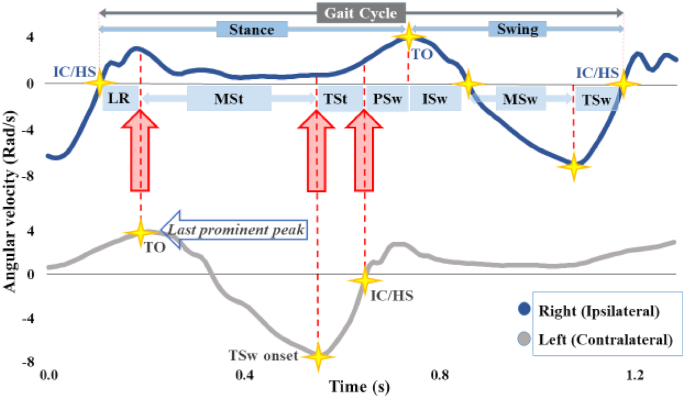
Gait-phase detection for the right side (ipsilateral) using left and right shank angular velocity around the mediolateral axis for a representative child who is typically developing. The onsets of 4 gait phases—loading response (LR), initial swing (ISw), midswing (MSw), and terminal swing (TSw)—are defined by peaks and zero-crossings of ipsilateral shank angular velocity: LR is defined by initial contact (IC)—also called heel strike (HS) in typical gait—and is detected as a positive-to-negative zero crossing; ISw is defined by toe-off (TO), detected as the last prominent peak in stance following IC/HS; MSw is detected as a negative-to-positive zero crossing; and TSw is detected as the absolute minimum in the gait cycle. The onsets of the remaining gait phases—midstance (MSt), terminal stance (TSt), and preswing (PSw)—are defined by contralateral gait events: MSt is defined by contralateral ISw onset; TSt is defined by contralateral TSw onset; and PSw is defined by contralateral IC/HS.
Training Protocol
Prior to training, stimulation current and pulse duration were determined to elicit functional movements for each muscle group while avoiding excessive discomfort. This procedure, known as thresholding, is explained in eAppendix D (available at https://academic.oup.com/ptj). As mentioned above, stimulation protocols (ie, which muscles to stimulate in which gait phase) were determined by 3 physical therapists who viewed videos of each participant's gait and determined which muscle/phase combinations to stimulate to reduce gait deviations. Initial stimulation patterns were based on typical muscle firing patterns observed during gait;20 and, they were modified based on clinical judgment of the 3 physical therapists and on the participant's stimulation thresholding results to produce the desired functional response. Stimulation protocols—including deviations from the physiologically derived activation pattern observed during gait in individuals who are typically developing—are indicated in eAppendix B.
Although we provide typical muscle activation patterns during gait for reference, we posit that modifications are necessary for FES application during gait in children with CP. Physical therapist–prescribed stimulation timing modifications to typical activation patterns are necessary because limb positioning at any 1 point in the gait cycle differs from positions in typical gait. Therefore, typical activation patterns cannot be expected to suffice for aberrant limb position as the limb transfers from gait phase to gait phase. Thus, the physical therapists determined when and to what muscle stimulation occurs, to improve the limb position at each gait phase to reduce deviations when transitioning to the next phase; FES patterns are influenced by the variation of gait patterns observed in CP gait and patient-specific responses to FES.
During training, each participant walked on a treadmill for 30 minutes, 3 times per week, for 12 weeks at speeds meeting Karvonen's criterion (between 60% and 80% of age-predicted maximum heart rate) and using a maximum heart rate of 194 bpm based on Verschuren et al.21,22 Each training session consisted of five 6-minute walking bouts separated by 5-minute rest periods. Each walking bout was further divided into 1-minute intervals, for which FES was alternately turned on and off. The last walking bout was divided into two 3-minute intervals: Participants walked on the treadmill with FES assistance for the first interval and walked overground without FES for the second interval. Participants were assessed for several measures before training, at the end of training, and 4 weeks following the end of training (follow-up).
