Abstract
We aimed to investigate associations between individual and concurrent (≥2) intakes of one-carbon cofactors vitamins B6 and B12, choline, betaine, and methionine and neural tube defect (NTD) outcomes among mothers meeting the folic acid recommendations. In the Slone Birth Defects Study (case-control design; North America, 1998–2015), mothers of 164 NTD cases and 2,831 nonmalformed controls completed food frequency questionnaires and structured interviews. Estimated intakes of one-carbon cofactors were dichotomized (high vs. low) for all except betaine (low or middle vs. high). We used logistic regression models to estimate odds ratios and 95% confidence intervals adjusted for center, age, and race. The analysis was restricted to mothers with estimated daily total folate intake of ≥400 μg during periconception. Fewer cases, compared with controls, had high intakes for each one-carbon cofactor except betaine, where the starkest contrast occurred in the middle group. Women with concurrent high intakes of B6, B12, choline, and methionine and moderate intake of betaine had approximately half the risk of an NTD-affected pregnancy (odds ratio = 0.49, 95% confidence interval: 0.23, 1.08). These findings suggest that, in the presence of folic acid, one-carbon cofactors—notably when consumed together—might reduce NTD risk. Additional research should inform any changes to clinical recommendations.
Keywords: folic acid, neural tube defects, one-carbon group transferases, primary prevention
Maternal periconceptional intake of folic acid reduces risk for neural tube defects (NTDs) (1–4). Despite fortification programs and clinical guidance to supplement with folate for NTD prevention, spina bifida, the most common type of NTD, affects approximately 36 of every 100,000 births in the United States (5), including among women who consume the recommended ≥400 μg of folic acid daily. Improved understanding of nonfolate-sensitive NTD etiology is needed to develop effective interventions for women who follow recommendations but might still be at risk.
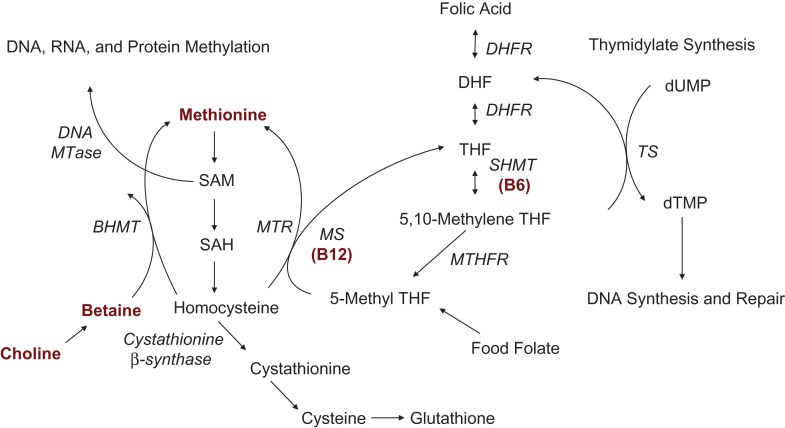
The precise mechanism by which folic acid prevents NTDs is unknown. Folic acid is metabolized as part of the one-carbon cycle (Figure 1) (6), the functionality of which is critical for processes involved in embryogenesis, including DNA methylation (7, 8). If folic acid prevents NTDs through one-carbon metabolism, further risk reduction might occur with intake of other one-carbon micronutrient cofactors (9). Lower NTD risk has been associated with vitamin B12 (10–12), vitamin B6 (10, 13), choline (14, 15), betaine (14, 16, 17), and methionine (18, 19); however, not all research found protective associations (20–22). Few studies specifically evaluated associations among women meeting the folic acid recommendations or joint exposures (14, 17, 20).
Figure 1.
Simplified version of the one-carbon cycle, modified from a systematic review by Crider et al. (6) and reproduced with permission from the authors. Refer to Web Appendix 1 for more information on the role of folate and its cofactors in the one-carbon cycle. Note: There are additional pathways that include the cofactors of interest but are not included in this depiction to simplify presentation. BHMT, betaine-homocysteine methyltransferase; DHF, dihydrofolate; DHFR, dihydrofolate reductase; dTMP, thymidylate; dUMP, deoxyuridylate; MS, methionine synthase; MTase, methyltransferase; MTHFR, methylenetetrahydrofolate reductase; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate; TS, thymidylate synthase.
The objective of this analysis was to investigate whether, among women who ingest at least 400 μg of folic acid daily, certain intakes of vitamins B6 and B12, choline, betaine, and methionine, individually or in combination, are associated with lower NTD risk, using data from the Slone Epidemiology Center Birth Defects Study for 1998–2015. We hypothesized that NTDs occur among some women who follow the folic acid recommendations because these women cannot reap its protective benefits without additional support from other one-carbon cofactors.
