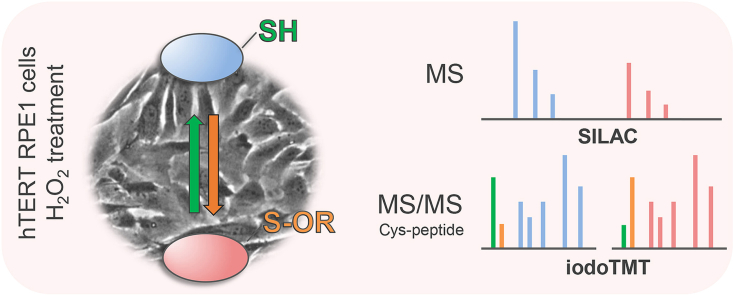
Abstract
Under normal conditions, the cellular redox status is maintained in a steady state by reduction and oxidation processes. These redox alterations in the cell are mainly sensed by protein thiol residues of cysteines thus regulating protein function. The imbalance in redox homeostasis may therefore regulate protein turnover either directly by redox modulating of transcription factors or indirectly by the degradation of damaged proteins due to oxidation. A new analytical method capable of simultaneously assessing cellular protein expression and cysteine oxidation would provide a valuable tool for the field of cysteine-targeted biology. Here, we show a workflow based on protein quantification using metabolic labeling and determination of cysteine oxidation using reporter ion quantification. We applied this approach to determine protein and redox changes in cells after 5-min, 60-min and 32-h exposure to H2O2, respectively. Based on the functional analysis of our data, we confirmed a biological relevance of this approach and its applicability for parallel mapping of cellular proteomes and redoxomes under diverse conditions. In addition, we revealed a specific pattern of redox changes in peroxiredoxins in a short time-interval cell exposure to H2O2. Overall, our present study offers an innovative, versatile experimental approach to the multifaceted assessment of cellular proteome and its redox status, with broad implications for biomedical research towards a better understanding of organismal physiology and diverse disease conditions.
Keywords: SILAC-iodoTMT labeling, Proteome, Redoxome, Cysteine, Liquid chromatography/mass spectrometry, Peroxiredoxin
Graphical abstract
Highlights
-
•
SILAC-iodoTMT is a valuable approach for cysteine-targeted mass spectrometry-based proteomics.
-
•
SILAC-iodoTMT approach enables monitoring protein expression changes and redox shift in parallel.
-
•
Global change in protein expression and redox imbalance were detected after prolonged time period post H2O2exposure.
-
•
Till 60 min after H2O2-treatment, only selective reduction of resolving peroxiredoxin cysteine residues is occurring.
-
•
A portion of these reduced cysteines is probably generated from modified cysteine residues inaccessible for TCEP reduction.
1. Introduction
Excessive levels of reactive oxygen species (ROS), such as high concentrations of H2O2, can attack bases in nucleic acids, amino acid side chains in proteins, and double bonds in unsaturated fatty acids resulting in damage of biomacromolecules [1]. Depending on H2O2 concentration and antioxidant capacity of a given cell type, such damage can trigger oxidative stress-response pathways leading to altered gene expression or increased proteolysis [2], stress-induced premature senescence [3,4], or cell death [5,6]. Therefore, accurate measurement of protein expression in addition to a determination of cysteine oxidation would provide an important layer of information when investigating not only H2O2-induced phenotypes but the response to oxidative stress in general.
Given its specificity to sulfhydryl groups, iodoacetyl tandem mass tag (iodoTMT) was efficiently used for quantification of reversibly modified cysteines [7,8]. However, the cysteine free thiols are very prone to artificial oxidation during sample preparation, which might diminish an accurate comparison between steady state and perturbed redox homeostasis. In this report, we modified iodoTMT method by combining it with Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC) [9] which enabled us to combine control and treated samples prior to iodoTMT labeling. Using a model of H2O2-induced oxidative stress we demonstrated that SILAC-iodoTMT labeling represents a powerful technique for simultaneous assessment of redox and proteome changes related to H2O2-treatment on a global scale. Furthermore, the combination of both SILAC and iodoTMT quantitative data can provide hints for the discrimination of apparent shifts in cysteine redox states caused by changes in peptide/protein abundances.
2. Material and methods
For a detailed description of all methods see the supplementary document.
2.1. Cell cultivation and treatment
SILAC labeling of hTERT RPE-1 cells was done by using l-arginine [13C6, 15N4] and l-lysine [13C6]. Cells were treated with 100 μM H2O2 and harvested after 5 min, 60 min, and 32 h together with the untreated control. The experiment was done in biological duplicate with swapped SILAC groups.