At any time during training or assessments, if the participant indicated or appeared to experience excessive exhaustion or discomfort, the research physical therapist was able to stop the treadmill immediately with an emergency stop button.
Measures and Data Analysis
Endurance was measured as the distance traversed during the 6-Minute Walk Test (6MWT), for which the participant walked as fast as she safely could for 6 minutes. Distance was measured using a surveyor's wheel. Peak oxygen uptake (peak 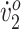 ) was measured via indirect calorimetry (Jaeger Oxycon Mobile, Hochenhausen, Germany) during an incremental walking test (treadmill speed was increased every minute until exhaustion). The oxygen cost of walking (O2 cost), an efficiency measure, was also computed using indirect calorimetry as the participant walked on the treadmill at her initial SSWS (SSWS was computed from the 10-Meter Walk Test, in which the participant walked overground for 14 m, and speed was computed as distance divided by time elapsed during the middle 10 m. Speed was averaged over 3 trials.). Peak
) was measured via indirect calorimetry (Jaeger Oxycon Mobile, Hochenhausen, Germany) during an incremental walking test (treadmill speed was increased every minute until exhaustion). The oxygen cost of walking (O2 cost), an efficiency measure, was also computed using indirect calorimetry as the participant walked on the treadmill at her initial SSWS (SSWS was computed from the 10-Meter Walk Test, in which the participant walked overground for 14 m, and speed was computed as distance divided by time elapsed during the middle 10 m. Speed was averaged over 3 trials.). Peak 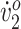 was normalized to body mass and the data were smoothed using a moving average filter. Kinematic and kinetic data were collected using instrumented motion capture (Motion Analysis Corp., Santa Rosa, CA, USA) while patients walked at their initial SSWSs on an instrumented treadmill (Bertec Corp, Columbus, OH, USA). Hip, knee, and ankle joint angles were computed from kinematic data and compared with averaged joint angles of 7 children/adolescents who were typically developing (mean [SD] age = 12.4 [2.15] y).15 Spatiotemporal measures (step width and stride length) were computed from kinetic data collected on the instrumented treadmill (at heel strike, defined as the onset of force on the ipsilateral force plate following a no-force period). Step width was computed as the medio-lateral distance between the proximal end (heel) of each foot from consecutive bilateral heel strikes. Stride length was computed as the distance between the proximal end of the foot between consecutive bilateral heel strikes. Kinematic and kinetic data were averaged over all complete gait cycles (from 15 to 30 cycles) and were analyzed in Visual 3d (C-Motion Inc., Rockville, MD, USA). Data were normalized to an average gait cycle.
was normalized to body mass and the data were smoothed using a moving average filter. Kinematic and kinetic data were collected using instrumented motion capture (Motion Analysis Corp., Santa Rosa, CA, USA) while patients walked at their initial SSWSs on an instrumented treadmill (Bertec Corp, Columbus, OH, USA). Hip, knee, and ankle joint angles were computed from kinematic data and compared with averaged joint angles of 7 children/adolescents who were typically developing (mean [SD] age = 12.4 [2.15] y).15 Spatiotemporal measures (step width and stride length) were computed from kinetic data collected on the instrumented treadmill (at heel strike, defined as the onset of force on the ipsilateral force plate following a no-force period). Step width was computed as the medio-lateral distance between the proximal end (heel) of each foot from consecutive bilateral heel strikes. Stride length was computed as the distance between the proximal end of the foot between consecutive bilateral heel strikes. Kinematic and kinetic data were averaged over all complete gait cycles (from 15 to 30 cycles) and were analyzed in Visual 3d (C-Motion Inc., Rockville, MD, USA). Data were normalized to an average gait cycle.