METHODS
Study design
The Slone Birth Defects Study had a case-control design and was conducted between 1976 and 2015 to investigate a wide range of exposures during pregnancy and risk for major birth defects. We restricted this analysis to interviews between 1998 and 2015 (postfortification). Research nurses administered standardized telephone interviews in English or Spanish with mothers of cases and controls within 6 months of delivery. The interviews asked about sociodemographic and reproductive factors, medical history, pregnancy complications, and behavioral factors, including diet and supplementation. All participants provided informed consent. Institutional review boards at the affiliated facilities approved the study. The analysis was approved by the Institutional Review Board at Boston University Medical Campus and Boston Medical Center (IORG0000222, FWA00000301, Protocol Number H-30154; original approval date January 19, 2011).
Subjects
Cases were live births, fetal deaths at or after 20 weeks, and elective terminations later than 12 weeks, ascertained from birth hospitals and tertiary care centers in the greater metropolitan areas of Boston, Massachusetts; Philadelphia, Pennsylvania; Toronto, Canada (until 2005); San Diego, California (starting in 2001); and Nashville, Tennessee (starting in 2012); as well as birth defect registries in Massachusetts (starting in 2003) and parts of New York State (starting in 2004). NTDs included spina bifida, anencephaly, and encephalocele confirmed by a clinical geneticist, after excluding known chromosomal anomalies, Mendelian-inherited disorders, recognized syndromes, amniotic bands, body wall defects, and conjoined twins. Controls were infants without major defects who were delivered at the same facilities as were the cases.
Nutrient assessment
Women completed a semiquantitative, modified Willett food frequency questionnaire that asked about diet during the 6 months prior to pregnancy, which we assumed would be representative of behaviors in the periconceptional period, when NTDs develop and before women change behaviors due to pregnancy. We estimated average daily micronutrient intakes by combining reported frequency and prescribed serving sizes and applying matrices from Harvard University (23) and the US Department of Agriculture (24).
Participants reported vitamin use, including type and brand (if known), start and stop dates, dose, and frequency. If the mother was unsure of an exact date and reported “sometime in the month,” we assigned the first and last day of the month for start and stop dates, respectively. Product information was linked to active ingredients using the Slone Drug Dictionary (25). Periconceptional supplementation was defined as any use, during the 28 days before through 28 days after the last menstrual period, of at least 1 product containing the one-carbon cofactors B6, B12, choline, betaine, or methionine; nonsupplementers were women who did not report any products containing these micronutrients during this period.
Statistical analyses
We restricted our analysis to participants whose estimated average daily total folic acid intake was ≥400 μg during the periconceptional period. We calculated total intake by summing supplements and diet, including natural folate (discounted by 30% due to its lower bioavailability) (26) and fortified foods.
To categorize each cofactor, we identified cutpoints that aligned with shifts in odds of NTD outcomes based on calorie-adjusted (27) estimated dietary intake in crude spline regression models among nonsupplementers (refer to Web Appendix 1, available at https://academic.oup.com/aje, specifically Web Figure 1, for more information). The resulting dichotomizations (B6: ≥2.2 mg, <2.2 mg; B12: ≥3 μg, <3 μg; choline: ≥200 mg, <200 mg; and methionine: ≥1.3 g) were interpreted as higher and lower intake, respectively; 3 categories were created for betaine (<40 mg, ≥40 and <70 mg, ≥70 mg), which were interpreted as lower, middle, and higher, respectively. Because exposure from vitamin supplements was likely as high as (or higher than) from diet (28), we grouped supplementers into the highest intake category if they did not meet the cutoff based on diet.
We estimated crude and adjusted odds ratios and 95% confidence intervals for associations between one-carbon cofactors and NTD risk overall, as well as spina bifida specifically, using unconditional logistic regression models. We interpreted odds ratios as relative risks given the rarity of NTDs. First, we estimated odds ratios for each individual cofactor. Participants in the lower intake group served as the reference for all comparisons, except betaine, for which the higher category served as the reference. We did not report any odds ratios for the lower intake group of betaine because its small size led to unstable estimates. In addition to evaluating individual intakes, we assessed concurrent (≥2) intakes of 2 or more cofactors by counting the number of one-carbon cofactors that fell into the higher (or middle for betaine) intake category. Odds ratios compared participants who had 2, 3, 4, or all 5 cofactors in the higher (middle for betaine) range to participants who had only 1 or no cofactors in these ranges; the reference group did not include participants who had intake in the lowest range for betaine, to align with the reference group in the individual intake analysis.
We considered established risk factors for NTDs as potential confounders, specifically maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), age (in years: <25, 25–34, ≥35), education (in years: <12, 12, >12), annual family income (in $: <10,000, 10,000–14,000, 15,000–24,000, 25,000–34,000, 35,000–44,000, ≥45,000—these exact ranges were the options provided to participants during the interview), and prepregnancy body mass index (calculated as weight (kg)/height (cm/100)2; underweight, <18.5; normal, 18.5–24.9; overweight, 25.0–29.9; obese, ≥30.0). We also considered study center and year of last menstrual period. Using a 10% change-in-estimate approach (29), we identified maternal age, race, and study center as a sufficient set of covariates to control for confounding in the adjustment models. Participants with missing data (<0.05%) were excluded from the adjustment models.
