Abstract
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) improve the clinical outcomes of EGFR-mutant non-small cell lung cancer (NSCLC) patients significantly, however, acquired resistance occurs almost inevitably. The underlying mechanisms of osimertinib resistance and treatment strategies after resistance remain largely unknown. Here we reported a case of lung adenocarcinoma patient who progressed on osimertinib with EGFR L718Q mutation in the absence of T790M mutation. The patient received icotinib as an exploratory treatment regimen for a short while with stable disease observed. Unfortunately, the therapy was discontinued due to intolerable hepatotoxicity. This is the first clinical report of the use of the effective EGFR-TKI treatment after L718Q-induced osimertinib resistance. The therapeutic regimens for NSCLC patients progressed on osimertinib still require large-scale investigation.
Keywords: Non-small cell lung cancer (NSCLC), epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), EGFR L718Q, osimertinib resistance
Introduction
Tremendous advances in precision medicine have changed the clinical therapeutic patterns of advanced non-small cell lung cancer (NSCLC). For epidermal growth factor receptor (EGFR)-mutant patients, the utilization of tyrosine kinase inhibitors (TKIs) has improved the progression-free survival (PFS) to 18 months (1). Osimertinib is a third-generation, irreversible EGFR-TKI that selectively inhibits both EGFR sensitizing mutations and T790M resistance mutations (2,3). Despite the initial encouraging therapeutic efficacy, all patients will develop resistance to osimertinib eventually. EGFR C797S is the most common molecular mechanism conferring resistance to osimertinib treatment (4). EGFR L718Q mutation is another resistance tertiary mutation. It was firstly reported in a metastatic NSCLC patient with coexisting EGFR L858R and T790M mutations who progressed on osimertinib treatment (5). Here, we reported a lung adenocarcinoma patient resistant to osimertinib who harbored a novel EGFR L718Q mutation and lost the previously detected EGFR T790M mutation. Soon after that, he received icotinib treatment for one month with stable disease observed. However, he discontinued the treatment due to liver toxicity. In this report, we also discussed the possible treatment options after progression on osimertinib.
Case presentation
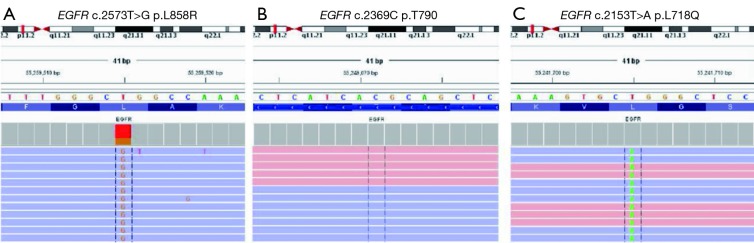
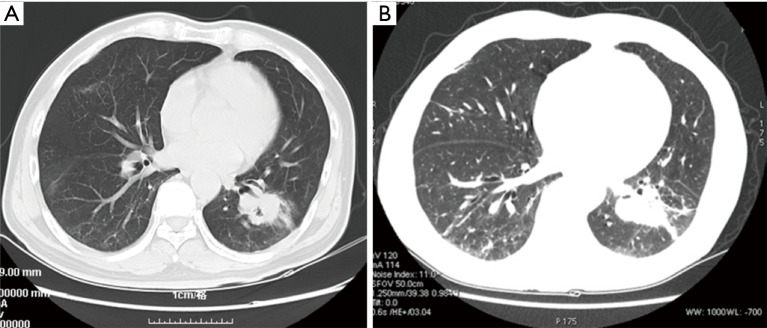
A 55-year-old Chinese man presented with pain of the lower right limb without apparent causes in January 2016. Chest radiography and computed tomography (CT) scan revealed a lesion in the left lower lung, enlarged lymph nodes in mediastina and left hilum of the lung and lesions in lumbar vertebrae, sacral vertebrae and right sacroiliac joint. Biopsy of the lesion confirmed the diagnosis of stage IV (T2aN2M1c) lung adenocarcinoma (with bone, brain and lymph nodes metastasis). Based upon the detection of EGFR L858R mutation, the patient received first-line therapy with gefitinib (250 mg/day) for 12 months. Periodically reexaminations every 2 months suggested the stable disease. He also underwent whole brain radiotherapy once and received zoledronic acid to block the activity of osteoclast cells during that 12 months. Pulmonary CT in February 2017 revealed enlarged tumor in left lower lobe, which can be defined as disease progression (PD). Meanwhile, ctDNA analysis verified the presence of T790M resistance mutation. Thus, he started osimertinib treatment in February 2017. Two months later, CT scan demonstrated that the lung primary lesion was obviously shrank and other lesions were stable. He continued osimertinib treatment until September 2017, when the lesions on lower left lung and lymph nodes of left hilum of the lung progressed. Percutaneous needle lung biopsy sample was sent to perform hybridization capture based next-generation sequencing (NGS) testing, which enables the simultaneous detection of somatic alterations of 59 cancer-associated genes. Three somatic mutations, including EGFR L858R, TP53 R175H and EGFR L718Q, were identified, while T790M mutation was not detected (Table 1, Figure 1). Upon review of several pre-clinical and clinical studies, patients harboring EGFR L858R and L718Q mutations may be sensitive to first- and second-generation EGFR-TKIs (6-8). The patient tried icotinib treatment. One month later, the examination of liver function showed that the aspartate transaminase (ALT) level elevated to 374 U/L and the alanine transaminase (AST) level elevated to 192 U/L. The patient received liver protective therapy and discontinued icotinib treatment. The level of tumor marker carcinoembryonic antigen (CEA) reduced from 402.25 to 184.90 ng/mL. CT in other hospital suggested stable disease (Figure 2). The combination of pemetrexed, cisplatin and recombinant human endostatin was administered as the following treatment.
