Abstract
Intraocular pressure (IOP) is a critical risk factor in glaucoma, and the available evidence derived from experimental studies in primates and rodents strongly indicates that the site of IOP-induced axonal damage in glaucoma is at the optic nerve head (ONH). However, the mechanisms that cause IOP-induced damage at the ONH are far from understood. A possible sequence of events could originate with IOP-induced stress in the ONH connective tissue elements (peripapillary sclera, scleral canal and lamina cribrosa) that leads to an increase in biomechanical strain. In consequence, molecular signaling cascades might be activated that result in extracellular matrix turnover of the peripapillary sclera, changing its biomechanical properties. Peripapillary sclera strain might induce reactive changes in ONH astrocytes and cause astrogliosis. The biological changes that are associated with ONH astrocyte reactivity could lead to withdrawal of trophic or metabolic support for optic nerve axons and cause their degeneration. Alternatively, the expression of neurotoxic molecules might be induced. Unfortunately, direct experimental in vivo evidence for these or other scenarios is currently lacking. The pathogenic processes that cause axonal degeneration at the ONH in glaucoma need to be identified before any regenerative therapy is likely to succeed. Several topics and emerging techniques should be pursued to enhance our understanding of the mechanisms that are behind axonal degeneration. Among them are: Advanced imaging techniques, the development of in vivo markers to identify axonal injury, the generation of molecular approaches for in vivo detection of mechanosensitivity and for molecular manipulation of the ONH, a more complete characterization of retinal ganglion cells, the use of organ cultures, 3D-bioprinting, and the engineering of microdevices that can measure pressure. Questions that need to be answered relate to the specific roles of astrogliosis, neuroinflammation, blood flow and intracranial pressure in axonal degeneration at the ONH.
1. Introduction
Intraocular pressure (IOP) is a critical causative risk factor that leads to damage of retinal ganglion cell (RGC) axons in glaucoma (CollaborativeNormal-TensionGlaucomaStudyGroup, 1998a, b; Kass et al., 2002; Leske et al., 2003; TheAGISInvestigators, 2000). Results from decades-old studies in monkeys, in which IOP was experimentally elevated, indicate that IOP-induced structural and functional alterations of RGC axons occur first within the optic nerve head (ONH) and precede changes in the retina and the RGC somata (Gaasterland et al., 1978; Quigley and Addicks, 1980; Quigley et al., 1981). More recent data from rat (Johnson et al., 2000; Johnson et al., 1996) and mouse (Danias et al., 2003; Howell et al., 2007; Schlamp et al., 2006) models with high IOP and glaucoma confirm the findings seen first in monkeys. Further compelling results come from a recent report using the DBA/2J model of hereditary mouse glaucoma (John et al., 1998): when DBA/2J mice were crossed with mutant mice that were deficient in Bax, a proapoptotic gene, the RGC somata and their proximal axons in the retina survived while RGC axons continued to degenerate at the ONH (Howell et al., 2007). Furthermore, DBA/2J mice were generated that harbored the Wallerian degeneration-Slow (WldS) allele, which is known to protect axons against injury (Lunn et al., 1989; Perry et al., 1991). The WldS mutation in DBA/2J mice had a strong protective effect on the survival of RGC axons (Howell et al., 2007). Overall, there is consensus that the ONH is the most likely site for initial RGC axonal damage in glaucoma, both in rodents and in primates. Nonetheless, the mechanism(s) that cause IOP-induced damage of RGC axons at the ONH are far from understood.