We conducted several sensitivity analyses. First, to address potential confounding, although possibly introducing collinearity, we adjusted for estimated average daily folate from supplements and diet, and in another set of models, we included all of the one-carbon cofactors in a single model and interpreted the β coefficients for each cofactor. Second, we restricted the analysis to women who were nonsupplementers for all cofactors (B6, B12, choline, betaine, and methionine). Third, instead of using spline-based categorizations, we constructed quartiles based on the distribution of calorie-adjusted dietary intake among the nonsupplementer controls, and for B6, B12, and choline (none available for betaine and methionine), we created dichotomizations for dietary intake among nonsupplementers based on the daily recommendations for women aged 19–50 years from the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine (previously the Institute of Medicine) (30). Last, we evaluated the potential linear association with each cofactor using the continuous, calorie-adjusted estimated daily dietary intake among nonsupplementers, computing odds ratios for a 1-standard-deviation unit increase in intake.
To estimate the strength of residual confounding required to fully attenuate to the null the point estimate for our strongest association from the main analysis, we conducted a simple bias analysis that considered the required difference in prevalence of a hypothetical unmeasured confounder between each cofactor group, as well as the magnitude of the association between the unmeasured confounder and NTD outcomes.
RESULTS
Subjects
Between 1998 and 2015, mothers of 494 NTD cases and 10,269 controls were interviewed, among whom 218 (44.1%) mothers of cases and 5,376 (52.4%) mothers of controls reported ≥400 μg of folic acid daily during the periconceptional period. Of those, we excluded 12 (5.5%) mothers of cases who met the exclusion criteria or had unconfirmed diagnoses and 33 (15%) mothers of cases and 609 (11%) mothers of controls with extreme caloric intakes (i.e., <500 or >3,800 kcal/day) or incomplete food frequency questionnaire data. Upon restriction to cases for whom a hospital-matched control could be identified, the final analysis included mothers of 164 NTD cases, 124 of whom were affected by spina bifida, and 2,831 controls (Web Figure 2).
In the analytical sample, cases’ mothers were more likely to be younger than 25 years (14.6% vs. 10.1%), black or Hispanic (22.5% vs. 15.2%), and overweight or obese (40.1% vs. 30.1%), and less likely to be college educated (81.7% vs. 86.2%), compared with controls’ mothers (Table 1).
Table 1.
Maternal Characteristics, Restricted to Women With Periconceptional Folic Acid Intake of ≥400 μg Daily, Slone Epidemiology Center Birth Defects Study, North America, 1998–2015
| Characteristic | Neural Tube Defect Cases (n = 164) | Controls (n = 2,831) | ||
|---|---|---|---|---|
| No. | %a | No. | %a | |
| Age, years | ||||
| <25 | 24 | 14.6 | 285 | 10.1 |
| 25–34 | 113 | 68.9 | 1,912 | 67.7 |
| ≥35 | 27 | 16.5 | 628 | 22.2 |
| Missing | 0 | 6 | ||
| Education, years | ||||
| <12 | 11 | 6.7 | 98 | 3.5 |
| 12 | 19 | 11.6 | 292 | 10.3 |
| ≥12 | 134 | 81.7 | 2,439 | 86.2 |
| Missing | 0 | 2 | ||
| Race/ethnicity | ||||
| White, non-Hispanic | 115 | 70.1 | 2,194 | 77.6 |
| Black, non-Hispanic | 13 | 7.9 | 123 | 4.4 |
| Hispanic | 24 | 14.6 | 304 | 10.8 |
| Other | 12 | 7.3 | 206 | 7.3 |
| Missing | 0 | 4 | ||
| Prepregnancy BMIb | ||||
| Underweight | 7 | 4.3 | 132 | 4.7 |
| Normal | 90 | 55.6 | 1,826 | 65.2 |
| Overweight | 40 | 24.7 | 534 | 19.1 |
| Obese | 25 | 15.4 | 308 | 11.0 |
| Missing | 2 | 31 | ||
| Study center | ||||
| Massachusetts | 31 | 18.9 | 1,260 | 44.5 |
| Philadelphia | 40 | 24.4 | 664 | 23.5 |
| Toronto | 52 | 31.7 | 202 | 7.1 |
| San Diego | 18 | 11.0 | 445 | 15.7 |
| New York State | 19 | 11.6 | 215 | 7.6 |
| Nashville | 4 | 2.4 | 45 | 1.6 |
| Missing | 0 | 0 | ||
| Year of last menstrual period | ||||
| 1997–2002 | 76 | 46.3 | 1,196 | 42.2 |
| 2003–2008 | 47 | 28.7 | 743 | 26.2 |
| 2009–2014 | 41 | 25.0 | 892 | 31.5 |
| Missing | 0 | 0 | ||
Abbreviation: BMI, body-mass-index.
a Column percentages were computed using the total number of participants in each group with the variable defined as the denominator; the number missing, if any, were reported separately. Due to rounding, some columns do not sum to exactly 100%.
b Maternal BMI was computed as (prepregnancy weight (kg))/(height (cm)/100)2; the BMI categories are defined as follows: underweight, <18.5; normal, 18.5–24.9; overweight, 25.0–29.9; and obese, ≥30.0.