Outcomes
The values for all measures at pretraining, posttraining, and follow-up are presented in Table 2. Participant 1’s walking speed began well below the reported mean economical walking speed for children with diplegic CP (0.85 m/s) and increased to within 4% of the mean. Participant 2’s initial walking speed was actually above the typical range for normal children (0.85–1.52 m/s).18 She maintained her walking speed above this range throughout the study. Participant 1’s peak 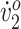 increased by more than 50%, from below “very poor” into the “good” range. In contrast, Participant 2’s peak
increased by more than 50%, from below “very poor” into the “good” range. In contrast, Participant 2’s peak 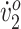 remained in the “good” range throughout the study.17 With her increased step width, scissoring was eliminated from Participant 1’s gait. Both participants either maintained or increased their improvements in most measures after training and at follow-up.
remained in the “good” range throughout the study.17 With her increased step width, scissoring was eliminated from Participant 1’s gait. Both participants either maintained or increased their improvements in most measures after training and at follow-up.
Kinematic data for left hip, knee, and ankle at pretraining, posttraining, and follow-up are shown in Figure 2. Improvements were seen in Participant 1’s hip angle (Fig. 2a) from pretraining to posttraining, most notably in decreased flexion from MSt through MSw, despite the absence of gluteus maximus stimulation (she could not tolerate it). Her knee extension (Fig. 2b) increased from TSw through MSt. Her ankle angle (Fig. 2c) improved in most gait phases, particularly with decreased flexion from LR through TSt. These improvements were largely maintained at follow-up, despite some regression to pretraining levels.
Participant 2’s initial kinematic data were more typical than Participant 1’s data, as expected given her lower GMFCS level and less-impaired gait. Her hip angle plotted throughout the gait cycle (Fig. 2d) resembled that of children who are typically developing. However, she displayed a near-constant 30 degrees of flexion above that of individuals who are typically developing. This offset persisted after training and at follow-up. Her knee extension (Fig. 2e) improved from MSw through TSt, and her ankle showed increased dorsiflexion throughout the gait cycle (Fig. 2f). She maintained most improvements at follow-up: Although her ankle angle deviations were even higher than at pretraining, she maintained a distinct dorsiflexion peak.
Discussion
Our goal was to apply an FES-assisted walking intervention to produce neurotherapeutic improvements in 2 adolescents with CP. The FES system used in this 12-week training study was capable of detecting all 7 phases of gait and stimulating up to 5 lower-extremity muscle groups in synchrony with gait phases. Each participant's protocol was individually tailored to address her gait deviations. Despite differences in gait deviations and GMFCS level, both participants showed improvements after the intervention. Improved pelvis/trunk alignment, pronounced ankle dorsiflexion peak in PSw, and more typical step width were observed in both participants after training.
Participant 1’s kinematic improvements (increased hip extension from MSt to MSw, increased knee extension from TSw to MSt, and decreased dorsiflexion during stance) indicate improved pelvis/trunk alignment. Her ankle dorsiflexion was more typical after training, including a more pronounced peak in PSw. This increase in peak dorsiflexion, also seen in Pool et al,9 potentially enabled greater push-off force, perhaps driving her increase in stride length. Unfortunately, although kinetic data were collected, unreliable handrail force data precluded the computation of push-off force for either participant. Increased knee extension from late swing to early stance likely also helped increase Participant 1’s stride length and could have helped improve her walking endurance. Similar (although not statistically significant) results were reported by van der Linden et al7 in an 8-week study investigating the effects of FES applied to the tibialis anterior during activities of daily living in children with CP. Increased stride length was also observed in patients with stroke, following a 12-week FES walking intervention.14 Although improved gait posture would be expected to result in more efficient walking (ie, decreased energy cost), Participant 1’s O2 cost did not improve, perhaps because we restricted her walking speed to her initial SSWS at her posttraining and follow-up assessments. However, her higher peak 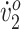 and lower respiratory exchange ratio at posttraining and follow-up indicated improved fitness. We hypothesize that if her SSWS increased, her walking efficiency curve would have moved to the right, resulting in a higher O2 cost at her slower initial SSWS. Although we could not verify this (we did not remeasure SSWS posttraining and at follow-up), her increased first-minute walking speed from the 6MWT (from 0.54 m/s at pretraining to 0.81 m/s posttraining) suggested a rightward shift in her walking efficiency curve, thus supporting our hypothesis. Scissoring was greatly reduced, because her step width increased toward the typical value for children walking on a treadmill.16 Increased step width was also noted by Al-Abdulwahab2 after stimulation of hip abductors and adductors in children with CP. It is interesting to note that we observed similar outcomes in Participant 1 without gluteal stimulation. Increases in walking speeds were also reported in patients with stroke, using similar intervention dosages to the current study (12 weeks of task-specific training).13,14 In contrast, van der Linden et al7 reported higher walking speeds following FES training during activities of daily living in children with CP. The potentially confounding effects of a rollator walker, used during the 6MWT at pretraining and posttraining assessments, should be acknowledged.