2. Intraocular pressure-induced changes at the optic nerve head
Quite intriguingly, both the mouse (Howell et al., 2007; Sun et al., 2009) and the rat ONH (Dai et al., 2012; Johansson, 1987; Pazos et al., 2015b) show distinct structural differences when compared with ONHs in primates, such as humans and monkeys. Specifically, in the primate eye, RCG axons pass through a meshwork of astrocyte-covered, capillary containing, connective tissue beams known as the Lamina cribrosa (LC) (Anderson, 1969; Burgoyne et al., 2005; Morrison et al., 1989; Quigley et al., 1990). The beams of the LC insert into the surrounding peripapillary sclera. In the rat and mouse eye, the scleral canal is surrounded by peripapillary sclera, but contains no connective tissue beams (Howell et al., 2007; Johansson, 1987; May and Lütjen-Drecoll, 2002; Sun et al., 2009). Just posterior to the peripapillary sclera, astrocytes form an enmeshing network termed the “glial lamina” through which the RGC axons pass (Dai et al., 2012; Howell et al., 2007; Sun et al., 2009). Within the glial lamina, astrocyte processes compartmentalize ganglion cell axons into bundles forming “glial tubes,” thereby giving the glial architecture of the ONH a honeycomb-like appearance in transverse section (Fig. 1), not unlike that seen in species with a connective tissue LC (Dai et al., 2012; Sun et al., 2009). Clearly, the fact that the same causative factor, increased IOP, induces ONH axonal degeneration both in rodents and primates despite their differences in ONH architecture indicates the involvement of common mechanism(s). Moreover, such mechanisms do not necessarily depend on the presence of a LC with connective tissue beams such as in the primate eye.
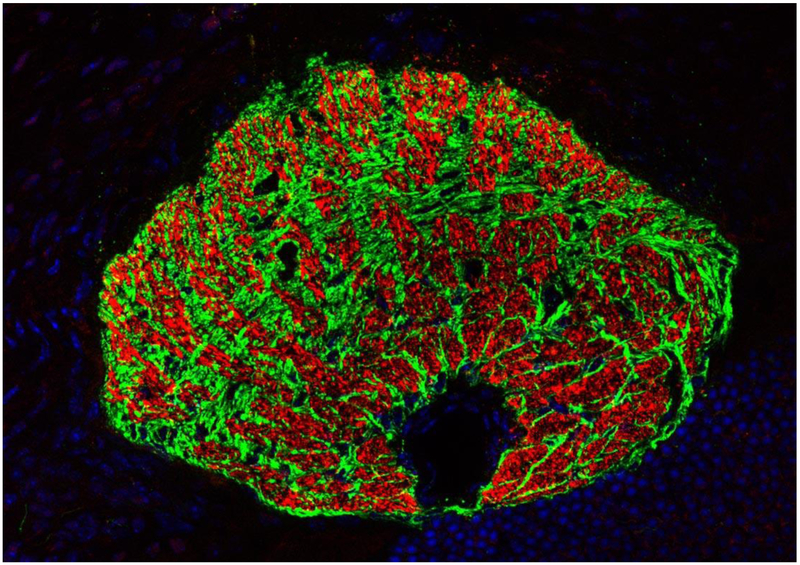
Figure 1. The glial lamina in the mouse eye.
Oblique cross section through the glial lamina shows that astrocytes (labeled in green) compartmentalize ganglion cell axons (labeled in red) into bundles forming glial tubes. The black area in the lower quadrant marks the position of the ophthalmic artery. Immunohistochemical staining for GFAP (green) and for neurofilament (red). Nuclei are labeled with DAPI (blue). Kindly provided by Rudolf Fuchshofer (University of Regensburg, Regensburg, Germany).
A possible candidate for the common mechanism might be an IOP-induced strain in the extracellular matrix components of the peripapillary sclera, a scenario which has been predicted and analyzed in both primates (Downs et al., 2008; Norman et al., 2011; Sigal and Ethier, 2009; Sigal et al., 2005) and mouse models (Cone-Kimball et al., 2013; Nguyen et al., 2013) with high IOP and glaucoma. The peripapillary sclera forms the boundary of the ONH neural canal and transmits biomechanical strain directly to the tissues and cells that connect with it at the scleral canal wall. In primates, this would be the astrocyte-covered connective tissue beams of the LC and in rodents it would be the endfeet of the glial lamina astrocytes. An increase in strain should lead to an enlargement of the canal and the ON within it. Indeed, such an enlargement has been indirectly (Chauhan et al., 2002; Guo et al., 2005) or directly (Pazos et al., 2015a) observed in rat experimental glaucoma. ON enlargement is expected to induce biomechanical stress and strain in the processes of the superficial ON astrocytes whose end feet are embedded in the collagenous extracellular matrix of the pial sheath that covers the ON. Indeed, a separation of those astrocyte end feet from the pial sheath in the superior quadrant of the ONH (just under its dorsal circumferential margin and opposite to the ophthalmic artery) has been reported in rats one week after an acute elevation in IOP (Dai et al., 2012; Li et al., 2015). John Morrison (Oregon Health and Science University, Portland, OR), in as yet unpublished work, reported quite comparable, albeit not identical, findings at the meeting. His laboratory used transmission electron microscopy to study the ONHs of rats with early experimental glaucoma and very mild optic nerve axon loss. A consistent finding within the superior ONH starting at Bruch’s membrane opening was empty spaces between apparent processes of astrocytes and the axons, and in many places the presence of axons with no association with cellular components. The spaces were polygonal, as if the astrocytes’ cellular processes had pulled away from the axons, and quite often a dying axon was observed in the middle of the space. The regional effect was strikingly consistent in that it was almost exclusively within the superior part of the nerve. The mechanisms underlying this superior susceptibility remain to be determined.
