Abstract
To find a lipase for synthesis of flavor esters in food processing, a total of 35 putative lipases from Aspergillus niger F0215 were heterologously expressed and their esterification properties in crude preparations were examined. One of them, named An-lipase with the highest esterification rate (23.1%) was selected for further study. The purified An-lipase had the maximal activity at 20 °C and pH 6.5 and the specific activity of 1293 U/mg. Sixty percent of the activity was maintained in a range of temperatures of 0–30 °C and pHs of 3.0–8.5. The highest hydrolysis activity of An-lipase was towards pNPC (C8), followed by pNPB (C4) and pNPA (C2), then pNPL (C12). Km, Vmax, kcat, and kcat/Km towards pNPC were 26.7 mmol/L, 129.9 mmol/(L h), 23.2 s−1, and 0.8/mM/s, respectively. The ethyl lactate, butyl butyrate, and ethyl caprylate flavor esters were produced by esterification of the corresponding acids with conversion efficiencies of 15.8, 37.5, and 24.7%, respectively, in a soybean-oil-based solvent system. In conclusion, An lipase identified in this study significantly mediated synthesis of predominant flavor esters (ethyl lactate, butyl butyrate, and ethyl caprylate) in a soybean-oil-lacking other toxic organic solvents, which has potential application in food industries.
Keywords: Aspergillus niger, Lipase, Flavor ester synthesis, Soybean-oil solvent
Introduction
Flavor esters are short-chain esters commonly found in various plant species. The predominant flavor esters are ethyl acetate, ethyl caproate, ethyl lactate, ethyl caprylate, and butyl butyrate, which have been applied in the food, cosmetic, detergent, chemical, and pharmaceutical industries with an estimated global market exceeding $US 22 billion per year (Brault et al. 2014). For example, ethyl lactate is an agrochemical product defined as Generally Recognized as Safe (GRAS) approved by Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) as a pharmaceutical ingredient and food additive (Villanueva-Bermejo et al. 2017). Ethyl caprylate can be used as flavor enhancer in the fermentation food industry such as some beverages (Hu et al. 2018). Butyl butyrate is also important in food and fragrance industry and contributes significantly to the aroma of apples, pineapples, bananas, apricots, and butter. It is also a valuable potential fuel source, which possesses excellent compatibility with other fuels such as aviation kerosene, petrol, and diesel (Duan et al. 2017).
Currently, flavor esters are either extracted from natural resources using microbial cultures, plant cell cultures, and enzyme-catalyzed reactions or artificially synthesized using chemical processes (Longo and Sanroman 2006; Mishra et al. 2013, 2014). The extracted flavor esters from natural resources are ester mixtures either with low extraction yield or with low ester concentrations, thus drastically increasing production costs. It is also subject to diverse problems, including raw materials cost and dependence on climate and agriculture conditions (De Barros et al. 2012). Chemical synthesis is currently an alternative way to obtain flavor esters, but often relies on harsh procedures requiring chemical solvents and high energy consumption, resulting in environmentally non-friendly processes with a very high carbon footprint (Brault et al. 2014; De Barros et al. 2011). The lack of substrate selectivity in the chemical process also creates racemic mixtures with undesirable side-reaction products that reduce synthesis efficiency and increase production costs (Brault et al. 2014; De Barros et al. 2011).
The use of enzymatic synthesis for flavor ester production has cost-effective, industrially attractive features, such as the mild reaction conditions, along with the high selectivity and specificity, which enable the synthesis of flavor molecules with high quality and purity (De Barros et al. 2011). Lipases from bacteria (Talon et al. 1996) and fungi (Martins et al. 2013) have been successfully investigated for flavor esters synthesis in organic solvent systems. Organic solvents such as heptane, n-hexane and n-nonane used in enzymatic flavor ester preparation are effective but expensive, toxic, and flammable (Ghamgui et al. 2004). Furthermore, safety concerns limit the use of some organic solvents in the production of food ingredients (Yu et al. 2019). For flavor ester preparation, enzymatic process in organic solvent-free system requires a simple mixture of reactants with no organic solvents to be rapidly added, thereby allowing for much higher substrate concentrations and increases in volume productivity (Ghamgui et al. 2004; Yu et al. 2019).
The overall aim of the present study was to find an enzyme, which can synthesize flavor esters in a solvent system lacking toxic solvents as an alternative to the solvent-free system. A novel lipase capable of synthesizing flavor esters in a soybean-oil-solvent system was identified from A. niger. The biochemical properties of An-lipase important for potential application were investigated.