Table 1. Somatic mutations detected by NGS.
| Gene | c.HGVS | g.HGVS | Exon/intron | Allele frequency (%) |
|---|---|---|---|---|
| TP53 | c.524G>A | p.R175H | EX5 | 33.8 |
| EGFR | c.2573T>G | p.L858R | EX21 | 33.0 |
| EGFR | c.2153T>A | p.L718Q | EX18 | 1.3 |
NGS, next-generation sequencing. c.HGVS, Human Genome Variation Society CDNA reference sequence (represented by prefix “c”); g.HGVS, Human Genome Variation Society Genomic reference sequences (represented by the prefix “g”).
Figure 1.
The integrated genome viewer revealed the mutational status of tumor after osimertinib therapy. (A) EGFR L858R mutation; (B) wild-type EGFR T790; (C) EGFR L718Q mutation.
Figure 2.
CT scan images demonstrated an increase in size of lesions on the inferior lobe of left lung and stabilization of nodules in right lung after 1-month treatment of icotinib. (A) Before treatment initiation; (B) after treatment.
Discussion
The mechanisms underlying resistance to osimertinib have been investigated by several studies (9,10). One study classified the mechanisms into three groups: (I) EGFR tertiary mutations (C797S, L792H, L718Q, etc.); (II) activation of bypass signaling pathways, such as MET amplifications; (III) histologic transformation (e.g., EMT, SCLC, etc.) (10). A clinical report firstly identified EGFR L718Q mutation in a patient with EGFRL858R/T790M-mutant lung adenocarcinoma who progressed on osimertinib. L718 is located within the p-loop and directly interacts with the aniline ring of osimertinib. L718Q mutation was shown to result in steric hindrance and lead to resistance (5). In a large cohort study, EGFR L718Q/V mutations were identified in seven cases (8%), among which five cases do not have co-existing T790M mutation (9). Despite the possible false negative result in T790M detection due to tumor heterogeneity, we report another case that EGFR L718Q mediated osimertinib resistance without T790M mutation.
Currently, there are no actionable treatment strategies to overcome osimertinib resistance caused by EGFRL858R/L718Q co-mutation. Previous cell line study demonstrated that EGFRL858R/L718Q cells remained sensitive to afatinib or the combination of gefitinib and WZ4002 (7). Recently, one in vitro study stated that T790M-negative cells expressing EGFRL858R/L718Q were resistant to gefitinib (9). Taken together, afatinib might be an optional inhibitor to overcome osimertinib resistance caused by EGFR L718Q mutation. Due to issues of drug accessibility at that time, the patient chose to receive icotinib for one month. Icotinib is similar to erlotinib in its structure, mechanisms of action and therapeutic effects (11). Unfortunately, he stopped icotinib treatment due to severe liver injury. For NSCLC patients with osimertinib resistance caused by EGFR tertiary mutations, further investigations are required to direct the treatment strategies.
Conclusions
Here we present the case of a patient with osimertinib resistance caused by EGFRL858R/L718Q co-mutation. The patient had an attempt to receive EGFR-TKI treatment after resistance with stable disease achieved. Treatment regimens after osimertinib resistance still require to be further explored.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 2.Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 3.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 4.Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. 10.1038/nm.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bersanelli M, Minari R, Bordi P, et al. L718Q Mutation as New Mechanism of Acquired Resistance to AZD9291 in EGFR-Mutated NSCLC. J Thorac Oncol 2016;11:e121-3. 10.1016/j.jtho.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 6.Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res 2005;11:5878-85. 10.1158/1078-0432.CCR-04-2618 [DOI] [PubMed] [Google Scholar]
- 7.Ercan D, Choi HG, Yun CH, et al. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 2015;21:3913-23. 10.1158/1078-0432.CCR-14-2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercan D, Xie T, Capelletti M, et al. Abstract 4832: Novel EGFR mutations that cause drug resistance to irreversible pyrimidine but not quinazoline based EGFR inhibitors. Cancer Res 2012;72:4832 10.1158/1538-7445.AM2012-4832 [DOI] [Google Scholar]
- 9.Yang Z, Yang N, Ou Q, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097-107. 10.1158/1078-0432.CCR-17-2310 [DOI] [PubMed] [Google Scholar]
- 10.Le X, Puri S, Negrao MV, et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin Cancer Res 2018;24:6195-203. 10.1158/1078-0432.CCR-18-1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan YS, He Q, Li M. Icotinib: activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother 2014;15:717-28. 10.1517/14656566.2014.890183 [DOI] [PubMed] [Google Scholar]