The forces that are transmitted to the ONH by the peripapillary sclera strain should depend on both the molecular structure and anatomic features (e.g. thickness) of the peripapillary sclera, which together define its stiffness. Following up on this thought, Harry Quigley’s laboratory has been studying the relationship between peripapillary scleral extracellular matrix stiffness and the susceptibility of RGC axons to experimental glaucoma damage in the mouse eye. To this end, glaucoma was induced by bead injection in the eyes of mice with CD1 or B6 genetic backgrounds. In general, CD1 mice are more susceptible to RGC death than B6 mice in this type of experimental glaucoma (Cone et al., 2010; Cone et al., 2012). While the peripapillary sclera became thinner in both mouse types with glaucoma, the remainder of the sclera uniformly thinned in CD1, but thickened in B6 (Nguyen et al., 2013) mice. Longer eyes, greater scleral strain in some directions at baseline, and generalized scleral thinning after glaucoma were characteristic of CD1 mice (Nguyen et al., 2013). In another set of experiments, the sclera of CD1 mice was cross-linked by subconjunctival injections of glyceraldehyde, a procedure that increased its stiffness (Kimball et al., 2014). When elevated IOP was experimentally induced, glyceraldehyde-treated eyes had greater RGC axon loss from elevated IOP than controls. However, in a pilot study in rats, using a different glaucoma model and scleral stiffening approach, opposite results have been reported (Gonzalez P, et al. IOVS 2015; 56: ARVO E-Abstract 2006).
3. The role of transforming growth factor-β signaling
Transforming growth factor-β (TGF-β) is secreted in noncovalent association with its latency-associated propeptide (LAP). This small latent complex covalently binds to latent transforming growth factor-β binding proteins (LTBPs) that are components of the extracellular matrix (ECM), which store and present TGF-β for subsequent activation (Hinz, 2015; Robertson et al., 2015). A major mechanism to activate latent TGF-β from ECM stores is the contraction of the resident ECM cells that is transmitted by integrins to the surrounding ECM (Buscemi et al., 2011; Klingberg et al., 2014). An increase in ECM strain and stiffening, just as observed in the peripapillary sclera in glaucoma, should further lower the threshold for TGF-β activation (Hinz, 2015). Higher amounts of active TGF-β may further contribute to ECM turnover and increase its stiffness. Supporting a role for TGF-β in the pathogenesis of structural ONH changes in glaucoma are observations of a substantial increase of active TGF-β2 in cadaveric ONH tissues from humans with glaucoma (Pena et al., 1999; Zode et al., 2011). Microarray studies profiling the ONH transcriptome of rat experimental glaucoma did not find evidence for an increase in transcription of TGF-β isoforms or of molecules obviously involved in TGF-β signaling processes (Johnson et al., 2007). Still, a posttranslational mechanism that involves an increase in TGF-β activation may well enhance TGF-β signaling without any requirements for an increase in transcription. Quite intriguingly, TGF-βs are potent signaling molecules that induce an astrogliotic reaction in brain astrocytes and may well do so in the glaucomatous glial lamina (Gris et al., 2007; Schachtrup et al., 2010; Sofroniew, 2009). Such a scenario might imply that active TGF-β induces or amplifies the reactive changes in ONH astrocytes that are triggered by an increase in ECM peripapillary sclera/LC stiffness. Taken together, a possible scenario of events could take its origin from an IOP-induced strain in the peripapillary sclera, which in turn causes the release of active TGF-β from extracellular matrix stores. TGF-β might then chronically act on extracellular matrix turnover in the peripapillary sclera and contribute to its increase in stiffness. Peripapillary sclera stiffening along with activated TGF-β would then induce reactive changes in ONH astrocytes and astrogliosis (Figure 2).