Materials and methods
Fungal strain and media
Aspergillus niger F0215 used in this study was isolated from a natural sample, identified and stored at the Culture and Information Center for Industrial Microorganisms of China Universities (CICIM-CU, Jiangnan University, China). Pichia pastoris GS115 and pPIC9K (Invitrogene) were used for expression of putative lipases from A. niger according to the manufacturer’s guidelines. Escherichia coli JM109 (Stratagene) was used as the host cell for plasmid propagation.
Screening of the enzyme in A. niger F0215 for flavor ester synthesis
Aspergillus niger F0215 was cultivated in a basal culture medium at 180 rpm and 28 °C for 120 h (Parra et al. 2008). The supernatant was collected by removing the mycelium by filtration. The supernatant was lyophilized and used as a crude enzyme preparation for further experiments.
Screening the activity of A. niger supernatant for flavor ester synthesis was carried out in 5 mL reaction mixture containing 1.2 M caprylic acid and 1.44 M ethyl alcohol dissolved in n-heptane. The supernatant powder (20 mg) was added and mixed. The mixtures were then incubated at 40 °C with constant shaking at 150 rpm for 12 h (Duan et al. 2017). The product pattern in the reaction mixture was analyzed by gas chromatography.
Preparation of the recombinant lipases
The total RNA from A. niger F0215 were recovered and cDNA was synthesized according to the methods as described (Niu et al. 2017). The PCR products of putative lipase genes were cloned into pPIC9K and then genetically transformed into P. pastoris GS115 to yield the lipase-expressing recombinants. The recombinant lipases were prepared according to Invitrogen Easyselect™ Pichia Expression Kit. When necessary, the enzyme was purified to homogeneity using ammonium sulfate precipitation, followed by removal of the salt with DP-10 Spin Adopter and finally G75 gel chromatography. The supernatant was lyophilized and used as a pure enzyme preparation for further experiments.
Enzyme assay and protein concentration determination
Lipase activity was assayed using p-nitrophenyl butyrate (pNPB) (Sigma) as a substrate (Yang et al. 2010). Briefly, the reaction mixture (500 μL) contained 400 μL 50 mM phosphate buffer pH 8.0, 50 μL suitably diluted enzyme, and 50 μL 20 mM pNPB dissolved in isopropanol. After 10 min reaction at 30 °C, the released p-nitrophenol (pNP) was quantified by measuring the absorbance at 410 nm. One unit of enzyme activity is defined as the amount of enzyme liberating 1 μmol pNP per min under the above conditions. The protein concentration was determined, using bovine serum albumin (V, purchased from Sigma) as standard.
Effects of pH and temperature on the activity and stability of An-lipase
For optimum temperature and pH determination, the activity of An-lipase was measured at 0–70 °C or pH 2.0–10.0. The thermostability or pH stability was determined based on the residual activity of the enzyme after incubating the lipase at 20–60 °C or pH 5.0–9.0 for 4 h. The influence of cations and complexing agent on enzyme activity was tested by mixing with various cations and EDTA in phosphate buffer (pH 7.0) at a final concentration of 1 mmol/L and incubating at 4 °C for 30 min or 1 h. The residual activity was measured using pNPB as the substrate at 30 °C for 10 min.
Substrate specificity and kinetic parameter of An-lipase
The substrate specificity towards various p-nitrophenyl esters was examined using the substrates p-nitrophenyl acetate (pNPA, C2), pNPB (C4), p-nitrophenyl caprylate (pNPC, C8), p-nitrophenyl laurate (pNPL, C12), and p-nitrophenyl palmitate (pNPP, C16) (all purchased from Sigma) at a final concentration of 1 mmol/L. The lipase activity was measured in phosphate buffer (pH 7.0) at 30 °C for 10 min.
The kinetic parameters, Km and Vmax, of lipase towards pNPB were determined using different substrate concentrations at 30 °C in 20 mM phosphate buffer, pH 7.0 for 5 min. kcat was calculated using the following equation:
where E enzyme concentration.
Flavor ester synthesis by An-lipase in solvent-free system
Flavor ester synthesis by An-lipase was carried out in a 5 mL reaction volume containing 0.65 mL (0.59 g) caprylic acid and 0.35 mL (0.28 g) ethyl alcohol, 0.75 mL (0.94 g) lactic acid and 0.25 mL (0.20 g) ethyl alcohol, 0.6 mL (0.58 g) butyric acid, and 0.4 mL (0.32 g) butanol in 4 mL soybean oil as a solvent with 20 mg lipase powder (activity 1293 U/mg protein), respectively. The reaction mixture was incubated at 30 °C and natural pH with shaking at 200 rpm for 12 h.
