Abstract
Breast density is a modifiable factor that is strongly associated with breast cancer risk. We sought to understand the influence of newer technologies of full-field digital mammography (FFDM) on breast density research and to determine whether results are comparable across studies using FFDM and previous studies using traditional film-screen mammography. We studied 24,840 screening-age (40–74 years) non-Hispanic white women who were participants in the Research Program on Genes, Environment and Health of Kaiser Permanente Northern California and underwent screening mammography with either Hologic (Hologic, Inc., Marlborough, Massachusetts) or General Electric (General Electric Company, Boston, Massachusetts) FFDM machines between 2003 and 2013. We estimated the associations of parity, age at first birth, age at menarche, and menopausal status with percent density and dense area as measured by a single radiological technologist using Cumulus software (Canto Software, Inc., San Francisco, California). We found that associations between reproductive factors and mammographic density measured using processed FFDM images were generally similar in magnitude and direction to those from prior studies using film mammography. Estimated associations for both types of FFDM machines were in the same direction. There was some evidence of heterogeneity in the magnitude of the effect sizes by machine type, which we accounted for using random-effects meta-analysis when combining results. Our findings demonstrate the robustness of quantitative mammographic density measurements across FFDM and film mammography platforms.
Keywords: breast cancer, mammographic density, mammography, risk factors
There is great interest in studying mammographic density because it is strongly associated with breast cancer risk (1, 2) and is modifiable (3). Epidemiologic studies have consistently found that women with high breast density have 4–6 times’ higher risk of breast cancer than women with low breast density (2, 3). The dense area (DA) of the breast appears radiopaque on a mammogram and is comprised of more stromal and epithelial tissue than the radiotranslucent nondense areas, which are comprised largely of fatty tissues (4). Percent density (PD) is a measure of the dense area of the breast as a percentage of total breast area.
Mammographic density is modifiable and generally decreases with age (5). A number of reproductive factors have been studied in relation to their association with mammographic density and breast cancer risk. Postmenopausal status has been strongly associated with a decrease in mammographic density (6). Lower mammographic density has also been associated with other reproductive factors, such as having more children, younger age at first birth, and younger age at menarche (7–10).
Most previous epidemiologic studies of mammographic density have used film-screen mammography, whereas over the last decade full-field digital mammography (FFDM) has largely replaced conventional film mammography. Furthermore, there are multiple types of FFDM machines currently in use, and technological differences may affect the visual appearance of dense tissue in the processed mammograms used for clinical interpretation and its association with breast cancer outcomes and risk factors. Recently, we (11) and others (12, 13) have shown that quantitative mammographic density measured on processed digital mammograms is strongly associated with breast cancer risk, with a similar direction and magnitude as the associations found when using film mammograms—providing some validation of the use of FFDM for research. However, there is a need for large-scale studies to verify the comparability of the associations between reproductive factors and quantitative mammographic density measured on FFDM to those established using digitized film mammograms.
We examined whether associations of reproductive factors with mammographic density, measured using Cumulus software (Canto Software, Inc., San Francisco, California) (14), on FFDM images from 24,840 screening-age women were similar to previously reported associations based on film-screen mammography. We also considered whether the associations differed for FFDM machines manufactured by Hologic, Inc. (Marlborough, Massachusetts) and the General Electric Company (GE) (Boston, Massachusetts), which are the two most common types of FFDM machines used in the United States.
METHODS
Study population
This study drew from participants in the Research Program on Genes, Environment and Health, a program developed and administered by the Division of Research of Kaiser Permanente Northern California (KPNC) (Oakland, California). The study included non-Hispanic white female participants who were genotyped and whose mammographic density was measured as part of a genome-wide association study of mammographic density, as described previously (11, 15). Women between the ages of 40 and 74 years were eligible to be included in this study if they had completed a health survey, provided a saliva sample for genotyping, and undergone at least 1 FFDM mammogram for breast cancer screening between 2003 and 2013.
Mammograms
Screening FFDM mammograms were identified in the women’s electronic health records and obtained from the KPNC imaging archive. FFDM mammograms came from 37 different mammography facilities within KPNC, with 1–5 machines per facility. Image selection and exclusion criteria have been described previously (16). Of the 24,840 women in the final cohort, 1,406 women (5.7%) had previously had breast cancer. We used the left craniocaudal view for the majority of the cohort. For women with previous breast cancer, we selected the craniocaudal view of the unaffected breast from the closest prediagnostic screening examination. We randomly selected the right craniocaudal view for approximately 10% of the women who did not have a personal history of breast cancer, to blind the reader to case/control status.
Density assessments
FFDM images, in Digital Imaging and Communications in Medicine format (National Electrical Manufacturers Association, Arlington, Virginia), were down-sampled to a pixel size of 200 μm for transfer to the Stanford Radiology 3D and Quantitative Imaging Laboratory (Stanford University, Stanford, California). We applied a median filter with a radius of 3 pixels to the processed Hologic FFDM images to make them appear more film-like and to improve reproducibility (11). Images were randomly assembled into batches, including random replicates for quality control. DA and PD density measurements were obtained using the Cumulus interactive threshold method (14) by a single expert radiological technologist trained in Cumulus assessments. Reader reproducibility was high for both types of images, with batch-adjusted Pearson R values of 0.952 for PD and 0.925 for DA on Hologic images and 0.961 for PD and 0.941 for DA on GE images (see Web Appendix 1, available at https://academic.oup.com/aje).