2.2. Sequential iodoTMT labeling and protein digestion
For each time interval, a control (Ctrl) cell lysate labeled in the light/heavy SILAC channel was mixed with an equal amount of H2O2-treated lysate of the corresponding heavy/light SILAC channel. Free –SH groups were labeled by the first iodoTMT label (TMT1). Subsequently, proteins were precipitated using cold (−20 °C) acetone. The pellet was re-dissolved in lysis buffer and incubated with Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) to reduce the reversibly oxidized cysteines [10]. Reduced cysteines were then labeled by second iodoTMT label (TMT2), and the reaction was quenched by 200 mM cysteine. After second acetone precipitation, proteins were digested using rLys-C (Wako) at 37 °C for 3 h followed by sequencing grade trypsin (Promega) digestion at 37 °C overnight at 1 : 50 ratio (enzyme/substrate). Digestion was stopped by the addition of trifluoroacetic acid (TFA), and precipitated SDC was removed by extraction in water-saturated ethyl acetate. Samples were desalted, and liquid chromatography/mass spectrometry (LC-MS) analysis was performed as described in the supplementary document. Survey MS and MS/MS spectra were processed in MaxQuant 1.6.6.1 [11]. For data evaluation, output files from MaxQuant were processed in Perseus 1.6.2.2 and R 3.1.3. The mass spectrometry proteomics data and MaxQuant output text files have been deposited to the ProteomeXchange Consortium via the PRIDE [12] partner repository with the dataset identifier PXD012504.
3. Results and discussion
3.1. Development of SILAC-iodoTMT method
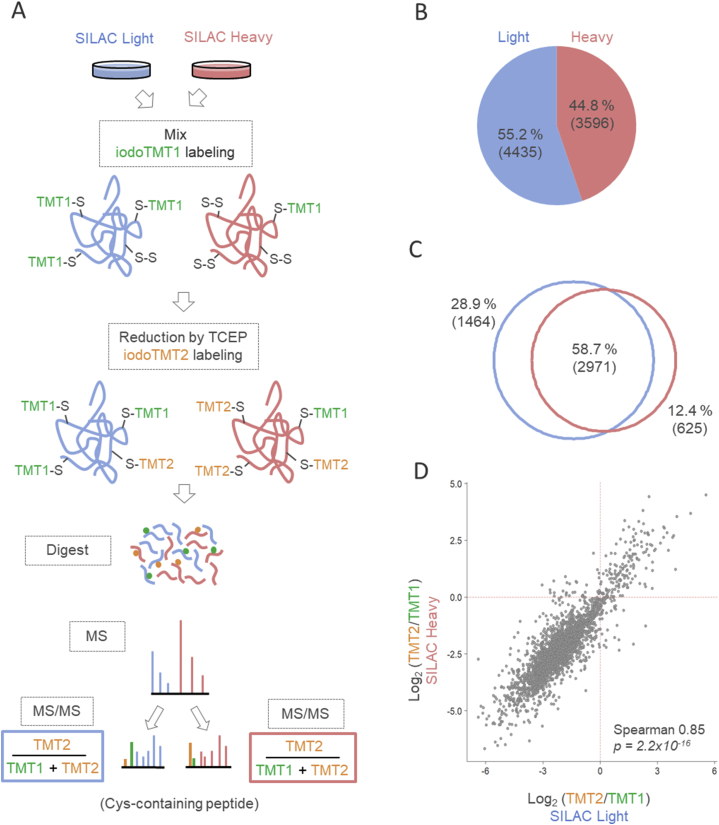
In this work, we introduced SILAC-iodoTMT labeling method to effectively capture the redox and proteome changes without cysteine enrichment, preserving thus information about the whole proteome. The workflow of this approach is depicted in Fig. 1A. The principal advantage of the described approach combining SILAC and iodoTMT labeling is that it enables to simultaneously quantify treatment-induced alterations in protein abundance and cysteine-containing peptide oxidation/reduction. While peptide SILAC precursor ratios correlate with changes in protein expression/degradation, MS/MS fragmentation spectra of cysteine-containing peptides include also signals of iodoTMT reporter ions proportional to the fraction of reduced/oxidized –SH groups. MS parameters fundamental for efficient LC-MS analysis (isolation window and normalized collision energy) were found to be a good compromise in terms of a sufficient abundance of iodoTMT reporters in MS/MS spectra of cysteine-containing peptides and high MS/MS identification rate (Supplementary Fig. S1). An important methodological requirement is that for the comparison of redox state between two experimental groups, both complete SILAC pairs (both light and heavy) of cysteine-containing peptides need to be fragmented and identified (Fig. 1A). Hence, about half of all identified cysteine-containing peptides should belong to heavy SILAC channel. Test LC-MS analysis of hTERT RPE-1 cells showed that heavy SILAC forms made up approximately 45% of the total identification count of cysteine-containing peptides (Fig. 1B). However, both SILAC variants were identified by MS/MS for the majority of iodoTMT-labeled peptides (Fig. 1C). Importantly, iodoTMT ratios obtained from the corresponding light and heavy SILAC peptides correlated well with Spearman correlation coefficient of 0.85 (Fig. 1D), as expected from the unperturbed sample. These results encouraged the application of SILAC-iodoTMT labeling for the monitoring of proteome redox state in hTERT RPE-1 cells under the conditions of oxidative stress.