The samples were extracted by hexane. The esters’ sample (1 μL) was analyzed using a gas chromatograph (Agilent 7890B, Agilent Technologies Inc., USA), equipped with a flame ionization detector and an HP-INNOWax column (30 m × 0.32 mm × 0.25 μm) with nitrogen as carrier gas and a split ratio of 10–1. The temperature of both injector and detector was maintained at 200 °C. The oven temperature was held at 50 °C for 8 min and then increased to 150 °C at a rate of 5 °C/min and held for 15 min. The standards of ethyl lactate, butyl butyrate, and ethyl caprylate were purchased from Merck (Shanghai, China). The conversion of acid was calculated based on the consumption of acid in the reaction using the following equation:
where A moles of acid in the reactant mixture and B initial moles of acid (Pires-Cabral et al. 2010).
Results and discussion
Screening an enzyme suitable for synthesis of flavor esters from A. niger F0215
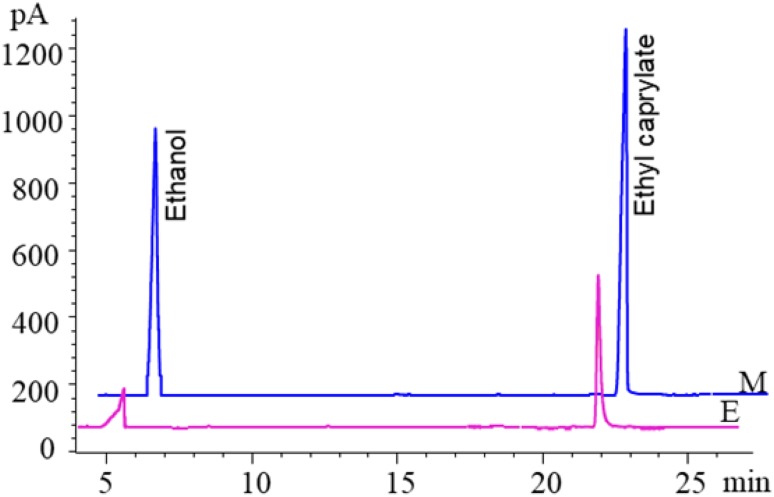
To illustrate the possibility of A. niger produced enzymes mediating flavor ester synthesis, the supernatant of A. niger F0215 fermentation was evaluated for the ability to synthesize flavor esters. The result showed that the A. niger F0215 fermentation supernatant contained the enzyme activity that was able to catalyze the synthesis of ethyl caprylate (Fig. 1). We tried to separate and purify these enzymes, but failed (data not shown).
Fig. 1.
Identification by gas chromatography of ethyl caprylate produced from caprylic acid and ethanol by the crude enzyme preparation of A. niger F0215. M: standards, E: A. niger F0215 fermentation supernatant treatment [the peak time of caprylic acid is about 38 min (not shown)]
Since the synthesis of flavor esters by enzymes has been reported previously (mainly belonging to several lipases and cutinases) (Duan et al. 2017; Koutinas et al. 2018), we, therefore, used bioinformatic methods to mine the A. niger CBS513.88 genome (EMBL AM270980-AM270998). A total of 35 putative lipases from A. niger F0215 were selected and expressed in P. pastoris GS115 (to be published). All expressed enzymes were purified, and of these, nine were found to be capable of esterification of caprylic acid. The esterification conversion efficiency of nine enzymes ranged between 1.3 and 23.1%. One enzyme with the best esterification ability was selected for further study. The DNA sequence encoding this enzyme was found to be the same as An14g02170 assigned in the A. niger CBS513.88 genome (designated as An-lipase in this study). BlastP searches showed that the deduced amino acid sequence of An-lipase shared the identity of 11.8% with the Lip1 from A. niger F044 (sequence identity DQ647700) (Yang et al. 2010) and 10.4% with the Lip1 from A. niger (FJ536288) (Yang et al. 2010), indicating that the lipase encoded by An14g02170 is a different lipase.