Data sources for characteristics of cohort
Age at mammography was determined on the basis of birthdate (demographic database) and date of the mammogram (mammography database). Body mass index (BMI; weight (kg)/height (m)2) measured at the patient visit closest to the mammogram date was obtained from the electronic health record. The Research Program on Genes, Environment and Health survey provided information on parity, age at first birth, age at menarche, family history of breast cancer, and menopause. The KPNC pharmacy database, which records all dispensed outpatient and inpatient prescriptions, was used to determine use of menopausal hormones within the 5 years prior to FFDM.
Literature review of published associations with PD and DA
We conducted a literature review of previously published studies of quantitative mammographic density to compare the associations obtained in this study using FFDM measurements with previously published associations obtained using digitized film mammograms. Four of the authors (S.E.A., N.U.O., R.M., and V.M.) independently searched for relevant articles using PubMed (National Library of Medicine, Bethesda, Maryland). To be included, the study had to have examined the associations of 1 or more reproductive factors (menopause, parity, age at first birth, and age at menarche) with the quantitative mammographic density outcome measures PD and/or DA. Studies examining mammographic density using the Wolfe classification (17) or the Breast Imaging Reporting and Data System classification (18) were excluded. For each study meeting our criteria, we extracted the effect estimates and reviewed the study design, study population, mammographic density assessment method, statistical model, and adjustment covariates. We then compared associations estimated in the present study with the previously published associations to evaluate the similarity of the estimates.
Statistical methods
To reduce skew and heteroscedasticity in model residuals, we applied a square-root transformation to PD measurements and a cube-root transformation to DA measurements. We adjusted for batch effects by fitting a regression model on batch and taking the residuals. We conducted F tests for differences in variance by machine type. We modeled the mammographic density outcomes of PD and DA using multivariable linear regression analyses. Model covariates were chosen a priori based on their established associations with mammographic density in prior studies (6) and included age, BMI, parity (number of children), age at first birth, family history of breast cancer, menopausal status, and use of menopausal hormones within the past 5 years. We included BMI and BMI2 in the DA model and BMI, BMI2, and BMI3 in the PD model, based on the best model fit as determined by Akaike’s Information Criterion (16). Missing covariate data from the survey were imputed using the Markov chain Monte Carlo approach (19). Menopausal status was modeled as a dichotomous variable. Age at first birth and parity were modeled categorically on the basis of data collected in the survey. We tested for a linear trend in associations across category levels.
Separate multivariable linear regression models were fitted for Hologic and GE mammograms. Heterogeneity in effect estimates was computed using the I2 statistic (20), and the statistical significance of the heterogeneity was tested formally by Cochran’s Q test (21). Random-effects meta-analysis was used to obtain combined parameter estimates (22). Parameter estimates and standard errors were transformed using the delta method to reflect change in PD and DA (23). We conducted a sensitivity analysis restricted to women without a history of breast cancer at the time of the mammogram. An α level of 0.05 was used as the cutoff for statistical significance. Regression analyses were implemented in SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina), and meta-analyses were implemented in R, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study cohort included 24,840 women with a mean age of 62 years and a mean BMI of 28. Most women were postmenopausal (81%). Digital mammograms were acquired predominantly on Hologic machines (84%), and the remainder on GE machines (16%). Table 1 gives descriptive characteristics for the full cohort and for the GE and Hologic subcohorts. There were small but statistically significant differences in mean age and BMI between women screened at clinics using Hologic machines and women screened at clinics using GE machines.
Table 1.
Characteristics of 24,840 Women in the Research Program on Genes, Environment and Health Who Underwent Full-Field Digital Mammography for Breast Cancer Screening, Kaiser Permanente Northern California, 2003–2013
| Covariate | Entire Cohort (n = 24,840) | Machine Manufacturer | ||||