Nutrient intake
In Web Appendix 1, Web Table 1 provides the distributions of and correlations between pairs of continuous, energy-adjusted estimated daily micronutrient intakes from diet of the one-carbon cofactors among the entire study population.
A majority of cases’ (51.8%) and controls’ (58.7%) mothers took supplements that contained both B6 and B12; supplementation of the other one-carbon cofactors was less common (for cases and controls, respectively: B6 alone, 0.6% vs. 3.0%; B12 alone, 1.2% vs. 1.0%; choline, 7.3% vs. 7.7%; methionine, 0.6% vs. 0.2%; and betaine, 0.6% vs. 0.8%). For each one-carbon cofactor, the proportion of women with higher intake was lower among cases compared with controls (B6, 59.8% vs. 70.9%; B12, 85.4% vs. 90.0%; choline, 87.2% vs. 90.5%; and methionine, 81.1% vs. 84.2%), except betaine which was lowest in the middle group (30.9% vs. 37.6%; Web Table 2). Participants who reported supplement use made up a large percentage of the highest categories for B6 and B12 (87.1% and 66.1%, respectively; Web Tables 3 and 4). The percentage of participants who reported supplementation in the highest categories for choline, methionine, and betaine was much smaller (8.5%, 0.3%, and 0.4%, respectively). For each one-carbon micronutrient, the top 5 sources from diet and supplementation are provided in Web Table 3.
Associations with NTDs
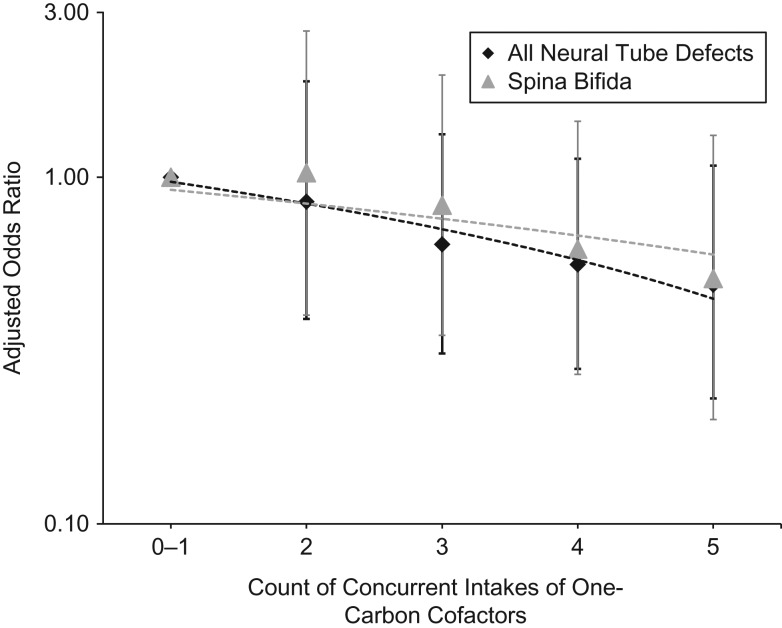
The adjusted odds ratios for NTD risk with the individual cofactors ranged from 0.73 (choline) to 0.86 (betaine), but all had wide confidence intervals that included 1 (refer to Web Table 2 and Web Figure 3). Upon restriction to spina bifida cases only, we observed similar results for the individual cofactors; while some estimates were slightly stronger (e.g., betaine) or weaker (e.g., methionine), the range of values within the confidence intervals were largely consistent with those from the all-NTDs analysis. The adjusted odds ratios for NTD risk with concurrent intake of multiple cofactors were all below 1 and moved further from the null as the number increased, in a dose-response pattern (Figure 2 and Web Table 5). Specifically, the estimated odds ratios were 0.85, 0.64, and 0.56 for 2, 3, and 4 concurrent intakes, respectively, although all 95% confidence intervals included 1. The strongest estimate—which equated to roughly a halving of NTD risk—was observed for participants whose intakes were in the highest respective categories for B6, B12, choline, and methionine and the middle category for betaine (adjusted odds ratio (OR) = 0.49, 95% confidence interval (CI): 0.23, 1.08). Upon restriction to spina bifida, the results for concurrent intakes were slightly attenuated compared with the all-NTDs results.