Biochemical properties of An-lipase
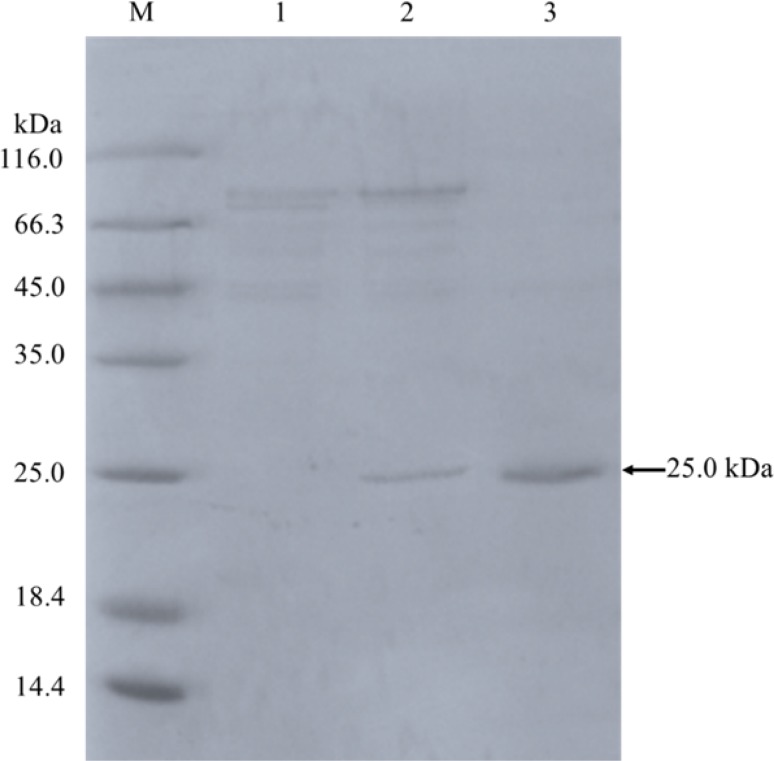
The recombinant An-lipase was purified to electrophoretic homogeneity using ammonium sulfate precipitation and G75 gel chromatography (Table 1, Fig. 2). The purified enzyme had the specific activity of 1293 U/mg and molecular weight of 25 kDa on SDS-PAGE, which matches with the predicted molecular mass of 24.8 kDa.
Table 1.
Purification of the recombinant An-lipase from A. niger F0215
| Purification step | Total protein (mg) | Total activity (U) | Specific activity (U/mg protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude enzyme solution | 77.5 | 2542 | 32.8 | 1.0 | 100 |
| Ammonium sulfate precipitation | 3.2 | 1679 | 525 | 16.0 | 66.1 |
| Sephadex G-75 | 0.7 | 905 | 1293 | 39.4 | 35.6 |
Fig. 2.
SDS-PAGE analysis of purified An-lipase from A. niger F0215. Lane M low-molecular weight standard protein markers; lane 1 crude GS115 solution; lane 2 crude enzyme solution; lane 3 purified An-lipase
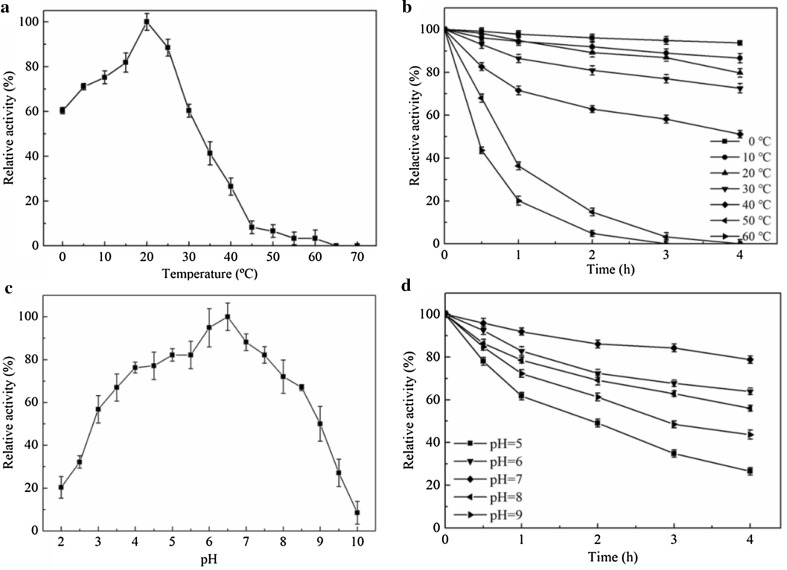
To establish the temperature and pH optima, the activity of purified enzyme was determined at a range of temperatures of 0–50 °C and pHs of 4.0–9.0. The temperature optimum was 20 °C and the activity gradually declined at temperatures above 30 °C and below 15 °C. The enzyme showed relatively high activity at 0 °C and retained more than 60% activity at 0 °C (Fig. 3a). An-lipase was stable at 0–40 °C, but lost activity rapidly at 50 and 60 °C (Fig. 3b). Its optimal temperature is similar to most of microbial lipases such as Yarrowia lipolytica NCIM 3639 (Sathish Yadav et al. 2011) and Geotrichum sp. SYBC WU-3 (Cai et al. 2009), but lower than those from Bacillus methylotrophicus PS3 (55 °C) (Sharma et al. 2017), Marinobacter sp. EMB5 (50 °C) (Hemamalini and Khare 2016) and Pichia lynferdii Y-7723 (35 °C) (Kim et al. 2010). Few microbial enzymes have catalytic activity at such low temperatures (Kim et al. 2010), suggesting that An-lipase might be of value in processes requiring low temperature, such as cheese ripening and alcoholic beverage production (Sumby et al. 2009).