|---|---|---|---|---|---|---|
| Hologica (n = 20,877) | GEb (n = 3,963) | |||||
| No. | % | No. | % | No. | % | |
| Age, yearsc | 61.5 (56.0–68.0) | 61.9 (56.0–69.0) | 59.3 (53.0–66.0) | |||
| Body mass indexc,d | 27.6 (23.2–30.7) | 27.7 (23.3–30.8) | 27.0 (22.8–30.0) | |||
| Menopausal status | ||||||
| Premenopausal | 4,835 | 19.5 | 3,941 | 18.9 | 894 | 22.6 |
| Postmenopausal | 20,005 | 80.5 | 16,936 | 81.1 | 3,069 | 77.4 |
| Parity (no. of children) | ||||||
| 0 (nulliparous) | 2,318 | 9.3 | 1,860 | 8.9 | 458 | 11.6 |
| 1 | 3,662 | 14.7 | 3,097 | 14.8 | 565 | 14.3 |
| 2 | 9,217 | 37.1 | 7,866 | 37.7 | 1,351 | 34.1 |
| ≥3 | 6,213 | 25.0 | 5,265 | 25.2 | 948 | 23.9 |
| Missing data | 3,430 | 13.8 | 2,789 | 13.4 | 641 | 16.2 |
| Age at first birth, years | ||||||
| N/A (nulliparous) | 2,318 | 9.3 | 1,860 | 8.9 | 458 | 11.6 |
| <20 | 2,584 | 10.4 | 2,208 | 10.6 | 376 | 9.5 |
| 20–24 | 6,734 | 27.1 | 5,710 | 27.3 | 1,024 | 25.8 |
| 25–29 | 5,502 | 22.2 | 4,693 | 22.5 | 809 | 20.4 |
| 30–34 | 2,748 | 11.1 | 2,329 | 11.2 | 419 | 10.6 |
| 35–40 | 1,133 | 4.6 | 948 | 4.5 | 185 | 4.7 |
| >40 | 252 | 1.0 | 216 | 1.0 | 36 | 0.9 |
| Missing data | 3,569 | 14.4 | 2,913 | 14.0 | 656 | 16.6 |
| Age at menarche, years | ||||||
| <10 | 491 | 2.0 | 422 | 2.0 | 69 | 1.7 |
| 10–11 | 4,617 | 18.6 | 3,893 | 18.6 | 724 | 18.3 |
| 12–13 | 13,177 | 53.1 | 11,037 | 52.9 | 2,140 | 54.0 |
| 14–15 | 4,147 | 16.7 | 3,500 | 16.8 | 647 | 16.3 |
| ≥16 | 920 | 3.7 | 748 | 3.6 | 172 | 4.3 |
| Never had a menstrual period | 10 | <0.1 | 9 | <0.1 | 1 | <0.1 |
| Missing data | 1,478 | 5.9 | 1,268 | 6.1 | 210 | 5.3 |
| Mammographic density | ||||||
| Percent density, %c | 21.0 (8.1–31.1) | 20.4 (8.0–30.0) | 24.3 (9.6–35.5) | |||
| Dense area, cm2 c | 28.0 (14.4–37.0) | 27.9 (14.5–36.5) | 28.9 (13.3–39.0) | |||
Abbreviations: GE, General Electric; N/A, not applicable.
a Hologic, Inc., Marlborough, Massachusetts.
b General Electric Company, Boston, Massachusetts.
c Values are expressed as mean (interquartile range).
d Weight (kg)/height (m)2.
Results from linear regression models
Table 2 shows the associations of reproductive factors (menopause, parity, age at first birth, and age at menarche) with PD and DA in our study. These associations represent the combined estimates from the random-effects meta-analysis of the associations from each machine type (as described above in “Statistical methods”). To facilitate interpretation, parameter estimates and standard errors from linear regression models for batch-corrected square-root PD and cube-root DA were transformed to estimate absolute differences in PD and DA.
Table 2.
Associationsa of Mammographic Percent Density With Reproductive Factors Among 24,840 Women in the Research Program on Genes, Environment and Health Who Underwent Full-Field Digital Mammography for Breast Cancer Screening, Kaiser Permanente Northern California, 2003–2013
| Reproductive Factor | Percent Density, %b | Dense Area, cm2 c | ||
|---|---|---|---|---|
| Estimated Difference | 95% CI | Estimated Difference | 95% CI | |
| Menopausal status | ||||
| Premenopausal | 3.50 | 0.62, 6.38 | 4.20 | 0.69, 7.71 |
| Postmenopausal | 0.00 | Referent | 0.00 | Referent |
| Parity | ||||
| 0 (nulliparous) | 1.16 | 0.60, 1.72 | 1.35 | 0.55, 2.14 |
| 1 | 0.32 | −0.09, 0.73 | 0.89 | −0.07, 1.86 |
| 2 | 0.00 | Referent | 0.00 | Referent |
| ≥3 | −1.11 | −2.87, 0.65 | −2.15 | −4.70, 0.40 |
| Age at first birth, years | ||||
| <20 | −0.54 | −1.00, −0.09 | −0.88 | −1.52, −0.23 |
| 20–24 | 0.00 | Referent | 0.00 | Referent |
| 25–29 | 0.25 | −0.54, 1.04 | 0.17 | −0.55, 0.90 |
| 30–34 | 0.75 | 0.25, 1.25 | 0.50 | −0.21, 1.20 |
| 35–39 | 1.11 | 0.39, 1.82 | 1.20 | 0.18, 2.22 |
| ≥40 | 1.89 | −0.15, 3.92 | 2.45 | 0.47, 4.43 |
| Age at menarche, years | ||||
| <10 | −1.19 | −2.15, −0.22 | −0.58 | −1.97, 0.81 |
| 10–11 | −0.46 | −0.84, −0.09 | −0.74 | −1.26, −0.22 |
| 12–13 | 0.00 | Referent | 0.00 | Referent |
| 14–15 | 0.87 | 0.47, 1.27 | 0.57 | 0.01, 1.13 |
| ≥16 | 1.69 | 0.91, 2.48 | 1.83 | 0.72, 2.95 |
Abbreviation: CI, confidence interval.
a Combined effects for Hologic (Hologic, Inc., Marlborough, Massachusetts) and General Electric (General Electric Company, Boston, Massachusetts) full-field digital mammography machines.
b Parameter estimates from the model for batch-corrected square root of percent density were transformed to estimate the difference in percent density. The model included age, body mass index, menopause, menopausal hormone therapy, family history of breast cancer, parity, age at first birth, and age at menarche.
c Parameter estimates from the model for cube root of dense area were transformed to estimate the difference in dense area. The model included age, body mass index, menopause, menopausal hormone therapy, family history of breast cancer, parity, age at first birth, and age at menarche.