Figure 2.
Associations between concurrent intakes of one-carbon cofactors and neural tube defect outcomes, Slone Epidemiology Center Birth Defects Study, North America, 1998–2015. The data are restricted to mothers reporting periconceptional folic acid of ≥400 μg daily. The figure displays estimated odds ratios (adjusted for maternal age, race, and study center) and 95% confidence intervals for concurrent (joint) intake of one-carbon cofactors and risk for all neural tube defects and spina bifida only. One-carbon cofactor intake estimates include both diet and supplementation, and counts represent the number of intakes within the respective primary comparison range for each cofactor (B6 ≥2.2 mg, B12 ≥3 μg, choline ≥200 mg, methionine ≥1.3 g, and betaine range of ≥40 to <70 mg).
Sensitivity and bias analyses
After adjustment for total folate intake, the estimates for the individual cofactors were largely unchanged; the estimates for concurrent intake were attenuated, although a suggestive dose-response pattern was still observed for 3, 4, and 5 counts (Web Table 6). Adjustment for other cofactors attenuated the results of the individual intakes analysis, especially for B12 and methionine, except betaine, which was unchanged (Web Table 2).
Exclusion of supplementers did not substantially change the findings, but confidence intervals were wider (Web Table 7).
Comparisons of the highest to lowest quartiles were similar to the main results for B6 and B12. For choline, betaine, and methionine, the comparisons of highest to lowest quartiles were essentially null. The estimates based on the daily recommendations were similar to the spline-based approach for B12 but not B6 or choline (Web Table 4). There was no association observed between the continuous estimated intake of each one-carbon cofactor and NTD outcomes, except for B6, but the odds ratio associated with a 1-standard-deviation increase was weaker than the odds ratio associated with the spline-based dichotomization.
Our bias analysis suggested that, assuming no other study error, an unmeasured confounder would have to be 6 times as prevalent among participants in the reference group compared with those with all high (middle for betaine) intakes, as well as be strongly associated with NTDs (OR ≥ 6), to fully attenuate the crude point estimate for concurrent intakes of all 5 cofactors from 0.37 to 0.98.
DISCUSSION
Main findings
In our analysis of women who met the recommendations for folic acid intake, we observed suggestive associations with individual and joint intake of one-carbon micronutrient cofactors and NTD outcomes. The data showed a dose-response pattern, although estimates were imprecise, with lowest risk observed among women who had higher estimated intakes of B6, B12, choline, and methionine and moderate intake of betaine. NTDs are a composite outcome, and each specific defect might have a unique set of risk factors; while we were able to explore only the spina bifida subtype due to small numbers of anencephaly and encephalocele cases, the association strength did not substantially change, supporting our hypothesis that these one-carbon cofactors might be important for NTD prevention in general among folic acid users.
Strengths and limitations
Ours is, to our knowledge, the first study of NTDs to capture case-control differences across the full distribution of dietary and supplemental intake of one-carbon cofactors, rather than relying on quantiles or prespecified values (e.g., recommended dietary allowances). It is also one of the first to consider concurrent intakes of one-carbon cofactors among folic acid users.
Our study relied on maternal self-report. Biomarkers can be considered more accurate, but for studies of NTDs, samples ideally need to be collected during periconception, which is often infeasible because it is before most women know they are pregnant. Indeed, most biospecimen investigations of B12 and NTDs collected samples in the second trimester (31). Once pregnancy is recognized, women often change their behaviors, including diet and supplementation, so the 6 months prior to pregnancy, as assessed in our study, might be more representative of behaviors during the NTD risk period in comparison with sample-based studies (28). Biomarkers reflect not only intake but also genetically determined metabolic variation, which at this point in time cannot be altered. In addition, while biomarkers might provide more accurate exposure measurement from an etiological perspective, diet and supplementation might be more meaningful in the formulation of clinical recommendations and policy. In general, food frequency questionnaires are not ideal for estimating absolute nutrient intake (although a validation study found that, compared with biomarkers and other dietary assessments (e.g., 24-hour recalls), the Willett questionnaire yielded valid energy-adjusted intake for certain nutrients, including folate (32); other one-carbon cofactors were not evaluated). We caution that absolute concentrations should not be extrapolated; however, values should be internally valid for comparing groups based on relative quantities. Adjustment for total caloric intake helps address systematic over- or underreporting. We did not consider specific doses of supplements because dose was unknown for some reported products; however, our results were similar when supplementers were excluded. Although NTD status was known at the time of maternal interview, recall bias is unlikely because the study question was not specified during data collection, and most women do not have good knowledge of micronutrient content in food. We believe that misclassification is likely nondifferential, leading to expected underestimates of the true associations. We restricted this analysis to controls born at the same facilities as the cases (variable ratio) to reduce the likelihood of selection bias; birth hospital was not considered a true confounder, so we did not do a matched analysis, but we did adjust for study center in the regression models. While residual confounding cannot be ruled out, adjustment for all measured confounders, including total estimated folate intake, led to minimal shift in the point estimates. Our bias analysis indicated that an unmeasured confounder would need to be very strong to fully explain our findings; we are not aware of any such confounder. That said, all estimates were imprecise, with wide confidence intervals that included 1. Maternal body mass index, race, or genetics could modify one-carbon metabolism (20, 33–35), limiting generalizability.