Fig. 3.
Enzyme activity and stability profiles of An-lipase from A. niger F0215 in various pH and temperature conditions. a Activity profile at various temperatures; b stability profile at various temperatures; c activity profile at various pHs; d stability profile at various pHs. Each point represents the mean ± standard error from three independent experiments
An-lipase was found to be functionally active in the pH range of 4.0–9.0 with optimal activity at pH 6.5 and showed approximately 80% residual activity at pH 4.0 and 7.5 (Fig. 3c). The optimal pH is similar to some microbial lipases (Sathish Yadav et al. 2011; Sharma et al. 2017; Kim et al. 2010), but lower than those of alkaline lipases from Geotrichum sp. SYBC WU-3 (pH 9.5) (Cai et al. 2009) and Marinobacter sp. EMB5 (pH 9.0) (Hemamalini and Khare 2016). The enzyme showed excellent stability over a wide pH range from acidic to alkaline (Fig. 3d), which is similar to the lipases of Geotrichum candidum (Muhammad et al. 2017). This broad-range stability may render it useful for industrial processes carried out in both acidic and alkaline environments.
The presence of cations in process reaction mixtures can increase or inhibit enzyme activity. Lipase from various sources has shown different degrees of sensitivity to cations and chemical agents (Ahmed Al-Tammar et al. 2016). The catalytic activity of An-lipase was not significantly altered by cations except for Fe3+, which had a moderate inhibitory effect (Table 2). This is similar to the behavior of lipase from B. methylotrophicus PS3 (Sharma et al. 2017). A lipase from G. candidum was inhibited by Ca2+, Mn2+, Cu2+, Mg2+, Hg2+, Cd2+, and Zn2+ (Muhammad et al. 2017), whereas the lipase from Marinobacter sp. EMB5 was slightly stimulated by Ca2+ (Hemamalini and Khare 2016). By contrast, the lipase from Marinobacter sp. EMB5 was inhibited by the presence of K+, Cu2+, Hg2+, and Ba2+ (Hemamalini and Khare 2016).
Table 2.
Effect of cations and EDTA on activity of An-lipase from A. niger F0215
| Metal ions | Relative activity (%)a |
|---|---|
| Control | 100.0 ± 0.9 |
| CoCl2 | 82.0 ± 0.9 |
| CuCl2 | 74.0 ± 0.8 |
| FeCl3 | 65.3 ± 1.0 |
| MnCl2 | 77.2 ± 0.8 |
| CaCl2 | 76.3 ± 0.9 |
| KCl | 95.5 ± 0.8 |
| MgCl2 | 79.6 ± 0.9 |
| ZnCl2 | 73.7 ± 0.8 |
| SnCl2 | 75.2 ± 1.0 |
| EDTA | 80.3 ± 1.0 |
aMean ± standard deviation of triplicate determinations
Substrate specificity of An-lipase
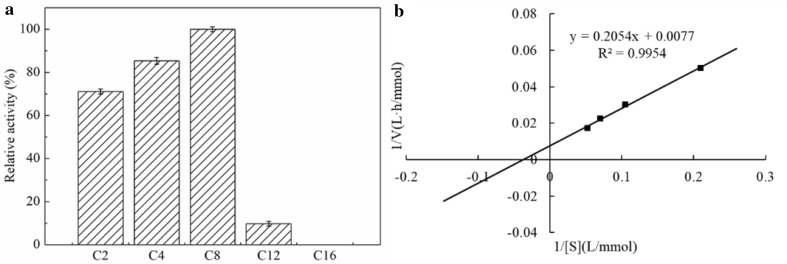
The An-lipase exhibited variable hydrolytic activity towards various p-nitrophenyl esters (Fig. 4a). The substrates pNPA (C2), pNPB (C4), and pNPC (C8) were more efficiently hydrolyzed than other esters. The highest activity of An-lipase was towards pNPC (C8), followed by pNPB (C4) and pNPA (C2), then pNPL (C12). This is similar to the behavior of Lipase A and Lipase B from Geotrichum sp. SYBC WU-3 (Cai et al. 2009), which preferred short-chain fatty acyl esters (C4). Other microbial lipases show different substrate specificities, such as Y. lipolytica NCIM 3639 lipase which preferred medium- and long-chain pNP esters (C8–C18) (Sathish Yadav et al. 2011). Furthermore, the Lip 1 and Lip 2 from A. niger preferred pNP caprate ester (C10) and pNP caprylate ester (C8), respectively (Yang et al. 2010).