Menopausal status, parity, age at first birth, and age at menarche all had statistically significant associations with breast density. We found that postmenopausal women had breast density lower than that of premenopausal women by approximately 3.5% for PD and 4.2 cm2 for DA. Nulliparity was associated with higher breast density, and both PD and DA decreased as the number of children increased. Older age at first birth was associated with higher breast density, such that women who first gave birth at age 40 years or more were estimated to have 2.4% higher PD and 3.3 cm2 higher DA than women who first gave birth before age 20 years. Older age at menarche was associated with higher PD, such that women whose age at menarche was ≥16 years were estimated to have 2.9% higher PD and 2.4 cm2 higher DA than women whose age at menarche was <10 years. We found similar results in a sensitivity analysis restricting the data to women without a history of breast cancer (Web Table 1).
Comparison with previous studies
We reviewed 13 previously published studies of the quantitative mammographic density measures PD and DA. Each study had examined the associations of mammographic density with 1 or more reproductive factors: menopause, parity, age at first birth, or age at menarche. Table 3 shows a summary of the results of each study. Further details on study design, mammographic density assessment methods, statistical models, and adjustment covariates are given in Web Table 2.
Table 3.
Results From Previous Studies of Associations Between Mammographic Density and Reproductive Factors, 2003–2017
| Study (First Author, Year (Reference No.)) and Density Measurea | Reproductive Factor | |||
|---|---|---|---|---|
| Menopause | Parity | Age at First Birth | Age at Menarche | |
| Burton, 2017 (24) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreased DA and PD in adjusted models. | |||
| SQRT PD | β = −0.46, P < 0.05 | |||
| SQRT DA | β = −0.55, P < 0.05 | |||
| Busana, 2016 (25) | Menopausal status (postmenopausal vs. pre- and perimenopausal) was associated with decreased PD in adjusted models. | Parous status (parous vs. nulliparous) was associated with decreased PD in adjusted models. | ||
| STD PD | β = −0.47, P < 0.001 | β = −0.43, P < 0.001 | ||
| Yaghjyan, 2016 (32) | Parous status (parous vs. nulliparous) was associated with decreased PD and DA in adjusted models among postmenopausal women. | Age at first birth (years; continuous) had a positive association with PD but not DA in adjusted models among postmenopausal women. | ||
| SQRT PD | β = −0.60, P < 0.05 | β = 0.03, P < 0.05 | ||
| SQRT DA | β = −0.66, P < 0.05 | β = 0.01, P > 0.05 | ||
| Parity (no. of children; continuous) had an inverse association with PD and DA in adjusted models among postmenopausal women. | ||||
| SQRT PD | β = −0.07, P < 0.05 | |||
| SQRT DA | β = −0.14, P < 0.05 | |||
| Couwenberg, 2014 (26) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreasing quintiles of PD in an unadjusted model. | Parous status (parous vs. nulliparous) was associated with decreasing quintiles of PD in an unadjusted model. | ||
| PD quintiles | P < 0. 001 | P < 0.001 | ||
| Parity (no. of children; continuous) was associated with decreasing quintiles of PD in an unadjusted model. | ||||
| PD quintiles | P < 0.001 | |||
| Lokate, 2013 (27) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreasing PD and DA over time. | Parity (no. of children; continuous) was associated with decreasing PD over time but not with DA. | Age at first birth (≥25 years vs. <25 years) had a suggestive association with increasing PD and DA over time (not formally tested). | |
| PD change | β = −0.88, P < 0.01 | β = −0.58, P < 0.05 | ||
| DA change | β = −1.42, P < 0.01 | β = −0.39, P > 0.05 | ||
| Nguyen, 2013 (9) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreased PD and DA in adjusted models. | Parity (no. of live births; continuous) had an inverse association with PD and DA in adjusted models. | Age at menarche (years; continuous) had no association with PD or DA in adjusted models. | |
| STD PD | β = −0.374, P < 0.001 | β = −0.142, P < 0.001 | β = −0.006, P > 0.05 | |
| STD DA | β = −0.162, P < 0.001 | β = −0.059, P < 0.001 | β = −0.010, P > 0.05 | |
| Tehranifar, 2011 (28) | Postmenopausal status (vs. pre- and perimenopausal) was not associated with PD or DA in adjusted models. | Parity (≥2 children vs. 0) was not associated with PD or DA in adjusted models. | Age at first birth (years; continuous) was not associated with PD or DA in adjusted models. | Age at menarche (≥13 years vs. ≤11 years) was associated with decreased DA but not PD in adjusted models. |
| PD | β = −1.68, P > 0.05 | β = −1.56, P > 0.05 | β = 0.05, P > 0.05 | β = −1.34, P > 0.05 |
| DA | β = −2.75, P > 0.05 | β = −4.13, P > 0.05 | β = 0.04, P > 0.05 | β = −6.10, P < 0.05 |
| Wong, 2011 (29) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreased PD in an adjusted model. | Parous status (parous vs. nulliparous) was associated with decreased PD in an adjusted model. | Age at first birth (<30 years vs. ≥30 years) was not associated with PD in an adjusted model. | Age at menarche (≥14 years vs. <14 years) was not associated with PD in an adjusted model. |
| PD | P < 0.001 | P < 0.001 | P > 0.05 | P > 0.05 |