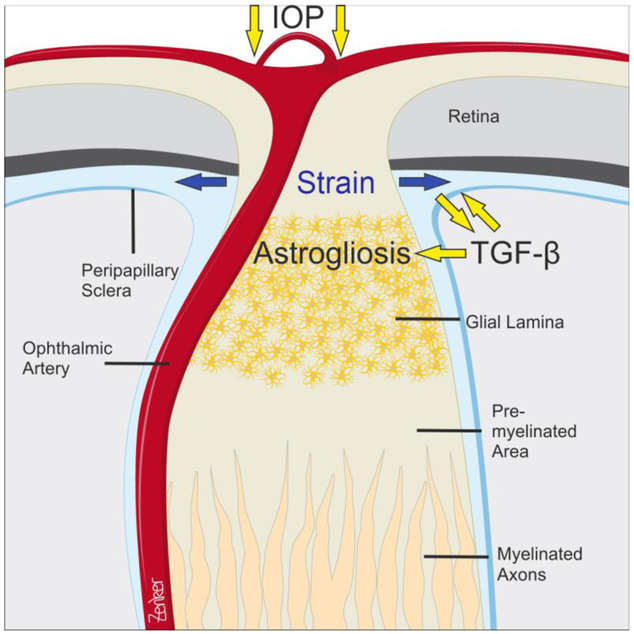
Figure 2. The mouse optic nerve head and its putative changes in glaucoma.
IOP-induced biomechanical strain in the peripapillary sclera is likely to induce the release of active TGF-β from extracellular matrix stores. TGF-β might then chronically act on extracellular matrix turnover in the peripapillary sclera and contribute to its increase in stiffness. Peripapillary sclera stiffening along with mechanical load and activated TGF-β may induces reactive changes in ONH astrocytes and astrogliosis. Drawing by Antje Zenker (University of Regensburg, Regensburg, Germany).
Recent data from Harry Quigley’s laboratory support an involvement of TGF-β signaling in axonal damage in glaucoma (Quigley et al., 2015). When mice with experimentally induced glaucoma or RGC degeneration following optic nerve crush were treated with losartan, RGC loss was significantly reduced in mice with glaucoma, but not in those with crush. Losartan altered the biomechanical response of the glaucomatous sclera by maintaining its creep rate in the normal range and reduced axonal transport blockade, both findings that indicate that glaucomatous damage was prevented through effects on the sclera and/or on astrocytes of the ONH. Losartan, an angiotensin II receptor antagonist used mainly to treat high blood pressure, is known to suppress TGF-β signaling overall (Habashi et al., 2006; Lavoie et al., 2005; Lim et al., 2001). Since losartan is widely prescribed, an obvious but difficult epidemiologic question to pursue is whether patients who are on losartan have a lower prevalence of glaucoma when controlled for age and untreated IOP. A second question is whether glaucoma patients on losartan have a lower rate of progression when controlled for age, treated IOP and the severity of glaucomatous damage.
4. The biological mechanisms that underlie axonal degeneration
The question of why axons degenerate at the ONH is complex and the attribution of a single specific causative insult (e.g. vascular or mechanical) in a given eye is unlikely. Instead, it is reasonable to assume that there are multiple contributing mechanisms, potentially present at all levels of IOP, which contribute to withdrawal of trophic or metabolic (nutrient) support and/or to the generation of molecules that are neurotoxic. Such a process might very well be influenced or driven by reactive changes in ONH astrocytes.