Fig. 1.
Sequential SILAC-iodoTMT labeling. (A) Scheme of labeling workflow. (B) Proportion of light and heavy SILAC-labeled cysteine-containing peptides identified by MS/MS with 13C615N4-Arg and 13C6-Lys as variable modifications. Data from two technical replicates were combined. (C) Venn diagram showing the proportion of cysteine-containing peptides with light and heavy SILAC counterpart fragmented. Data from two technical replicates were combined. (D) Correlation of iodoTMT log-ratios of the respective light and heavy SILAC cysteine-containing peptides. Data were averaged from two technical replicates.
3.2. Quantification of late global protein and redox changes post H2O2-treatment
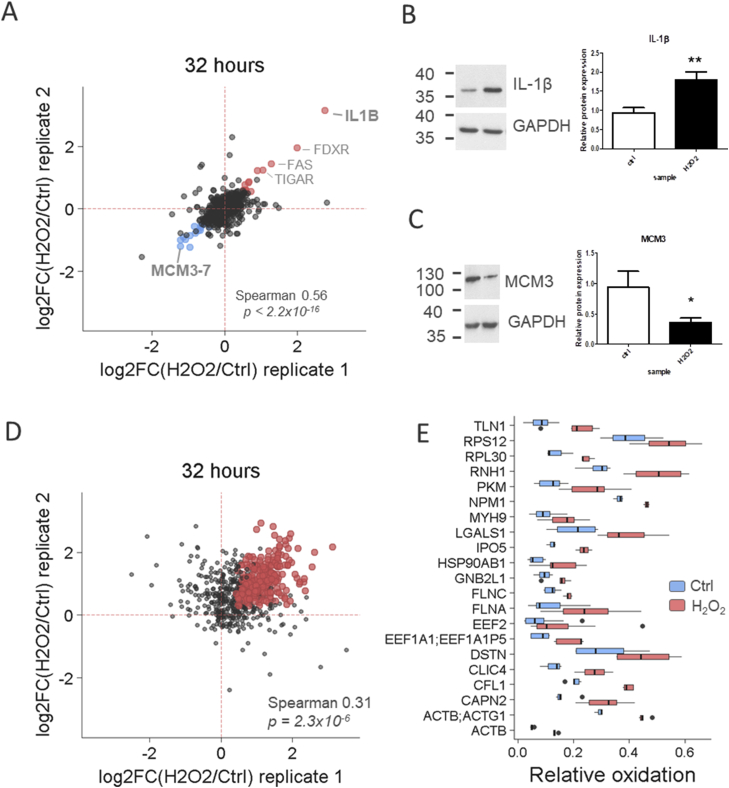
To evaluate the applicability of SILAC-iodoTMT approach for simultaneous redox and proteome measurement, we selected a sublethal H2O2 dose (100 μM) and harvested the treated hTERT RPE-1 cells (Supporting Results and Discussion) in the following time-interval after H2O2 exposure; 5 min, 60 min, and 32 h. We found no changes in protein levels related to H2O2-treatment at early time points (5 and 60 min; Supplementary Fig. S3A). However, the significant protein expression changes and increase of intracellular ROS were detected 32 h post treatment (Fig. 2A, Supplementary Fig. S2A). The most upregulated proteins included a pro-inflammatory cytokine interleukin-1β (IL1B, Fig. 2B), p53-dependent proteins such as ferredoxin reductase (FDXR), TP53-induced glycolysis and apoptosis regulator (TIGAR), and p53-independent apoptosis inductor Fas. On the contrary, members of the DNA replication licensing MCM complex involved in cell cycle progression were the most downregulated (Fig. 2C) [[13], [14], [15], [16], [17]]. Enrichment analysis revealed consistently up- or down-regulated GOBP classes including DNA damage response, DNA replication, or regulation of apoptosis (Supplementary Figs. S3B and C). Altogether, the described protein expression changes in 32-h-sample correlated well with the observed H2O2-induced phenotype (Supplementary Fig. S2B) and confirmed biological relevance of our data on proteome level.
Fig. 2.