Fig. 4.
a Substrate specificity of An-lipase from A. niger F0215 to various p-nitrophenyl esters was examined using the substrates p-nitrophenyl acetate (C2), p-nitrophenyl butyrate (C4), p-nitrophenyl caprylate (C8), p-nitrophenyl laurate (C12) and p-nitrophenyl palmitate (C16) in phosphate buffer (pH 7.0) at 37 °C for 10 min. b Lineweaver–Burke plot of the inverse of An-lipase activity and the inverse of p-nitrophenyl caprylate concentration yielded Km and Vmax of 26.7 mmol/L and 129.9 mmol/(L h), respectively
The kinetic parameters of the An-lipase were determined on pNPC. Km, Vmax, kcat, and kcat/Km towards pNPC were 26.7 mmol/L, 129.9 mmol/(L h), 23.2 s−1, and 0.8/mM/s, respectively (Fig. 4b).
Synthesis of flavor ester mediated by An-lipase
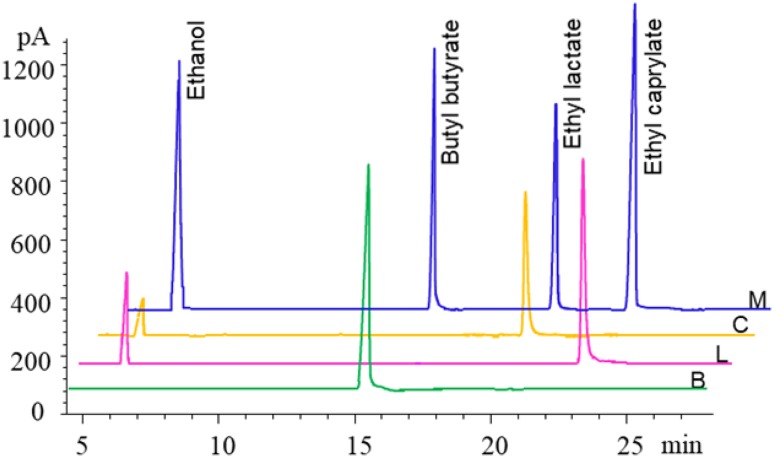
To illustrate the ability of An-lipase to facilitate flavor ester synthesis in the soybean-oil-solvent system, the An-lipase was used in the synthesis of flavor esters. The result showed that An-lipase exhibited significant esterification efficiency for flavor esters (ethyl lactate, butyl butyrate, and ethyl caprylate) (Fig. 5). The product yields of ethyl lactate, butyl butyrate, and ethyl caprylate was 15.8, 37.5, and 24.7%, respectively.
Fig. 5.
GC profiles of synthesis of flavor esters by An-lipase from A. niger F0215 in soybean-oil-solvent system. M: Standards (5 mg/mL butyl butyrate, ethyl caprylate, and ethyl lactate, respectively), B: synthesis of butyl butyrate by An-lipase [The peak time of butyric acid is about 30 min (not shown). Butanol flowed out of the reagent peak], L: synthesized ethyl lactate by An-lipase [The peak time of lactic acid is about 35 min (not shown)], C: synthesized ethyl caprylate by An-lipase [The peak time of caprylic acid is about 38 min (not shown)]
Fermented foods consist of a consortium of microorganisms, which are present as natural indigenous microbiota in uncooked plant or animal substrates and in the environment. Furthermore, starter culture(s) containing functional microorganisms can also be added to modify the substrates biochemically and organoleptically into edible products that are culturally and socially acceptable to the consumers (Tamang et al. 2016). Among them, esters are the dominant compounds responsible for volatile aroma and flavor and have been detected in some fermented foods (Wu et al. 2015). The predominant flavor esters are ethyl acetate, ethyl caproate, ethyl lactate, ethyl caprylate, and butyl butyrate, which are mainly formed during the vigorous phase of primary fermentation by enzymatic chemical condensation of organic acids (acetate, lactate, butyrate, etc.) and alcohols (ethanol, butanol, etc.) (Pires et al. 2014). An-lipase exhibited significant esterification efficiency for flavor esters ethyl lactate, butyl butyrate, and ethyl caprylate in a soybean-oil-solvent system. Therefore, the addition of the lipase during food fermentation is another option to be considered to improve the flavor and reduce the acid value of fermented foods.