It is important to emphasize that at the present time it is not clear if and/or to what extent the reactive changes of glaucomatous ONH astrocytes contribute to axonal damage. While it has often been assumed that such reactive changes are detrimental for the health of ONH axons, clear evidence for this assumption is missing. Reactive astrogliosis, a process whereby astrocytes undergo varying molecular and morphological changes, is an ubiquitous, but poorly understood hallmark of central nervous system pathologies (Burda and Sofroniew, 2014; Sofroniew, 2009; Sofroniew and Vinters, 2010). Recent studies provide compelling evidence that various signaling mechanisms trigger different molecular, morphological and functional changes in reactive astrocytes in a manner that reflects the typical graduated responses of reactive astrogliosis. More specifically, STAT3 appears to be a critical regulator of certain aspects of reactive astrogliosis. Mice with a conditional deficiency of STAT3 signaling in astrocytes do not develop characteristic signs of astrocyte reactivity. Instead, they show an attenuation of GFAP upregulation, no astrocyte hypertrophy, and a pronounced disruption of astroglial scar formation after spinal cord injury. The lack of astrocyte reactivity in this system is not associated with beneficial effects, but is rather detrimental as it is correlates with an increased spread of inflammation and lesion volume, and partially attenuated motor recovery (Herrmann et al., 2008). Comparable studies would be extremely helpful to understand the role of ONH astrogliosis in ON axonal damage and to define if reactive changes are beneficial or detrimental for the health of optic nerve axons.
One possible scenario of how OHN astrocytes could contribute to axonal damage is a glaucoma-related defect in their syncytial network maintained by gap junctions. The astrocyte syncytium is thought to be very important for neuronal homeostasis as it enables astrocytes to rapidly dissipate small molecules such as potassium and glutamate and to prevent their potentially detrimental accumulation (Seifert et al., 2006). There is published evidence that gap junction intercellular communication in human ONH astrocytes is likely to be compromised when cells are exposed to an increase of hydrostatic pressure in vitro (Malone et al., 2007). However, newer data strongly indicate that the biological changes observed in this model are not caused by hydrostatic pressure, but rather by low gas tension, a parameter of no obvious relevance for glaucoma (Lei et al., 2011). Thus, as of now, it is not clear if astrocyte connectivity within the astrocyte network is changed in glaucoma.
Another open question is whether reactive ONH astrocytes in glaucoma are retracting their processes from optic nerve axons within their bundles and/or whether they withdraw their processes from the adjacent capillaries. In this regard, it is important to mention that it is currently not known if ONH astrocytes extend processes to the laminar beam capillary endothelial cells and/or pericytes. LC capillaries are unique among the capillaries in the central nervous system, inasmuch as they are surrounded by a compact sheath of fibrillar ECM (Anderson, 1969; Kuhnt, 1879). The laminar beam astrocytes may also be unique among astrocytes in the central nervous system, if they do not send processes through the laminar beam ECM to contact the laminar beam capillary, endothelial cell or pericyte.
If laminar beam and axon bundle astrocyte process withdrawal is happening, affected astrocytes may be executing a self-survival program that inadvertently results in inadequate support of axonal energetic, trophic and phagocytic requirements. In addition to determining if astrocytes retract their processes in glaucoma, it would also be important to clarify if they migrate away from the beams and/or if they proliferate. To answer all those questions, cell-specific markers for ONH astrocytes would be very helpful. In addition, the available means of measuring how axon/astrocyte interactions directly affect axonal physiology are currently limited. In a recent report, a quite intriguing example of ONH axon/astrocyte interaction was discovered. Davis and colleagues demonstrated that a surprisingly large proportion of RGC axonal mitochondria are normally degraded by the astrocytes of the ONH, a process that was termed transmitophagy and that might well be altered in glaucoma (Davis et al., 2014).
5. How relevant/feasible is axonal regeneration in glaucoma?
There is no doubt that degenerated ON axons can only be replaced by regenerated new ones. However, in glaucoma, such regeneration has to occur through the profoundly deformed and remodeled LC environment, which may well be “toxic” to axonal regrowth as well as presenting a significant physical barrier to axonal extension. In other words, if factors persist at the LC that originally caused or contributed to axonal degeneration, axonal regeneration is extremely unlikely to succeed. Despite the importance of this problem to axonal regeneration in glaucoma, thoughts about identifying and repairing the underlying causative problem in the LC do not appear to figure prominently in current conversations about axonal recovery in models of glaucoma. The most commonly used model in axonal repair research is optic nerve crush, a severe and acute form of compression that results in profound acute axonal damage. This is very different from the kind of injury that occurs in glaucoma. In clinical experience, damage in glaucoma more closely resembles ON damage following chronic ON compression seen in tumors and trauma where axons are known to recover robustly. However, while there are documented reports of visual field recovery following glaucoma surgery to lower intraocular pressure, this phenomenon is unusual in everyday clinical practice. Without doubt, the mechanisms of axonal damage in glaucoma must be understood before it will be possible to determine how to alter the ONH environment in a way that will allow ON axonal regeneration. Nevertheless, studies of regeneration in the crush model should be encouraged as a means of optimizing regrowth to necessary visual system targets such as the lateral geniculate nucleus. These strategies could then be further developed in unilateral chronic IOP elevation models of experimental glaucoma.