Hydrogen peroxide-induced changes in protein expression and cysteine thiol oxidation/reduction in 32 h after H2O2-treatment. (A) Correlation of protein expression between two biological replicates in 32 h time-interval is shown. The most significant protein expression changes (one-sample t-test, Benjamini-Hochberg FDR < 0.05, |log2FC| > 2SD) were marked with blue (downregulated) or red (upregulated) dots. Confirmation of expression changes of IL-1β (B) and MCM3 (C) using immunoblotting. Mean and standard deviation from three independent biological replicates are shown. Protein expression was normalized on corresponding loading control (GAPDH). Statistical significance was calculated by two-sided unpaired t-test, ** p-value < 0.01, * p-value < 0.05. (D) Global increase of cysteine oxidation was detected 32 h after H2O2-treatment. Correlation between two biological replicates in 32-h time-interval is shown. Hydrogen peroxide-sensitive cysteine peptides significantly upregulated in both biological replicates (two-sample t-test, permutation-based FDR < 0.05) were marked with red dots. (E) The most significant examples of hydrogen peroxide-sensitive proteins are shown (paired t-test p-value < 0.02, mean difference > 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Several studies analyzing sensitivity of cellular thiols to hydrogen peroxide in the early post-recovery phase have been already published [[18], [19], [20]]. However, these studies focused on the very early time points such as 5, 10, or 15 min. Due to SILAC-iodoTMT method, we could investigate redox and protein expression imbalances that might potentially develop as a secondary or late/delayed response to oxidative damage in a much longer time frame. As shown in Fig. 2D, a global redox change was detected after 32 h post treatment as demonstrated by a significant shift of median relative oxidation levels in Ctrl and H2O2-treated sample (Supplementary Fig. S3D). The list of the most significant redox-sensitive proteins included e.g. ribosomal proteins, translation elongation factors, glycolytic enzymes, and chaperones (Fig. 2E). Importantly, most of these H2O2-sensitive proteins have been already listed in the redox database RedoxDB [21] and in the previously reported redoxomes [18,22], providing an important confirmation of biological relevance of our data.
3.3. Selective reduction of resolving peroxiredoxin cysteine residues early (5 and 60 min) post H2O2-treatment
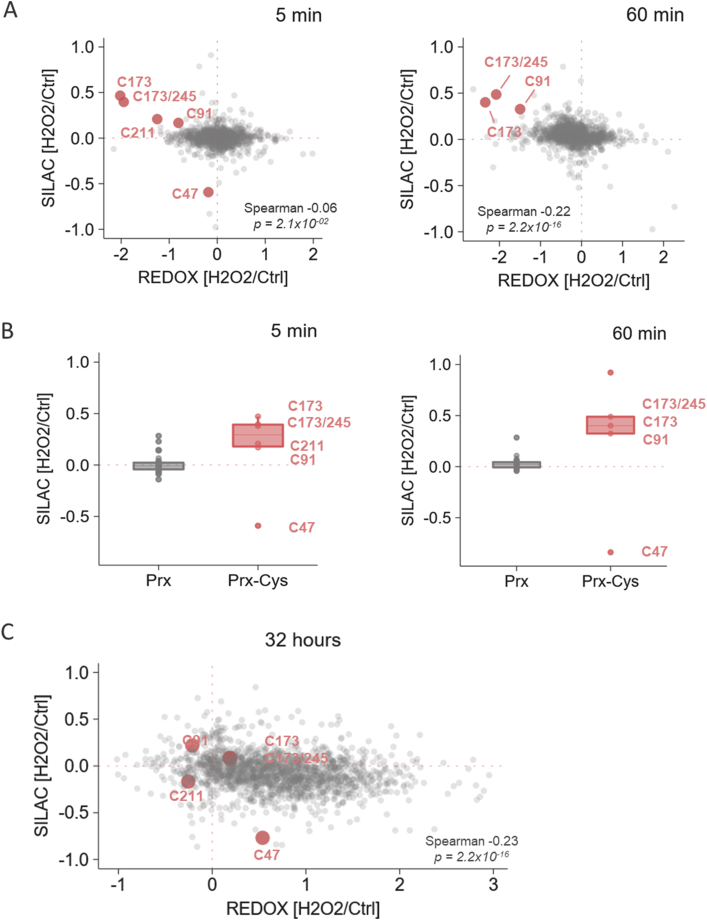
Peroxiredoxins (Prx) are primarily employed as fast H2O2 scavengers [23]. In our results, peroxiredoxins were the only proteins of which resolving (CR) and peroxidatic (CP) cysteine residues were significantly changed in redox state in 5 and 60 min after H2O2-treatment (Table 1, see Supplementary document). Surprisingly, we found these peroxiredoxin cysteine residues apparently shifted to the ‘reduced’ form (Supplementary Fig. S3E). To explain somewhat contradictory findings that these cysteines are reduced by H2O2-treatment, we correlated cysteine redox states with changes in abundance of the respective peptides. Despite unchanged protein expression, SILAC H2O2/Ctrl log-ratios of Prx peptides carrying reduced CR (C173, C173/245, C211) and Cys 91 (C91) were higher than zero (Fig. 3A). It indicated that these peptides were upregulated as soon as 5 min after H2O2-treatment in contrast to other non-cysteinyl peptides from Prx proteins (Fig. 3B) or other proteins (see Supplementary document, Supplementary Fig. S4). We hypothesize that it might point to the ‘mobilization’ of CR cysteines in a fraction of Prx proteins due to stressful condition (Fig. 4A). In absence of H2O2, this ‘shielded’ portion of Prx CR in vivo would not be amenable to TCEP reduction after lysis (e.g. concealed by modification) and therefore inapproachable for iodoTMT labels. In this assumption, H2O2-treatment may stimulate cells to mobilize these CR by transforming them into active reduced forms. Thus, increasing MS signal in H2O2 SILAC channel and boosting the abundance of the reporter ion correspond with the reduced form of these Prx peptides.