and lower respiratory exchange ratio at posttraining and follow-up indicated improved fitness. We hypothesize that if her SSWS increased, her walking efficiency curve would have moved to the right, resulting in a higher O2 cost at her slower initial SSWS. Although we could not verify this (we did not remeasure SSWS posttraining and at follow-up), her increased first-minute walking speed from the 6MWT (from 0.54 m/s at pretraining to 0.81 m/s posttraining) suggested a rightward shift in her walking efficiency curve, thus supporting our hypothesis. Scissoring was greatly reduced, because her step width increased toward the typical value for children walking on a treadmill.16 Increased step width was also noted by Al-Abdulwahab2 after stimulation of hip abductors and adductors in children with CP. It is interesting to note that we observed similar outcomes in Participant 1 without gluteal stimulation. Increases in walking speeds were also reported in patients with stroke, using similar intervention dosages to the current study (12 weeks of task-specific training).13,14 In contrast, van der Linden et al7 reported higher walking speeds following FES training during activities of daily living in children with CP. The potentially confounding effects of a rollator walker, used during the 6MWT at pretraining and posttraining assessments, should be acknowledged.
Participant 2’s improvements were not as ubiquitous, likely due to higher initial walking function and fitness level (as indicated by higher pretraining peak 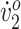 ). Although her excessive hip flexion did not abate, she showed increased knee extension between MSw and TSt and more pronounced peak dorsiflexion in PSw. These changes could have improved her posture during push-off. Participant 2’s initial ankle dorsiflexion closely matched typical dorsiflexion, particularly during swing. Increased dorsiflexion also facilitated foot clearance in the swing phase. Her ankle dorsiflexion deviations regressed toward pretraining levels at follow-up. This could be explained by the following: (1) her ankle angle—although similar in shape to pretraining throughout the gait cycle—was offset toward dorsiflexion by about 4.5 degrees; and (2) her hip and knee flexion regressed slightly at posttraining. Therefore, to maintain the same ankle motion, her ankle was forced into greater dorsiflexion. Despite this regression, she maintained a more pronounced peak dorsiflexion at follow-up. Participant 2 initially had a wider stance than typical. She reduced her step width by the end of the study, indicating improved balance. In fact, both participants’ step width converged toward the typical value of 0.105 m for treadmill walking for children who are typically developing (Participant 1, from 0.068 m to 0.106 m; Participant 2, from 0.172 m to 0.126 m).18 Although Participant 2’s improved biomechanics did not result in increased walking distance or peak
). Although her excessive hip flexion did not abate, she showed increased knee extension between MSw and TSt and more pronounced peak dorsiflexion in PSw. These changes could have improved her posture during push-off. Participant 2’s initial ankle dorsiflexion closely matched typical dorsiflexion, particularly during swing. Increased dorsiflexion also facilitated foot clearance in the swing phase. Her ankle dorsiflexion deviations regressed toward pretraining levels at follow-up. This could be explained by the following: (1) her ankle angle—although similar in shape to pretraining throughout the gait cycle—was offset toward dorsiflexion by about 4.5 degrees; and (2) her hip and knee flexion regressed slightly at posttraining. Therefore, to maintain the same ankle motion, her ankle was forced into greater dorsiflexion. Despite this regression, she maintained a more pronounced peak dorsiflexion at follow-up. Participant 2 initially had a wider stance than typical. She reduced her step width by the end of the study, indicating improved balance. In fact, both participants’ step width converged toward the typical value of 0.105 m for treadmill walking for children who are typically developing (Participant 1, from 0.068 m to 0.106 m; Participant 2, from 0.172 m to 0.126 m).18 Although Participant 2’s improved biomechanics did not result in increased walking distance or peak 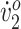 , her O2 cost improved considerably. The intervention appears to have fine-tuned her walking biomechanics and, in turn, increased her walking efficiency.