| Butler, 2008 (8) | Perimenopausal status (vs. premenopausal) was not associated with PD in an adjusted model. | Parity (no. of births; ≥3 vs. 0) had a suggestive association with decreased PD in adjusted models. | Age at first birth (years; continuous) was not associated with PD in an adjusted model. | Age at menarche (>13 years vs. <12 years) had a suggestive association with increased PD in an adjusted model. |
| PD | β = −1.63, P > 0.05 | β = −5.16, P < 0.05 | P > 0.05 | β = 3.32, P = 0.09 |
| Modugno, 2006 (33) | Parity (no. of births; continuous) had an inverse association with PD in an adjusted model. | Age at first birth (years; continuous) was not associated with PD in an adjusted model. | Age at menarche (years; continuous) had no association with PD in an adjusted model. | |
| PD | β = −0.28, P < 0.001 | β = −0.33, P > 0.05 | β = −0.056, P > 0.05 | |
| Haars, 2005 (10) | Age at menopause (years; continuous) had a positive association with PD and DA in adjusted models. | Parity (no. of children; continuous) had an inverse association with PD in an adjusted model. | Age at first birth (years; categorical) was not associated with PD or DA in adjusted models. | Age at menarche (years; continuous) had a positive association with DA but not with PD in adjusted models. |
| PD | β = 0.80, P < 0.05 | β = −2.49, P < 0.001 | P > 0.05 | β = 1.00, P > 0.05 |
| DA | β = 0.84, P < 0.05 | β = −2.94, P < 0.001 | P > 0.05 | β = 1.55, P < 0.05 |
| Heng, 2004 (30) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreased PD and DA in adjusted models. | Parous status (parous vs. nulliparous) was associated with decreased PD but not DA in adjusted models. | Age at first delivery (years; continuous) was positively associated with PD and DA in adjusted models. | Age at menarche (years; continuous) was not associated with PD or DA in adjusted models. |
| PD | β = −5.37, P < 0.001 | β = −3.15, P < 0.05 | β = 0.35, P < 0.05 | β = 0.22, P > 0.05 |
| DA | β = −4.09, P < 0.001 | β = −1.37, P > 0.05 | β = 0.34, P < 0.05 | β = 0.44, P > 0.05 |
| Parity (no. of children; continuous) had an inverse association with PD and DA in adjusted models. | ||||
| PD | β = −1.20, P < 0.01 | |||
| DA | β = −1.19, P < 0.01 | |||
| Gapstur, 2003 (31) | Menopausal status (postmenopausal vs. premenopausal) was associated with decreased PD in adjusted models. | Parity (no. of births; continuous) was not associated with PD in adjusted models. | Age at first birth (years; categorical) was not associated with PD in unadjusted models. The association was not analyzed in adjusted models. | Age at menarche (years; categorical) was not associated with PD in unadjusted models. The association was not analyzed in adjusted models. |
| PD | β = −5.19, P < 0.01 | β = −0.08, P > 0.05 | P = 0.60 | P = 0.69 |
Abbreviations: DA, dense area; PD, percent density; SQRT, square-root–transformed; STD, standardized.
a See full details in Table 1.
The association of menopausal status with mammographic density in our study was consistent with previously published studies using film mammograms. In 9 studies that evaluated menopausal status, investigators all reported that postmenopausal women had lower mammographic density than premenopausal women (9, 24–31). Effect estimates associated with being postmenopausal ranged from a 0.88% decrease in PD to a 5.37% decrease and from a 1.42-cm2 decrease in DA to a 4.09-cm2 decrease. Our effect estimates of a 3.5% decrease in PD and a 4.2-cm2 decrease in DA for postmenopausal women were similar to estimates reported in these previous studies.
Our findings on the relationship between parity and mammographic density were also consistent with those of prior studies using film mammograms. Parous status versus nulliparous status was associated with decreased mammographic density in 5 prior studies (25, 26, 29, 30, 32), and number of children was inversely associated with mammographic density in 7 studies (9, 10, 26, 27, 30, 32, 33). Three additional studies of parity and mammographic density also found inverse associations, but the associations were not statistically significant (8, 28, 31). Effect estimates associated with parity ranged from a 0.28% decrease in PD per child to a 2.49% decrease per child and from a 1.19-cm2 decrease in DA per child to a 2.94-cm2 decrease per child. Our effect estimates of an approximately 0.76% decrease in PD and a 1.17-cm2 decrease in DA per child were similar to the estimates reported in these previous studies.
The association of age at first birth with mammographic density across 9 previous studies (8, 10, 27–33) has been less consistent than the association of menopause or parity with mammographic density. Age at first birth had a positive and statistically significant association with mammographic density in 2 studies (30, 32). Of the 7 studies that did not find a statistically significant association between age at first birth and mammographic density, researchers in 2 studies reported positive effects (27, 28), those in 1 study reported an inverse effect (33), and those in 4 studies reported finding no association but did not report the specific effect estimates or their direction (8, 10, 29, 31). Our effect estimates of an approximately 0.10% increase in PD and a 0.13-cm2 increase in DA per year of age at first birth were consistent with, though slightly smaller than, the reported associations of a 0.35% increase in PD and a 0.34-cm2 increase in DA per year of age at first birth in the study by Heng et al. (30).