Interpretation
In 2012, the National Birth Defects Prevention Study compared spina bifida cases with controls using quartiles of nutrient intakes and stratified by folic acid supplementation (20). Among folic acid supplementers, for the highest versus lowest quartiles, the investigators observed a suggestive protective association for choline (adjusted OR = 0.83, 95% CI: 0.50, 1.38), but odds ratios were close to the null for B6 (OR = 1.09, 95% CI: 0.69, 1.73), B12 (OR = 0.90, 95% CI: 0.60, 1.36), betaine (OR = 0.94, 95% CI: 0.65, 1.35), and methionine (OR = 1.02, 95% CI: 0.63, 1.65). The discrepancy with our findings might be due to their use of quartiles, which require assumptions that could be violated, notably when the underlying distribution of continuous values is skewed (36, 37).
A few other investigations evaluated the joint effects of folic acid and one-carbon cofactors, and none, to our knowledge, reviewed intake of all one-carbon cofactors at once. The California Birth Defects Monitoring Program (1989–1991) found that women with high intake of choline paired with high intake of betaine, folate, or methionine had lower NTD risk (14). A study of Mexican Americans (1995–2000) found stronger associations with dietary choline and betaine individually when folic acid was ≥400 μg in comparison with <400 μg; the investigators did not explore joint effects of betaine and choline (17). The Texas Neural Tube Defects Project (1995–2000) found some evidence of interaction between B12 and methionine (18), where the lowest odds ratio for NTDs was observed among women in the highest quartiles for both serum B12 and dietary methionine intake; the analysis did not stratify by folic acid.
In our study, we detected higher risk of an NTD-affected pregnancy with higher compared with moderate betaine intake. Shaw et al. (15) also detected a possible slight increase in NTD risk with high betaine intake. More research is necessary before conclusions can be drawn regarding potential risk posed by high betaine concentrations, given that both studies yielded fairly imprecise estimates.
Conclusion
Our findings suggest that, beyond the benefits provided by folic acid, higher intakes of vitamins B6 and B12, choline, and methionine and moderate intake of betaine might be associated with additional NTD risk reduction. Despite imprecise estimates, we observed a trend of decreasing risk of NTDs with increasing concurrent intakes, with the degree of benefit related to the number of one-carbon cofactors. Our research supports the hypothesis that folic acid prevents NTDs through one-carbon metabolism. Our findings add to the evidence stressing the importance maternal nutrition in fetal development and, more specifically, how diet and/or supplementation of one-carbon micronutrients could contribute to NTD risk reduction, potentially improving outcomes among pregnancies that would otherwise be susceptible to folate-resistant NTDs. In support of this theory, a recent study in mice with the solute carrier family 25 member 32 gene (SLC25A32) silenced found that NTDs could be prevented by formate supplementation (38). Future research with more precise exposure measurement is needed to confirm these findings before clinical guidance can be developed to specify recommended amounts of intake and the acceptable forms (diet, supplementation, or a combination) for NTD prevention.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts (Julie M. Petersen, Samantha E. Parker, Martha M. Werler); National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia (Krista S. Crider, Sarah C. Tinker); and the Slone Epidemiology Center at Boston University, Boston, Massachusetts (Allen A. Mitchell).
This work was supported by the Centers for Disease Control and Prevention (grant U01 DD001184-02) and the National Heart, Lung, and Blood Institute (grant K01 HL133600 to S.E.P.).