, her O2 cost improved considerably. The intervention appears to have fine-tuned her walking biomechanics and, in turn, increased her walking efficiency.
Participant 1 demonstrated crouch gait and the tendency for scissoring of the legs across midline. Her FES stimulation protocol differed from reference typical gait activation patterns for the hip, knee, and ankle muscles. Specifically, FES was not applied to the gluteus muscles during LR, MSt, or TSw because the participant could not tolerate gluteus maximus stimulation, nor was FES applied during PSw or TSw because it was determined that the participant's own muscle activation during these phases was sufficient. To reduce crouch during stance, FES was applied to the quadriceps femoris at LR and MSt (as typical). Her hamstrings were not stimulated (1) to avoid counteracting quadriceps function, and (2) because it is very difficult to achieve 2-joint function of the hamstring with FES. Dorsiflexors were not stimulated at LR because the participant's ankles were either neutral or slightly plantarflexed, thus eccentric activation of the dorsiflexors to prevent foot slap at LR was not needed. Dorsiflexors were not stimulated during PSw but rather during ISw, MSw, and TSw, to mitigate foot drag. Plantarflexors were stimulated to generate push-off force and advancement of the center of mass over the base of support, as seen with typical walking.
Participant 2 demonstrated a wide-based, stiff-legged jump gait. The gluteus muscles were stimulated during TSt to enhance hip extension and position the leg for better swing-through and longer stride. As a result of greater swing-through, gluteus maximus stimulation was not necessary during PSw. Similarly, quadriceps stimulation during PSw might not have been necessary because the quadriceps was in a more lengthened position (or perhaps, with the leg in extension, gravity helped to initiate leg swing). To enhance knee extension during MSw, gluteus maximus stimulation was added in this phase. Participant 2’s ability to activate her quadriceps precluded stimulation of the quadriceps femoris during MSt. Similarly, hamstring stimulation was unnecessary during LR, MSw, and TSw. Dorsiflexor and plantarflexor muscles required stimulation as per typical muscle activation during gait.
Neurotherapeutic improvements were observed in ankle and knee angles for both participants. Although both participants regressed toward their pretraining kinematics at follow-up, many of the improvements seen posttraining carried over—in particular, improved pelvis/trunk alignment and pronounced ankle dorsiflexion peak in PSw. Improvements were specific to each participant's walking function, fitness, and gait pathology, indicating that a customized FES prescription—a unique capability of our FES system—can be more effective than 1-size-fits-all approaches such as that used in Pool et al9 and Damiano et al12 (which did not show neurotherapeutic improvements in joint angles or spatiotemporal measures). The improvements we saw might have partially resulted from task specificity: despite the fact that both Pool et al and Damiano et al used high-dose stimulation (6 hours per day for 8 weeks and 3 months, respectively), their interventions were non–task specific, that is, FES device use was focused on activities of daily living as opposed to a specific functional task such as walking or cycling.