6. Emerging techniques
There are several key developments and/or emerging techniques that could be exploited in more depth to further our understanding of the biological mechanisms behind glaucomatous damage.
Advanced imaging techniques.
Such techniques may increase the ability to study retinal ganglion cell (RGC) axonal damage in animal models of glaucoma and in human patients, and are being used and developed by a number of groups. A promising approach is the use of confocal microscopy to image the retinal nerve fiber layer and to quantify its reflectance in order to obtain information about axonal activity changes in glaucoma. The method has already been used successfully in rat retinal explants (Huang et al., 2016; Huang et al., 2013; Huang et al., 2012). Further, adaptive optics and polarization-sensitive optical coherence tomography have also been used to image vessels or photoreceptors of human retinas in vivo (Felberer et al., 2014; Felberer et al., 2015). Such techniques may be improved to visualize RGC and their axons in the retinae of human patients.
Signal recording.
In the United States, The Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (http://www.braininitiative.nih.gov) is part of a US Presidential focus aimed at revolutionizing our understanding of the human brain. There are several key questions from the BRAIN Initiative with potential overlap with glaucoma: 1) how does one record, over long periods of time, very high density signals from as many cell types in the brain (or eye) as possible; and 2) how does one engineer new microdevices that can measure pressure? Relevant measurement techniques should include non-electrode based approaches. For example, measuring calcium signaling in the retina with in vivo imaging has been made possible through the generation of mutant mice that express fluorescent calcium indicator proteins, such as GCaMP3, in genetically defined neuronal populations (Dana et al., 2014; Zariwala et al., 2012). This method allows imaging of light-evoked responses in retinal ganglion cells (Zariwala et al., 2012). The optical properties of retinal tissues in multiple species and the potential of using adaptive optics to non-invasively image single ganglion cells has been discussed recently (Prasse et al., 2013).
3D Bioprinting.
To study the effects of biomechanics on ONH astrocytes, advances in 3D bioprinting could be harnessed to print a scaffold that mimics the architecture of the LC connective tissue beams. The scaffold could be used to grow cells in more realistic 3D cell culture conditions, thus allowing the imposition of realistic biomechanical insults and other physical cues. In this cell culture-based approach, stimuli could be better controlled, albeit at the cost of less in vivo fidelity. Possible limitations include the ability to construct a high enough resolution scaffold using current printing technology, and determining the correct substrate to use so that beams within the scaffold would have appropriate physiological and biophysical properties. Another limitation is our lack of knowledge on the exact 3D in vivo configuration of the LC. There is the possibility that this problem will be solved by further progress in the technology of optical coherence tomography (OCT) and/or scanning laser ophthalmoscopy (SLO). Still, the challenge of overcoming blood vessel shadowing in these imaging modalities is expected to remain.
7. Questions and topics that should be addressed within the next five years
Several questions and topics should be addressed with high priority in the next five years, as putative answers and developments have the distinct potential to give new and novel insights into the mechanisms that are behind axonal damage in glaucoma.
How relevant is neuroinflammation?
Recent studies show that radiation treatment of DBA/2J mice with hereditary glaucoma inhibits monocyte entry into the ONH and simultaneously prevents IOP-induced neuronal damage (Howell et al., 2012). Also other data from the same group indicate a neuroinflammatory involvement in the pathogenesis of this type of glaucoma (Howell et al., 2014; Howell et al., 2013). However, protective effects of radiation on IOP-induced axonal damage are not observed in rats with experimentally induced unilateral early glaucoma (hypertonic saline injection model) (Johnson et al., 2015). The mechanisms underlying both findings need to be clarified, preferentially by repeating them in other animal models, including models with both inducible and hereditary forms of glaucoma.