Conclusion
A novel lipase with attractive synthesis of flavor esters in a soybean-solvent system was identified from A. niger. The enzyme showed properties of tolerance to cold, acid, and alkaline conditions with the optimum temperature and pH of 20 °C and 6.5, respectively. The enzyme exhibited significant esterification efficiency for synthesis of the flavor esters, ethyl lactate, butyl butyrate, and ethyl caprylate in a soybean-oil-solvent system. These excellent properties make it an attractive candidate for food processing, such as cheese ripening, alcoholic beverage production, and other fermented foods production.
Acknowledgements
This research was supported by the Intergovernmental International Scientific and Technological Innovation Cooperation Program, MOST, China (Grant No.: 2018YFE0100400) and the Raising Program of Innovation Team for Tianjin Colleges and Universities, Tianjin, China (Grant No.: TD12-5002) to ZXWANG.
Compliance with ethical standards
Conflict of interest
The authors declare that a Chinese Innovative Patent based on the use of this enzyme or its mutants for improving the flavor of fermented foods is under application.
Contributor Information
Fuping Lu, Phone: +86 22 6060 1958, Email: lfp@tust.edu.cn.
Zheng-Xiang Wang, Phone: +86 22 6060 2770, Email: zxwang0519@tust.edu.cn.
References
- Ahmed Al-Tammar K, Omar O, Abdul Murad AM, Abu Bakar FD. Expression and characterization of a cutinase (AnCUT2) from Aspergillus niger. Open Life Sci. 2016;11:29–38. [Google Scholar]
- Brault G, Shareck F, Hurtubise Y, Lépine F, Doucet N. Short-chain flavor ester synthesis in organic media by an Escherichia coli whole-cell biocatalyst expressing a newly characterized heterologous lipase. PLoS ONE. 2014;9:1–9. doi: 10.1371/journal.pone.0091872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wang L, Liao X, Ding Y, Sun J. Purification and partial characterization of two new cold-adapted lipases from mesophilic Geotrichum sp. SYBC WU-3. Process Biochem. 2009;44:786–790. doi: 10.1016/j.procbio.2009.03.011. [DOI] [Google Scholar]
- De Barros DPC, Fernandes P, Cabral JMS, Fonseca LP. Synthetic application and activity of cutinase in an aqueous, miniemulsion model system: hexyl octanoate synthesis. Catal Today. 2011;173:95–102. doi: 10.1016/j.cattod.2011.05.039. [DOI] [Google Scholar]
- De Barros DPC, Azevedo AM, Cabral JMS, Fonseca LP. Optimization of flavor esters synthesis by Fusarium solani pisi cutinase. J Food Biochem. 2012;36:275–284. doi: 10.1111/j.1745-4514.2010.00535.x. [DOI] [Google Scholar]
- Duan X, Liu Y, You X, Jiang Z, Yang S, Yang S. High-level expression and characterization of a novel cutinase from Malbranchea cinnamomea suitable for butyl butyrate production. Biotechnol Biofuels. 2017;10:233. doi: 10.1186/s13068-017-0912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamgui H, Karra-Chaˆbouni M, Gargouri Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: a comparative study between n-hexane and solvent-free system. Enzyme Microb Technol. 2004;35:355–363. doi: 10.1016/j.enzmictec.2004.06.002. [DOI] [Google Scholar]
- Hemamalini R, Khare SK. Purification and characterization of active aggregates of an organic solvent tolerant lipase from Marinobacter sp. EMB5. Insights Enzyme Res. 2016;1:1–8. [Google Scholar]
- Hu K, Jin G-J, Mei W-C, Li T, Tao Y-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018;239:495–501. doi: 10.1016/j.foodchem.2017.06.151. [DOI] [PubMed] [Google Scholar]
- Kim HR, Kim IH, Hou CT, Kwon KI, Shin BS. Production of a novel cold-active lipase from Pichia lynferdii Y-7723. J Agric Food Chem. 2010;58:1322–1326. doi: 10.1021/jf903430t. [DOI] [PubMed] [Google Scholar]
- Koutinas M, Yiangou C, Osório NM, Ioannou K, Canet A, Valero F, Ferreira-Dias S. Application of commercial and non-commercial immobilized lipases for biocatalytic production of ethyl lactate in organic solvents. Bioresour Technol. 2018;247:496–503. doi: 10.1016/j.biortech.2017.09.130. [DOI] [PubMed] [Google Scholar]
- Longo MA, Sanroman MA. Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotechnol. 2006;44:335–353. [Google Scholar]