These outcomes provide preliminary evidence that an FES-assisted treadmill training intervention customized to a patient's individual gait deviations can improve the walking ability of children with CP. Although any patient can be intolerant of stimulation, it is especially important to determine if child and adolescent patients can tolerate stimulation. They might have little or no experience with muscular stimulation and could be averse to the experience. When determining functional stimulation thresholds, it can be helpful to slowly acclimate child and adolescent patients to stimulation by slowly ramping up to functional levels. This case suggests that FES can be effective even if stimulation cannot be tolerated to all targeted muscles.
Supplementary Material
Contributor Information
Ahad Behboodi, Department of Physical Therapy, University of Delaware, 540 S. College Ave, Newark, DE 19713 (USA).
Nicole Zahradka, InHealth Measurement Corps, Johns Hopkins University, Baltimore, Maryland.
James Alesi, Department of Physical Therapy, University of Delaware.
Henry Wright, Department of Physical Therapy, University of Delaware; and Shriners Hospitals for Children, Philadelphia, Pennsylvania.
Samuel C K Lee, Department of Physical Therapy, University of Delaware; and Shriners Hospitals for Children, Philadelphia, Pennsylvania.
Author Contributions and Acknowledgments
Concept/idea/research design: N. Zahradka, H. Wright, S.C.K. Lee
Writing: A. Behboodi, N. Zahradka, J. Alesi, H. Wright, S.C.K. Lee
Data collection: A. Behboodi, N. Zahradka, J. Alesi, H. Wright, S.C.K. Lee
Data analysis: A. Behboodi, N. Zahradka, J. Alesi, H. Wright, S.C.K. Lee
Project management: H. Wright, S.C.K. Lee
Fund procurement: S.C.K. Lee
Providing participants: N. Zahradka, H. Wright
Providing facilities/equipment: S.C.K. Lee
Providing institutional liaisons: S.C.K. Lee
Clerical/secretarial support: H. Wright
Consultation (including review of manuscript before submitting): H. Wright, S.C.K. Lee
The authors sincerely thank the patients and families who participated in this training program, without whom this work would not be possible.
Ethics Approval
This case report meets all Health Insurance Portability and Accountability (HIPPA) requirements of Shriners Hospitals for Children regarding disclosure of protected health information.
Funding
This work was supported by grants from Shriners Hospitals for Children (71011-PHL and 71011-18-PHL) and by the Foundation for the National Institutes of Health (P30 GM103333).
Role of the Funding Source
Shriners Hospitals for Children (SHC) provided funding for development and testing of the gait phase detection and stimulation system used in this case report. SHC also provided funding for subject recruiting and for the writing of this case report. The NIH Center of Biomedical Research Excellence (COBRE) provided funding for biomechanics facilities.