Results from previous studies evaluating age at menarche and mammographic density were inconsistent. In 5 studies, investigators reported finding no evidence of an association between age at menarche and mammographic density (9, 29–31, 33). Tehranifar et al. (28) reported that older age at menarche was associated with decreased mammographic density, but only the association with DA was statistically significant. Consistent with our findings, 2 previous studies found a positive trend between PD and age at menarche. Butler et al. (8) reported that PD increased with increasing age at menarche among pre- and perimenopausal women in unadjusted models, and the trend was suggestive but not statistically significant in fully adjusted models. Haars et al. (10) reported a positive association between age at menarche and DA of 1.55 cm2 per year of age at menarche; for PD, the estimated 1.0% change per year of age at menarche was not statistically significant. Our effect estimates of an approximately 0.36% change in PD and 0.30-cm2 change in DA per year of age at menarche are within the range of estimates reported in these two studies.
Comparison of results for GE and Hologic machines
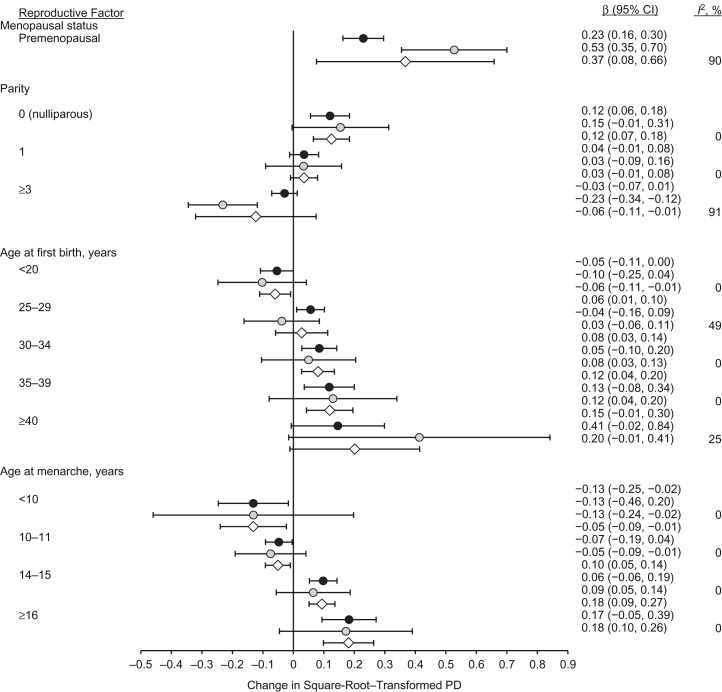
Figure 1 shows the parameter estimates and 95% confidence intervals for the association of each reproductive factor with transformed PD based on the separate regression models for women with Hologic and GE images and the combined estimate from the random-effects meta-analysis. The trends of association for each reproductive factor showed the same pattern among women with Hologic images and women with GE images, although there were some small differences in the magnitudes of the estimated associations. Menopausal status had a strong association with PD among women with both types of images, although the magnitude of this effect also showed high heterogeneity (I2 = 90%; P = 0.002), and the two estimated 95% confidence intervals did not overlap. Parity showed a clear inverse trend with PD for women with both Hologic and GE images; we found no heterogeneity in the estimated effects for nulliparous women or women with 1 child (I2 = 0%) as compared with 2 children, yet we found high heterogeneity in the estimated effect for 3 or more children (I2 = 91%, P = 0.001). Age at first birth showed a clear positive trend with PD for both Hologic and GE images, although the magnitude of the trend appeared slightly stronger in GE images. The parameter estimates for age at first birth showed low-to-moderate heterogeneity (I2 values ranging from 0% to 49%). Age at menarche showed a clear inverse trend with PD among women screened using either GE or Hologic machines, with no heterogeneity in effect estimates (I2 = 0%). Other small differences we observed in the distributions and regression models of PD and DA by machine type are described in Web Appendix 2, Web Table 3, and Web Figure 1.
Figure 1.
Associations of mammographic percent density (PD) with reproductive factors among screening-age (40–74 years) non-Hispanic white women undergoing full-field digital mammography, by machine manufacturer (black circles indicate Hologic machines (Hologic, Inc., Marlborough, Massachusetts); gray circles indicate General Electric machines (General Electric Company, Boston, Massachusetts)) and overall (results combined via meta-analysis, indicated by white diamonds), Kaiser Permanente Northern California, 2003–2013. Reference categories: menopausal status—postmenopausal; parity—2 children; age at menarche—12–13 years; age at first birth—20–24 years. Bars, 95% confidence intervals (CIs).
DISCUSSION
Our study found that reproductive factors, including menopausal status, parity, age at first birth, and age at menarche, were all associated with quantitative mammographic density measured using Cumulus software on processed FFDM images. The associations identified in this study were consistent with associations reported in previous studies of quantitative mammographic density that used film mammograms, illustrating the robustness of density measures acquired from 2 different digital mammography platforms. When comparing women screened using Hologic machines with women screened using GE machines, we found that associations of reproductive factors with mammographic density were similar in magnitude and direction and that small differences by manufacturer could be handled through appropriate statistical modeling.
Comparison with previous studies
Menopausal status had the strongest association with mammographic density in previously published studies using film mammograms, as well as in our study using processed FFDM images. Associations of parity, age at first birth, and age at menopause with mammographic density were smaller in magnitude than those for menopausal status in both our study and previous studies using film mammograms. Overall, the similarity in magnitude and direction of the associations found in our study and previous studies based on film mammograms shows that quantitative mammographic density measures obtained using Cumulus software on processed FFDM images are robust and suitable for research.