- Martins AB, Friedrich JLR, Cavalheiro JC, Garcia-Galan C, Barbosa O, Ayub MAZ, Fernandez-Lafuente R, Rodrigues RC. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour Technol. 2013;134:417–422. doi: 10.1016/j.biortech.2013.02.052. [DOI] [PubMed] [Google Scholar]
- Mishra S, Sachan A, Sachan SG. Production of natural value-added compounds: an insight into the eugenol biotransformation pathway. J Ind Microbiol Biotechnol. 2013;40:545–550. doi: 10.1007/s10295-013-1255-9. [DOI] [PubMed] [Google Scholar]
- Mishra S, Sachan A, Vidyarthi AS, Sachan SG. Transformation of ferulic acid to 4-vinyl guaiacol as a major metabolite: a microbial approach. Rev Environ Sci Biotechnol. 2014;13:377–385. doi: 10.1007/s11157-014-9348-0. [DOI] [Google Scholar]
- Muhammad A, Ali S, Bokhari I, Ali N, Mukhtar H, Nawaz A, Imran M. Purification and characterization of extracellular lipase by Geotrichum candidum of dairy origin. Pak J Bot. 2017;49:757–761. [Google Scholar]
- Niu D, Tian X, Mchunu NP, Jia C, Singh S, Liu X, Prior BA, Lu F. Biochemical characterization of three Aspergillus niger β-galactosidases. Electron J Biotechnol. 2017;27:37–43. doi: 10.1016/j.ejbt.2017.03.001. [DOI] [Google Scholar]
- Parra LP, Reyes F, Acevedo JP, Salazar O, Andrews BA, Asenjo JA. Cloning and fusion expression of a cold-active lipase from marine Antarctic origin. Enzyme Microb Technol. 2008;42:371–377. doi: 10.1016/j.enzmictec.2007.11.003. [DOI] [Google Scholar]
- Pires EJ, Teixeira JA, Brányik T, Vicente AA. Yeast: the soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl Microbiol Biotechnol. 2014;98:1937–1949. doi: 10.1007/s00253-013-5470-0. [DOI] [PubMed] [Google Scholar]
- Pires-Cabral P, Fonseca MMRD, Ferreira-Dias S. Esterification activity and operational stability of Candida rugosa lipase immobilized in polyurethane foams in the production of ethyl butyrate. Biochem Eng J. 2010;48:246–252. doi: 10.1016/j.bej.2009.10.021. [DOI] [Google Scholar]
- Sathish Yadav KN, Adsul MG, Bastawde KB, Jadhav DD, Thulasiram HV, Gokhale DV. Differential induction, purification and characterization of cold active lipase from Yarrowia lipolytica NCIM 3639. Bioresour Technol. 2011;102:10663–10670. doi: 10.1016/j.biortech.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Sharma P, Sharma N, Pathania S, Handa S. Purification and characterization of lipase by Bacillus methylotrophicus PS3 under submerged fermentation and its application in detergent industry. J Genet Eng Biotechnol. 2017;15:369–377. doi: 10.1016/j.jgeb.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby KM, Matthews AH, Grbin PR, Jiranek V. Cloning and characterization of an intracellular esterase from the wine-associated lactic acid bacterium Oenococcus oeni. Appl Environ Microbiol. 2009;75:6729–6735. doi: 10.1128/AEM.01563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon R, Montel MC, Berdague JL. Production of flavor esters by lipases of Staphylococcus warneri and Staphylococcus xylosus. Enzyme Microb Technol. 1996;19:620–622. doi: 10.1016/S0141-0229(96)00075-0. [DOI] [Google Scholar]
- Tamang JP, Holzapfel WH, Watabane K. Review: diversity of microorganisms in global fermented foods and beverages. Front Microbiol. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Bermejo D, Reglero G, Fornari T. Recent advances in the processing of green tea biomolecules using ethyl lactate: a review. Trends Food Sci Technol. 2017;62:1–12. doi: 10.1016/j.tifs.2016.12.009. [DOI] [Google Scholar]
- Wu R, Yu M, Liu X, Meng L, Wang Q, Xue Y, Wu J, Yue X. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int J Food Microbiol. 2015;211:23–31. doi: 10.1016/j.ijfoodmicro.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Yang J, Sun J, Yan Y. lip2, a novel lipase gene cloned from Aspergillus niger exhibits enzymatic characteristics distinct from its previously identified family member. Biotechnol Lett. 2010;32:951–956. doi: 10.1007/s10529-010-0238-4. [DOI] [PubMed] [Google Scholar]
- Yu H, Lee M-W, Shin H, Park KM, Chang PS. Lipase-catalyzed solvent-free synthesis of erythorbyl laurate in a gas-solid-liquid multiphase system. Food Chem. 2019;271:445–449. doi: 10.1016/j.foodchem.2018.07.134. [DOI] [PubMed] [Google Scholar]