We thank Dawn Jacobs, Fiona Rice, Rita Krolak, Kathleen Sheehan, Moira Quinn, Clare Coughlin, Laurie Cincotta, Nancy Rodriquez-Sheridan, Ileana Gatica, Laine Catlin Fletcher, Joan Shander, Mark Abcede, Judy Jean, and Julia Venanzi for their assistance in data collection; Nastia Dynkin for computer programming; the staff of the Massachusetts Department of Public Health, Center for Birth Defects Research and Prevention, and the Massachusetts Registry of Vital Records; Dr. Charlotte Druschel and the New York State Health Department; and Drs. Christina Chambers and Kenneth Jones of the University of California, San Diego. We also thank the medical and nursing staff at all participating hospitals for assistance with case ascertainment: Baystate Medical Center, Beth Israel Deaconess Medical Center, Boston Medical Center, Brigham & Women’s Hospital, Brockton Hospital, Cambridge Hospital, Caritas Good Samaritan Medical Center, Charlton Memorial Hospital, Children’s Hospital, Emerson Hospital, Falmouth Hospital, Haverhill-Hale Hospital, Jordan Hospital, Kent Hospital, Lawrence General Hospital, Lowell General Hospital, Melrose-Wakefield Hospital, Metro West Medical Center-Framingham, Mt. Auburn Hospital, New England Medical Center, Newton-Wellesley Hospital, North Shore Medical Center, Rhode Island Hospital, Saints Memorial Medical Center, South Shore Hospital, Southern New Hampshire Medical Center, St. Elizabeth’s Medical Center, St. Luke’s Hospital, St. Vincent Hospital, University of Massachusetts Memorial Health Care, Women & Infants’ Hospital, Abington Memorial Hospital, Albert Einstein Medical Center, Alfred I. duPont Hospital for Children, Bryn Mawr Hospital, Chester County Hospital, Children’s Hospital of Philadelphia, Christiana Care Health Services, Community Hospital, Crozer-Chester Medical Center, Doylestown Hospital, Frankford Hospital, Hahnemann University Hospital, The Hospital of the University of Pennsylvania, Lankenau Hospital, Lancaster General Hospital, Lehigh Valley Hospital, Nanticoke Memorial Hospital, Pennsylvania Hospital, Sacred Heart Hospital, St. Christopher’s Hospital for Children, St. Mary Medical Center, Temple University Health Sciences Center, Reading Hospital & Medical Center, Thomas Jefferson University Hospital, Grand River Hospital, Guelph General Hospital, Hamilton Health Sciences Corporation, The Hospital for Sick Children, Humber River Regional Hospital–Church Site, Humber River Regional Hospital–Finch Site, Joseph Brant Memorial Hospital, Lakeridge Health Corporation, London Health Sciences Center, Mt. Sinai Hospital, North York General Hospital, Oakville Trafalgar Memorial Hospital, Scarborough Hospital–General Division, Scarborough Hospital–Grace Division, St. Joseph’s Health Centre–London, St. Joseph’s Health Centre Toronto, St. Joseph’s Healthcare–Hamilton, St. Michael’s Hospital, Sunnybrook & Women’s College Health Sciences Center, Toronto East General Hospital, Toronto General Hospital, Trillium Health Center, William Osler Heath Centre, York Central Hospital, York County Hospital, Alvarado Hospital, Balboa Naval Medical Center, Camp Pendleton Naval Hospital, Children’s Hospital and Health Center, Kaiser Zion Medical Center, Palomar Medical Center, Pomerado Hospital, Scripps Mercy Hospital, Scripps Memorial Hospital–Chula Vista, Scripps Memorial Hospital–Encinitas, Scripps Memorial Hospital–La Jolla, Sharp Chula Vista Hospital, Sharp Coronado Hospital, Sharp Grossmont Hospital, Sharp Mary Birch Hospital, Tri-City Medical Center, and University of California San Diego Medical Center. We particularly thank all the mothers who participated in the study.
Versions of this analysis were presented at the 10th International Conference on Neural Tube Defects, October 2–4, 2017, Austin, Texas; the Semi-Annual Student and Post-Doc Committee Novel Methods Web Conference hosted by the Society for Epidemiologic Research (SERdigital), November 8, 2017; and the National Birth Defects Prevention Network 21st Annual Meeting, Advances and Opportunities for Birth Defects Surveillance, Research and Prevention, March 12–14, 2018, Atlanta, Georgia.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- NTD
neural tube defect
- OR
odds ratio
REFERENCES
- 1. Berry RJ, Li Z, Erickson JD, et al. . Prevention of neural-tube defects with folic acid in China. China-US Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341(20):1485–1490. [DOI] [PubMed] [Google Scholar]
- 2. Kirke PN, Daly LE, Elwood JH. A randomised trial of low dose folic acid to prevent neural tube defects. The Irish Vitamin Study Group. Arch Dis Child. 1992;67(12):1442–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 4. Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257–1261. [PubMed] [Google Scholar]
- 5. Atta CA, Fiest KM, Frolkis AD, et al. . Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am J Public Health. 2016;106(1):e24–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crider KS, Yang TP, Berry RJ, et al. . Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. [DOI] [PubMed] [Google Scholar]