Table 1.
Peroxiredoxin cysteine-containing peptides significantly reduced after 5 and/or 60 min of H2O2-treatment (+) in both biological replicates. Each peptide is designated by Uniprot identifiers (protein and gene name) and sequence. Position and type of cysteines (CP/CR) are introduced.
| Protein Name | Gene Name | Sequence | Cys position | Activity | 5 min | 60 min |
|---|---|---|---|---|---|---|
| Q06830 | PRDX1 | HGEVCPAGWKPGSDTIKPDVQK | 173 | CR | + | – |
| Q06830; Q13162 | PRDX1; PRDX4 | HGEVCPAGWK | 173, 245 | CR | + | + |
| P30048-2 | PRDX3 | AFQYVETHGEVCPANWTPDSPTIK | 211 | CR | + | + |
| P30041 | PRDX6 | DINAYNCEEPTEK | 91 | – | + | + |
| P30041 | PRDX6 | DFTPVCTTELGR | 47 | CP | + | + |
Fig. 3.
Cysteine reduction in peroxiredoxin peptides was related to higher intensity of H2O2-treated sample in SILAC channel. (A) Scatter plot of SILAC (y-axis) and ox/red (x-axis) ratio of successfully quantified cysteine-containing peptides in 5 and 60 min after H2O2-exposure. Significantly reduced peroxiredoxin cysteine-containing peptides were marked in red. Data were combined from two biological replicates. (B) H2O2/Ctrl SILAC ratio of peroxiredoxin peptides with the cysteine residue (Prx-Cys) and with the absence of cysteine (Prx) 5 and 60 min post H2O2-treatment. Intensity of cysteinyl Prx peptides was different in H2O2 and Ctrl SILAC channel in contrast to Prx peptides without cysteine, whose SILAC ratio is unchanged in both 5 and 60 min after H2O2-treatment. The peptide with peroxidatic C47 of Prx 6 had apparent decreased intensity in H2O2 SILAC channel compared to resolving cysteine residues listed in Table 1 (C) Scatter plot of SILAC ratio (y-axis) and relative oxidation (x-axis) of successfully quantified cysteine-containing peptides in 32-h sample. Peroxiredoxin cysteine-containing peptides significantly reduced in 5 and 60 min samples are marked in red. Data were combined from two biological replicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
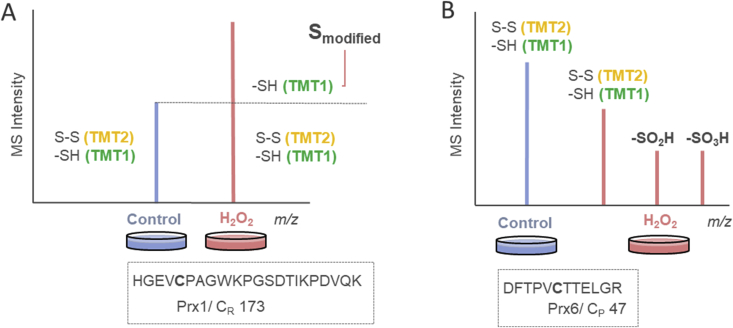
Fig. 4.
SILAC intensity of resolving cysteine-containing peptide “HGEVCPAGWKPGSDTIK-PDVQK” of Prx 1 (A) and peroxidatic cysteine-containing peptide “DFTPVCTTELGR” of Prx 6 (B). An increase of MS1 intensity in H2O2 channel might be caused by a proportion of Cys 173 moieties, which are inaccessible for TCEP reduction and iodoTMT labeling. On the contrary, we identified peroxidatic Cys 47 residue modified with dioxy (-SO2H) and trioxy (-SO3H) sites, which probably caused a reduced intensity of cysteines with free thiols and disulfide bond in H2O2 channel.
We could only speculate, how or if these CR are modified in the absence of a stress-related condition. TCEP is an efficient thiol-free reducing agent strong enough to reduce all reversible cysteine oxidation states [10]. However in Ctrl samples, the unknown modification probably protected CR against TCEP reduction following by cysteine labeling by TMT2 (Fig. 1A). Peroxidatic cysteine residues are prone to over-oxidation to CP-SO2H and CP-SO3H [24], while higher oxidation state of CR has been reported only in few studies [25,26]. Based on these findings, we performed an independent search to quantify higher oxidation states of thiols. By implementation of the –SO2H and –SO3H sites as variable modification in MaxQuant search together with iodoTMT label, we identified only peroxidatic Cys 47-peptide modified by iodoTMT label and in diox- and triox-state as illustrated in Fig. 4B. This peptide was distinctly downregulated up to 32 h post treatment based on SILAC H2O2/Ctrl ratio (Fig. 3A, B, and C), suggesting that H2O2 might decrease irreversibly the yield of CP susceptible to iodoTMT labeling. However, we did reveal neither –SO2H/-SO3H sites, nor other modification except iodoTMT label of CR in Prx. Thus the peroxiredoxin “shield” for resolving cysteines still remains to be identified. Nevertheless, Fig. 3 showed that the presented methodology of SILAC-iodoTMT labeling is able to discriminate functionally different cysteine sites.