Comparison of results for GE and Hologic machines
In this study, we found that the associations of reproductive factors with mammographic density were similar between women with processed mammograms acquired on Hologic and GE FFDM machines. We also observed some minor differences in the models for mammographic density among women with Hologic images versus GE images. Specifically, after transformation and batch correction, there were small differences in the unadjusted mean values and standard deviations for PD and DA, the variance explained by model covariates, and the distribution of residuals from our regression models. In addition, we found that while most associations of reproductive factors with mammographic density exhibited no heterogeneity by manufacturer, a few associations exhibited substantial heterogeneity.
Overall, these small differences suggest that using separate regression models for women with Hologic and GE images and combining the estimates using random-effects meta-analysis is an appropriate strategy for assessing multiple factors influencing mammographic density. Fitting a single model with images from both FFDM manufacturers would have caused us to incorrectly assume that the estimates of associations and residual variances did not exhibit any differences by machine type. Furthermore, random-effects meta-analysis is preferred over fixed-effects meta-analysis when there is between-group heterogeneity in association estimates (22). Modeling approaches that combine estimates without accounting for these between-group differences could lead to underestimation of the variance in the association, making confidence intervals too narrow.
By the design of our study, we know that all covariate data were collected in the same way for the entire cohort, that the same statistical models with the same covariates were fitted, and that all images were assessed using Cumulus software by a single experienced reader with a high level of reproducibility. Different target-filter combinations and processing software used by different FFDM manufacturers can affect the appearance of processed images used for clinical interpretation and may contribute to differences in mammographic density measurements, even when they are obtained by the same highly skilled reader. Because different types of FFDM machines were located at different mammography clinics, it is also possible that demographic differences between clinic populations contributed to the small differences found for the GE and Hologic subcohorts of women; for example, we noted that women screened at clinics using Hologic FFDM machines tended to be slightly older and to have slightly higher BMIs than women screened at clinics using GE machines. However, all key confounders were accounted for in these models, and any differences in the associations would have to have arisen from differences in unmeasured confounders that are also associated with FFDM machine type.
Strengths and limitations
This study had several strengths and limitations. To our knowledge, this is the largest study of mammographic density measured using Cumulus on FFDM images to date. Furthermore, all mammographic density measurements were conducted by a single reader with proven outstanding reliability. Other strengths are that the cohort had a wide range of covariate values across age and BMI, included both premenopausal and postmenopausal women, and was drawn from a population-based sample rather than a case-control study; all of these factors increase the generalizability of the results. However, the cohort included only non-Hispanic white women because it was ancillary to a genome-wide association study; thus, it was not representative of all racial/ethnic groups.
Another strength of the study is that the two most commonly used types of FFDM machines in the United States were included, and we were able to assess similarities and differences of the associations of reproductive factors with Cumulus density measurements by machine type. However, GE and Hologic machines were not assigned at random; rather, the different types of machines were located at different mammography clinics. Thus, the GE and Hologic groups of women may have represented slightly different underlying study populations that reflected local demographic differences among women living near different KPNC clinics.
Conclusions
In this large cohort study, menopausal status, parity, age at first birth, and age at menarche were all associated with quantitative mammographic density measures based on processed FFDM images. The associations were similar in magnitude and direction to those of previous studies that used quantitative density measurements based on film mammograms. Our findings convincingly demonstrate the robustness of mammographic density measurements obtained using the Cumulus computer-assisted thresholding method on processed images acquired from 2 widely used FFDM platforms. Furthermore, small differences by machine type can be addressed through appropriate statistical methodologies that use separate regression models when needed and that account for heterogeneity when combining results. As mammography platforms rapidly evolve with new technological advancements, investigators studying mammographic density may face additional challenges in the interpretation of new mammographic density measurements in a research context. Thus, it is important to compare and validate these new measurements with regard to the established associations between mammographic density, risk factors, and breast cancer risk.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Research, Kaiser Permanente Northern California, Oakland, California (Stacey E. Alexeeff, Ninah Achacoso, Luana Acton, Laurel A. Habel); Optum360, UnitedHealth Group, Inc., Las Vegas, Nevada (Nnaemeka U. Odo); Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York (Russell McBride, Joseph H. Rothstein, Weiva Sieh); Department of Health Research and Policy, Division of Epidemiology, School of Medicine, Stanford University, Stanford, California (Valerie McGuire, Alice S. Whittemore); Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, New York (Joseph H. Rothstein, Weiva Sieh); Department of Radiology, School of Medicine, Stanford University, Stanford, California (Jafi A. Lipson, Rhea Y. Liang, Daniel L. Rubin); Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada (Martin J. Yaffe); and Department of Biomedical Data Science, School of Medicine, Stanford University, Stanford, California (Alice S. Whittemore, Daniel L. Rubin).
W.S. and L.A.H. are co–senior authors.
This study was supported by the National Cancer Institute (grants R01 CA166827 (Principal Investigator, W.S.), K07 CA143047 (Principal Investigator, W.S.), and R01 CA168893 (Principal Investigator, L.A.H.)).