- 8. James P, Sajjadi S, Tomar AS, et al. . Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int J Epidemiol. 2018;47(6):1910–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li K, Wahlqvist ML, Li D. Nutrition, one-carbon metabolism and neural tube defects: a review. Nutrients. 2016;8(11):pii:E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carmichael SL, Yang W, Shaw GM. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth Defects Res A Clin Mol Teratol. 2010;88(8):670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu Q, Li Y, Cui ZL, et al. . Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Paediatr. 2012;101(11):e486–e490. [DOI] [PubMed] [Google Scholar]
- 12. Ray JG, Wyatt PR, Thompson MD, et al. . Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18(3):362–366. [DOI] [PubMed] [Google Scholar]
- 13. Candito M, Rivet R, Herbeth B, et al. . Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: a multicenter case-control study. Am J Med Genet A. 2008;146A(9):1128–1133. [DOI] [PubMed] [Google Scholar]
- 14. Shaw GM, Carmichael SL, Yang W, et al. . Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160(2):102–109. [DOI] [PubMed] [Google Scholar]
- 15. Shaw GM, Finnell RH, Blom HJ, et al. . Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 2009;20(5):714–719. [DOI] [PubMed] [Google Scholar]
- 16. Benevenga NJ. Consideration of betaine and one-carbon sources of N5-methyltetrahydrofolate for use in homocystinuria and neural tube defects. Am J Clin Nutr. 2007;85(4):946–949. [DOI] [PubMed] [Google Scholar]
- 17. Lavery AM, Brender JD, Zhao H, et al. . Dietary intake of choline and neural tube defects in Mexican Americans. Birth Defects Res A Clin Mol Teratol. 2014;100(6):463–471. [DOI] [PubMed] [Google Scholar]
- 18. Graham A, Brender JD, Sharkey JR, et al. . Dietary methionine intake and neural tube defects in Mexican-American women. Birth Defects Res A Clin Mol Teratol. 2010;88(6):451–457. [DOI] [PubMed] [Google Scholar]
- 19. Shaw GM, Velie EM, Schaffer DM. Is dietary intake of methionine associated with a reduction in risk for neural tube defect-affected pregnancies? Teratology. 1997;56(5):295–299. [DOI] [PubMed] [Google Scholar]
- 20. Chandler AL, Hobbs CA, Mosley BS, et al. . Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth Defects Res A Clin Mol Teratol. 2012;94(11):864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mills JL, Fan R, Brody LC, et al. . Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. Am J Clin Nutr. 2014;100(4):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molloy AM, Kirke P, Hillary I, et al. . Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch Dis Child. 1985;60(7):660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harvard T.H. Chan School of Public Health Nutrition Department Semi-quantitative food frequency questionnaire nutrient tables. https://regepi.bwh.harvard.edu/health/nutrition.html. Accessed February 4, 2018.
- 24. Papp C, Adám Z, Tóth-Pál E, et al. . Risk of recurrence of craniospinal anomalies. J Matern Fetal Med. 1997;6(1):53–57. [DOI] [PubMed] [Google Scholar]
- 25. Kelley KE, Kelley TP, Kaufman DW, et al. . The Slone Drug Dictionary: a research driven pharmacoepidemiology tool [abstract]. Pharmacoepidemiol Drug Saf. 2003;12(suppl 1):S168–S169. [Google Scholar]
- 26. Elkin AC, Higham J. Folic acid supplements are more effective than increased dietary folate intake in elevating serum folate levels. BJOG. 2000;107(2):285–289. [DOI] [PubMed] [Google Scholar]
- 27. Willett W. Chapter 11: Implications of Total Energy Intake of Epidemiologic Analysis, Energy-Adjustment and Measurement Error. In: Nutritional Epidemiology: Oxford Scholarship Online; 2013; http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199754038.001.0001/acprof-9780199754038-chapter-11. Accessed March 1, 2019.
- 28. Willett W. Chapter 13: Issues in Analysis and Presentation of Dietary Data. In: Nutritional Epidemiology: Oxford Scholarship Online; 2013; http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199754038.001.0001/acprof-9780199754038-chapter-13. Accessed March 1, 2019.
- 29. Kleinbaum DG, Kupper L, Morgenstern H. Epidemiologic Research: Prinicples and Quantitative Methods. New York, NY: Nostrand Reinhold; 1982. [Google Scholar]
- 30. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline Dietary Reference Intakes (DRI) for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed]
- 31. Ray JG, Blom HJ. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM. 2003;96(4):289–295. [DOI] [PubMed] [Google Scholar]
- 32. Yuan C, Spiegelman D, Rimm EB, et al. . Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao L, Wang Y, Zhang R, et al. . Association of neural tube defects with gene polymorphisms in one-carbon metabolic pathway. Childs Nerv Syst. 2018;34(2):277–284. [DOI] [PubMed] [Google Scholar]
- 34. Nathanielsz PW, Yan J, Green R, et al. . Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep. 2015;3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawrence JM, Watkins ML, Chiu V, et al. . Do racial and ethnic differences in serum folate values exist after food fortification with folic acid? Am J Obstet Gynecol. 2006;194(2):520–526. [DOI] [PubMed] [Google Scholar]
- 36. Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol. 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenland S, Rothman K. Data description and summarization: choice of categories In: Rothman K, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008:217–218. [Google Scholar]
- 38. Kim J, Lei Y, Guo J, et al. . Formate rescues neural tube defects caused by mutations in Slc25a32. Proc Natl Acad Sci U S A. 2018;115(18):4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.