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Mattern-Baxter K, Bellamy S, Mansoor JK. Effects of intensive locomotor treadmill training on young children with cerebral palsy. Pediatr Phys Ther. 2009;21:308–318. [DOI] [PubMed] [Google Scholar]
- 2. Al-Abdulwahab SS. Electrical stimulation improves gait in children with spastic diplegic cerebral palsy. NeuroRehabilitation. 2011;29:37–43. [DOI] [PubMed] [Google Scholar]
- 3. Pool D, Valentine J, Bear N, Donnelly CJ, Elliott C, Stannage K. The orthotic and therapeutic effects following daily community applied functional electrical stimulation in children with unilateral spastic cerebral palsy: a randomised controlled trial. BMC Pediatr. 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmick J. Clinical use of neuromuscular electrical stimulation for children with cerebral palsy, part 1: lower extremity. Phys Ther. 1993;73:505–513.; discussion 523-527. [DOI] [PubMed] [Google Scholar]
- 5. Comeaux P, Patterson N, Rubin M, Meiner R. Effect of neuromuscular electrical stimulation during gait in children with cerebral palsy. Pediatr Phys Ther. 1997;9:103–109. [Google Scholar]
- 6. Ho C-L, Holt KG, Saltzman E, Wagenaar RC. Functional electrical stimulation changes dynamic resources in children with spastic cerebral palsy. Phys Ther. 2006;86:987–1000. [PubMed] [Google Scholar]
- 7. van der Linden ML, Hazlewood ME, Hillman SJ, Robb JE. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr Phys Ther. 2008;20:23–29. [DOI] [PubMed] [Google Scholar]
- 8. Damiano DL, Kelly LE, Vaughn CL. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995;75:658–667.; discussion 668-671. [DOI] [PubMed] [Google Scholar]
- 9. Pool D, Blackmore AM, Bear N, Valentine J. Effects of short-term daily community walk aide use on children with unilateral spastic cerebral palsy. Pediatr Phys Ther. 2014;26:308–317. [DOI] [PubMed] [Google Scholar]
- 10. Bailes AF, Caldwell C, Clay M, Tremper M, Dunning K, Long J. An exploratory study of gait and functional outcomes after neuroprosthesis use in children with hemiplegic cerebral palsy. Disabil Rehabil. 2016;8288:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Robinson BS, Williamson EM, Cook JL, Harrison KS, Lord EM. Examination of the use of a dual-channel functional electrical stimulation system on gait, balance and balance confidence of an adult with spastic diplegic cerebral palsy. Physiother Theory Pract. 2015;31:214–220. [DOI] [PubMed] [Google Scholar]
- 12. Damiano DL, Prosser LA, Curatalo LA, Alter KE. Muscle plasticity and ankle control after repetitive use of a functional electrical stimulation device for foot drop in cerebral palsy. Neurorehabil Neural Repair. 2013;27:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hakansson NNA, Kesar TTM, Reisman DTet al. Effects of fast functional electrical stimulation gait training on mechanical recovery in poststroke gait. Artif Organs. 2011;35:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32:1594–1603. [DOI] [PubMed] [Google Scholar]
- 15. Zahradka N. When and what to stimulate? An evaluation of a custom functional electrical stimulation system and its neuroprosthetic effect on gait in children with cerebral palsy. http://udspace.udel.edu/handle/19716/23624. July 12, 2018. Accessed November 28, 2018. [Google Scholar]
- 16. Stolze H, Kuhtz-Buschbeck JP, Mondwurf Cet al. Gait analysis during treadmill and overground locomotion in children and adults. Electroencephalogr Clin Neurophysiol. 1997;105:490–497. [DOI] [PubMed] [Google Scholar]
- 17. Heyward VH. The Physical Fitness Specialist Certification Manual. In: Advanced Fitness Assessment and Exercise Prescription. 3rd ed. Dallas, TX: The Cooper Institute for Aerobics Research; 1997:48. [Google Scholar]
- 18. Rose J, Gamble JG, Burgos A, Medeiros J, Haskell WL. Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev Med Child Neurol. 1990;32:333–340. [DOI] [PubMed] [Google Scholar]
- 19. Behboodi A, Wright H, Zahradka N, Lee SCK. Seven phases of gait detected in real-time using shank attached gyroscopes. Conf Proc IEEE Eng Med Biol Soc. 2015:5529–5532. [DOI] [PubMed] [Google Scholar]
- 20. Levine D, Richards J, Whittle MW. Whittle's Gait Analysis. 5th ed.Churchill Livingstone; 2012. [Google Scholar]
- 21. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins. 2013. [DOI] [PubMed] [Google Scholar]
- 22. Verschuren O, Maltais DB, Takken T. The 220-age equation does not predict maximum heart rate in children and adolescents. Dev Med Child Neurol. 2011;53:861–864. [DOI] [PubMed] [Google Scholar]
- 23. Fowler EG, Staudt LA, Greenberg MB, Oppenheim WL. Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev Med Child Neurol. 2009;51:607–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.