We are grateful to the Kaiser Permanente Northern California members who generously agreed to participate in the Kaiser Permanente Research Program on Genes, Environment and Health. We thank Mark Westley and Marvella Villaseñor at the Kaiser Permanente Division of Research, Marc Sofilos and Shannon Walters at the Stanford Radiology 3D and Quantitative Imaging Laboratory, and Anoma Gunasekara and Gordon Mawdsley at Sunnybrook Health Sciences Center for their technical expertise and assistance.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- DA
dense area
- FFDM
full-field digital mammography
- GE
General Electric
- KPNC
Kaiser Permanente Northern California
- PD
percent density
REFERENCES
- 1. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. [DOI] [PubMed] [Google Scholar]
- 2. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 3. Boyd NF, Rommens JM, Vogt K, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. [DOI] [PubMed] [Google Scholar]
- 4. Ghosh K, Brandt KR, Reynolds C, et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat. 2012;131(1):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schetter SE, Hartman TJ, Liao J, et al. Differential impact of body mass index on absolute and percent breast density: implications regarding their use as breast cancer risk biomarkers. Breast Cancer Res Treat. 2014;146(2):355–363. [DOI] [PubMed] [Google Scholar]
- 6. Huo CW, Chew GL, Britt KL, et al. Mammographic density—a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat. 2014;144(3):479–502. [DOI] [PubMed] [Google Scholar]
- 7. Brisson J, Sadowsky NL, Twaddle JA, et al. The relation of mammographic features of the breast to breast cancer risk factors. Am J Epidemiol. 1982;115(3):438–443. [DOI] [PubMed] [Google Scholar]
- 8. Butler LM, Gold EB, Greendale GA, et al. Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN). Breast Cancer Res Treat. 2008;112(1):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen TL, Schmidt DF, Makalic E, et al. Explaining variance in the Cumulus mammographic measures that predict breast cancer risk: a twins and sisters study. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2395–2403. [DOI] [PubMed] [Google Scholar]
- 10. Haars G, van Noord PA, van Gils CH, et al. Measurements of breast density: no ratio for a ratio. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2634–2640. [DOI] [PubMed] [Google Scholar]
- 11. Habel LA, Lipson JA, Achacoso N, et al. Case-control study of mammographic density and breast cancer risk using processed digital mammograms. Breast Cancer Res. 2016;18:Article 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fowler EE, Vachon CM, Scott CG, et al. Automated percentage of breast density measurements for full-field digital mammography applications. Acad Radiol. 2014;21(8):958–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vachon CM, Fowler EE, Tiffenberg G, et al. Comparison of percent density from raw and processed full-field digital mammography data. Breast Cancer Res. 2013;15:Article R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byng JW, Boyd NF, Fishell E, et al. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–1638. [DOI] [PubMed] [Google Scholar]
- 15. Kvale MN, Hesselson S, Hoffmann TJ, et al. Genotyping informatics and quality control for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics. 2015;200(4):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexeeff SE, Odo NU, Lipson JA, et al. Age at menarche and late adolescent adiposity associated with mammographic density on processed digital mammograms in 24,840 women. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37(5):2486–2492. [DOI] [PubMed] [Google Scholar]
- 18. American College of Radiology Breast Imaging Reporting and Data System. 2nd ed Reston, VA: American College of Radiology; 1995. [Google Scholar]
- 19. Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall, Inc.; 1997. [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 23. Cox C. Delta method In: Armitage P, Colton T, eds. Encyclopedia of Biostatistics. 2nd ed Chichester, United Kingdom: John Wiley & Sons Ltd.; 2005:1540–1542. [Google Scholar]
- 24. Burton A, Maskarinec G, Perez-Gomez B, et al. Mammographic density and ageing: a collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017;14(6):e1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Busana MC, Eng A, Denholm R, et al. Impact of type of full-field digital image on mammographic density assessment and breast cancer risk estimation: a case-control study. Breast Cancer Res. 2016;18:Article 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Couwenberg AM, Verkooijen HM, Li J, et al. Assessment of a fully automated, high-throughput mammographic density measurement tool for use with processed digital mammograms. Cancer Causes Control. 2014;25(8):1037–1043. [DOI] [PubMed] [Google Scholar]
- 27. Lokate M, Stellato RK, Veldhuis WB, et al. Age-related changes in mammographic density and breast cancer risk. Am J Epidemiol. 2013;178(1):101–109. [DOI] [PubMed] [Google Scholar]
- 28. Tehranifar P, Reynolds D, Flom J, et al. Reproductive and menstrual factors and mammographic density in African American, Caribbean, and white women. Cancer Causes Control. 2011;22(4):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong CS, Lim GH, Gao F, et al. Mammographic density and its interaction with other breast cancer risk factors in an Asian population. Br J Cancer. 2011;104(5):871–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heng D, Gao F, Jong R, et al. Risk factors for breast cancer associated with mammographic features in Singaporean Chinese women. Cancer Epidemiol Biomarkers Prev. 2004;13(11):1751–1758. [PubMed] [Google Scholar]
- 31. Gapstur SM, López P, Colangelo LA, et al. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1074–1080. [PubMed] [Google Scholar]
- 32. Yaghjyan L, Colditz GA, Rosner B, et al. Reproductive factors related to childbearing and mammographic breast density. Breast Cancer Res Treat. 2016;158(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Modugno F, Ngo DL, Allen GO, et al. Breast cancer risk factors and mammographic breast density in women over age 70. Breast Cancer Res Treat. 2006;97(2):157–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.