4. Conclusions
In this work, we introduced SILAC-iodoTMT workflow as an effective tool for mapping changes of protein level as well as a shift in cysteine oxidation and reduction. Using SILAC-iodoTMT we efficiently quantified global protein and redox changes up to 32 h after H2O2-treatment. The biological relevance of our data proved the applicability of SILAC-iodoTMT to simultaneously quantify changes in protein and redox imbalance. In addition, we revealed a selective reduction of active cysteine residues of peroxiredoxin 1, 3, 4, and 6 relatively early (5 and 60 min) after exposure to H2O2. We presume there is a portion of cysteine resolving residues modified under normal condition and selectively reduced upon the stressful condition such as H2O2 exposure. Altogether, the combination of SILAC and iodoTMT presented in our work enabled us to highlight functional cysteines involved in H2O2 scavenging and quantify their redox dynamics.
Acknowledgements
This work was supported by Grant Agency of the Czech Republic (Project No. 15-03379S), Institutional Grant (Project No. RVO 68378050) and MH CZ - DRO UHHK (Project No. 00179906). We would like to thank Marketa Vancurova for her excellent technical support.
Abbreviations
- SILAC
stable isotope labeling with amino acids in cell culture
- iodoTMT
iodoacetyl tandem mass tag
- FDR
false discovery rate
- LC-MS
liquid chromatography/mass spectrometry
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- SDC
sodium deoxycholate
- ACN
acetonitrile
- TFA
trifluoroacetic acid
- FA
formic acid
- IW
isolation window
- NCE
normalized collision energy
- EDTA
ethylenediaminetetraacetic acid
- TEAB
triethylammonium bicarbonate
- Ctrl
control
- Cys
cysteine
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101227.
Contributor Information
Jiri Bartek, Email: jb@cancer.dk.
Zdenek Hodny, Email: hodny@img.cas.cz.
Declaration of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu J.M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell Mol. Med. 2010;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; J.M. Lu, P.H. Lin, Q. Yao, C. Chen, Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems, J Cell Mol Med 14(4) (2010) 840-860. [DOI] [PMC free article] [PubMed]
- 2.Davies K.J. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83(3–4):301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]; K.J. Davies, Degradation of oxidized proteins by the 20S proteasome, Biochimie 83(3-4) (2001) 301-310. [DOI] [PubMed]
- 3.Toussaint O., Houbion A., Remacle J. Aging as a multi-step process characterized by a lowering of entropy production leading the cell to a sequence of defined stages. II. Testing some predictions on aging human fibroblasts in culture. Mech. Ageing Dev. 1992;65(1):65–83. doi: 10.1016/0047-6374(92)90126-x. [DOI] [PubMed] [Google Scholar]; O. Toussaint, A. Houbion, J. Remacle, Aging as a multi-step process characterized by a lowering of entropy production leading the cell to a sequence of defined stages. II. Testing some predictions on aging human fibroblasts in culture, Mech Ageing Dev 65(1) (1992) 65-83. [DOI] [PubMed]
- 4.Chen Q., Ames B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. Unit. States Am. 1994;91(10):4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]; Q. Chen, B.N. Ames, Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells, Proceedings of the National Academy of Sciences 91(10) (1994) 4130-4134. [DOI] [PMC free article] [PubMed]
- 5.Holze C., Michaudel C., Mackowiak C., Haas D.A., Benda C., Hubel P., Pennemann F.L., Schnepf D., Wettmarshausen J., Braun M., Leung D.W., Amarasinghe G.K., Perocchi F., Staeheli P., Ryffel B., Pichlmair A. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018;19(2):130–140. doi: 10.1038/s41590-017-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; C. Holze, C. Michaudel, C. Mackowiak, D.A. Haas, C. Benda, P. Hubel, F.L. Pennemann, D. Schnepf, J. Wettmarshausen, M. Braun, D.W. Leung, G.K. Amarasinghe, F. Perocchi, P. Staeheli, B. Ryffel, A. Pichlmair, Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway, Nature Immunology 19(2) (2018) 130-140. [DOI] [PMC free article] [PubMed]
- 6.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29(3–4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]; J. Chandra, A. Samali, S. Orrenius, Triggering and modulation of apoptosis by oxidative stress, Free Radic Biol Med 29(3-4) (2000) 323-333. [DOI] [PubMed]
- 7.Qu Z., Meng F., Bomgarden R.D., Viner R.I., Li J., Rogers J.C., Cheng J., Greenlief C.M., Cui J., Lubahn D.B., Sun G.Y., Gu Z. Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. J. Proteome Res. 2014;13(7):3200–3211. doi: 10.1021/pr401179v. [DOI] [PMC free article] [PubMed] [Google Scholar]; Z. Qu, F. Meng, R.D. Bomgarden, R.I. Viner, J. Li, J.C. Rogers, J. Cheng, C.M. Greenlief, J. Cui, D.B. Lubahn, G.Y. Sun, Z. Gu, Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents, J Proteome Res 13(7) (2014) 3200-3211. [DOI] [PMC free article] [PubMed]
- 8.Pan K.T., Chen Y.Y., Pu T.H., Chao Y.S., Yang C.Y., Bomgarden R.D., Rogers J.C., Meng T.C., Khoo K.H. Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia. Antioxidants Redox Signal. 2014;20(9):1365–1381. doi: 10.1089/ars.2013.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]; K.T. Pan, Y.Y. Chen, T.H. Pu, Y.S. Chao, C.Y. Yang, R.D. Bomgarden, J.C. Rogers, T.C. Meng, K.H. Khoo, Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia, Antioxid Redox Signal 20(9) (2014) 1365-1381. [DOI] [PMC free article] [PubMed]
- 9.Ong S.E., Blagoev B., Kratchmarova I., Kristensen D.B., Steen H., Pandey A., Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002;1(5):376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]; S.E. Ong, B. Blagoev, I. Kratchmarova, D.B. Kristensen, H. Steen, A. Pandey, M. Mann, Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics, Mol Cell Proteomics 1(5) (2002) 376-386. [DOI] [PubMed]
- 10.Wojdyla K., Rogowska-Wrzesinska A. Differential alkylation-based redox proteomics – lessons learnt. Redox Biol. 2015;6:240–252. doi: 10.1016/j.redox.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; K. Wojdyla, A. Rogowska-Wrzesinska, Differential alkylation-based redox proteomics - Lessons learnt, Redox biology 6 (2015) 240-252. [DOI] [PMC free article] [PubMed]
- 11.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]; J. Cox, M. Mann, MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification, Nat Biotechnol 26(12) (2008) 1367-1372. [DOI] [PubMed]
- 12.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., Pérez E., Uszkoreit J., Pfeuffer J., Sachsenberg T., Yilmaz S., Tiwary S., Cox J., Audain E., Walzer M., Jarnuczak A.F., Ternent T., Brazma A., Vizcaíno J.A. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47(D1):D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Y. Perez-Riverol, A. Csordas, J. Bai, M. Bernal-Llinares, S. Hewapathirana, D.J. Kundu, A. Inuganti, J. Griss, G. Mayer, M. Eisenacher, E. Perez, J. Uszkoreit, J. Pfeuffer, T. Sachsenberg, S. Yilmaz, S. Tiwary, J. Cox, E. Audain, M. Walzer, A.F. Jarnuczak, T. Ternent, A. Brazma, J.A. Vizcaino, The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47(D1) (2019) D442-D450. [DOI] [PMC free article] [PubMed]
- 13.Hwang P.M., Bunz F., Yu J., Rago C., Chan T.A., Murphy M.P., Kelso G.F., Smith R.A., Kinzler K.W., Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001;7(10):1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]; P.M. Hwang, F. Bunz, J. Yu, C. Rago, T.A. Chan, M.P. Murphy, G.F. Kelso, R.A. Smith, K.W. Kinzler, B. Vogelstein, Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells, Nat Med 7(10) (2001) 1111-1117. [DOI] [PMC free article] [PubMed]
- 14.Liu G., Chen X. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene. 2002;21(47):7195–7204. doi: 10.1038/sj.onc.1205862. [DOI] [PubMed] [Google Scholar]; G. Liu, X. Chen, The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis, Oncogene 21(47) (2002) 7195-7204. [DOI] [PubMed]
- 15.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]; K. Bensaad, A. Tsuruta, M.A. Selak, M.N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb, K.H. Vousden, TIGAR, a p53-inducible regulator of glycolysis and apoptosis, Cell 126(1) (2006) 107-120. [DOI] [PubMed]
- 16.Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]; N. Itoh, S. Yonehara, A. Ishii, M. Yonehara, S. Mizushima, M. Sameshima, A. Hase, Y. Seto, S. Nagata, The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis, Cell 66(2) (1991) 233-243. [DOI] [PubMed]
- 17.Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013;304(8):E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; M.N. Balasubramanian, E.A. Butterworth, M.S. Kilberg, Asparagine synthetase: regulation by cell stress and involvement in tumor biology, Am J Physiol Endocrinol Metab 304(8) (2013) E789-E799. [DOI] [PMC free article] [PubMed]
- 18.Brandes N., Reichmann D., Tienson H., Leichert L.I., Jakob U. Using quantitative redox proteomics to dissect the yeast redoxome. J. Biol. Chem. 2011;286(48):41893–41903. doi: 10.1074/jbc.M111.296236. [DOI] [PMC free article] [PubMed] [Google Scholar]; N. Brandes, D. Reichmann, H. Tienson, L.I. Leichert, U. Jakob, Using quantitative redox proteomics to dissect the yeast redoxome, J Biol Chem 286(48) (2011) 41893-41903. [DOI] [PMC free article] [PubMed]
- 19.Araki K., Kusano H., Sasaki N., Tanaka R., Hatta T., Fukui K., Natsume T. Redox sensitivities of global cellular cysteine residues under reductive and oxidative stress. J. Proteome Res. 2016;15(8):2548–2559. doi: 10.1021/acs.jproteome.6b00087. [DOI] [PubMed] [Google Scholar]; K. Araki, H. Kusano, N. Sasaki, R. Tanaka, T. Hatta, K. Fukui, T. Natsume, Redox Sensitivities of Global Cellular Cysteine Residues under Reductive and Oxidative Stress, J Proteome Res 15(8) (2016) 2548-2559. [DOI] [PubMed]
- 20.Fu L., Liu K.K., Sun M.A., Tian C.P., Sun R., Morales Betanzos C., Tallman K.A., Porter N.A., Yang Y., Guo D.J., Liebler D.C., Yang J. Systematic and quantitative assessment of hydrogen peroxide reactivity with cysteines across human proteomes. Mol. Cell. Proteom. 2017;16(10):1815–1828. doi: 10.1074/mcp.RA117.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]; L. Fu, K.K. Liu, M.A. Sun, C.P. Tian, R. Sun, C. Morales Betanzos, K.A. Tallman, N.A. Porter, Y. Yang, D.J. Guo, D.C. Liebler, J. Yang, Systematic and quantitative assessment of hydrogen peroxide reactivity with cysteines across human proteomes, Mol Cell Proteomics 16(10) (2017) 1815-1828. [DOI] [PMC free article] [PubMed]
- 21.Sun M.A., Wang Y., Cheng H., Zhang Q., Ge W., Guo D. RedoxDB--a curated database for experimentally verified protein oxidative modification. Bioinformatics. 2012;28(19):2551–2552. doi: 10.1093/bioinformatics/bts468. [DOI] [PubMed] [Google Scholar]; M.A. Sun, Y. Wang, H. Cheng, Q. Zhang, W. Ge, D. Guo, RedoxDB--a curated database for experimentally verified protein oxidative modification, Bioinformatics 28(19) (2012) 2551-2552. [DOI] [PubMed]
- 22.Go Y.M., Duong D.M., Peng J., Jones D.P. Protein cysteines map to functional networks according to steady-state level of oxidation. J. Proteom. Bioinform. 2011;4(10):196–209. doi: 10.4172/jpb.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]; Y.M. Go, D.M. Duong, J. Peng, D.P. Jones, Protein Cysteines Map to Functional Networks According to Steady-state Level of Oxidation, Journal of proteomics & bioinformatics 4(10) (2011) 196-209. [DOI] [PMC free article] [PubMed]
- 23.Netto L.E., Antunes F. The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cells. 2016;39(1):65–71. doi: 10.14348/molcells.2016.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]; L.E. Netto, F. Antunes, The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction, Mol Cells 39(1) (2016) 65-71. [DOI] [PMC free article] [PubMed]
- 24.Neumann C.A., Cao J., Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8(24):4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]; C.A. Neumann, J. Cao, Y. Manevich, Peroxiredoxin 1 and its role in cell signaling, Cell Cycle 8(24) (2009) 4072-4078. [DOI] [PMC free article] [PubMed]
- 25.Barranco-Medina S., Lazaro J.J., Dietz K.J. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583(12):1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]; S. Barranco-Medina, J.J. Lazaro, K.J. Dietz, The oligomeric conformation of peroxiredoxins links redox state to function, FEBS Lett 583(12) (2009) 1809-1816. [DOI] [PubMed]
- 26.Wu C., Dai H., Yan L., Liu T., Cui C., Chen T., Li H. Sulfonation of the resolving cysteine in human peroxiredoxin 1: a comprehensive analysis by mass spectrometry. Free Radic. Biol. Med. 2017;108:785–792. doi: 10.1016/j.freeradbiomed.2017.04.341. [DOI] [PMC free article] [PubMed] [Google Scholar]; C. Wu, H. Dai, L. Yan, T. Liu, C. Cui, T. Chen, H. Li, Sulfonation of the resolving cysteine in human peroxiredoxin 1: A comprehensive analysis by mass spectrometry, Free Radic Biol Med 108 (2017) 785-792. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.