RGC characterization.
Although much progress has been made during the past decades, there remains a need for a better understanding of the basic circuitry, synaptic function and anatomical types of human RGCs. A major advance in the field would be for researchers and clinicians to gain the ability to image RGC health/function in real time in situ. Potentially, fluorescent markers could be developed for this purpose, with the critical cell population to image being composed of RGCs on the path to cell death. Such an approach would help to identify thresholds for intervention with a neuroprotective therapy, and provide means of assessing the outcome of that therapy. Limitations exist in developing neuroprotective therapies because the disease process generally occurs over a long period of time; thus, by the time changes become apparent in the RGC population, disease progression may be too far advanced for intervention to be successful. Development of tools for non-invasive, reliable measurement of one (or more) early functional RGC changes would be greatly welcomed. There are some rudimentary functional tests, but these are presently not very reliable. Using high-frequency AO/OCT, Don Miller’s group is imaging the nerve fiber bundles in healthy eyes in ways that may lead to the detection of sick ganglion cell axons without the need for a contrast agent to assess transport (Kocaoglu et al., 2011).
How relevant is intracranial pressure?
Recent evidence indicates a potential role for intracranial pressure in the pathogenesis of at least some forms of glaucoma (Berdahl et al., 2008a; Berdahl et al., 2008b). Better understanding of this important issue requires the development of a non-invasive way to accurately measure intracranial pressure. Current techniques are either highly invasive or do not have the 1-2 mmHg accuracy that will be required for true translational impact.
How relevant is blood flow?
Blood flow remains a poorly understood aspect of glaucoma pathogenesis. There is need for better ways to measure local blood flow, especially within the short posterior ciliary arteries as they pass through the peripapillary sclera, and within the vessels in the laminar beams of the ONH.
Are there in vivo markers to identify axonal injury?
There is a need to develop reliable in vivo markers that can identify axonal injury within the nerve head. A fundamental problem with existing animal models of glaucoma is that the metrics used to assess injury in the ONH do not allow identification of specific areas of damage. The identification of areas with specific abnormalities or functional deficits would be an extremely important breakthrough, since this would allow researchers to focus on the very earliest changes in the most susceptible axons, not on those that occur after several weeks of elevated pressure.
In vivo detection of mechanosensitivity.
While it appears that virtually every cell type is mechanosensitive, very little is known about mechanotransduction and mechanosensitivity in the ONH. The creation of cell type-specific, biological mechanosensors connected to a suitable reporter system would greatly advance this area of research, and in fact would have impact far beyond the field of glaucoma. The mechanosensors could consist of fluorescent indicator proteins expressed in cells that are under high biomechanical strain in genetically modified mice.
To this end, the coding sequences of appropriate reporter proteins such as green fluorescent protein could be placed under control of promoter elements that are recognized by mechanosensitive transcription factors (Wang et al., 2015). This approach would be essentially comparable to that (mentioned above) in mutant mice, which express fluorescent calcium indicator proteins. The expression of such proteins could be targeted to specific cell populations such as astrocytes.
Molecular manipulation of the ONH.
We must understand how to specifically manipulate the biology of the ONH in animal models in vivo. To this end, methods need to be identified that allow direct targeting of ONH cells such as astrocytes or peripapillary fibroblasts in mutant mice. For example, are there ONH cell-specific promoters that drive transgenic expression of Cre-recombinase or other molecules that are used to target gene expression in mutant mice? This could provide an important tool for answering critical questions about ONH biology.
Organ cultures.
The field should seek to develop tissue/organ culture preparations for the ONH, peripapillary retina, and peripheral orbital optic nerve that parallel anterior chamber tissue culture models (Johnson and Tschumper, 1987), which are now widely used to study aqueous humor dynamics and/or trabecular meshwork function in porcine, monkey and human preparations.
Widespread access of adaptive optics (AO)-OCT.
Making AO-OCT more widely accessible for imaging the ONH would allow a more localized detection of change in animal models of glaucoma and in patients. Imaging of the ONH in humans is underway, but very few centers currently have this capability and only a very small number are employing it in animal models.
Databases.
With ongoing improvements in our ability to collect massive amounts of data, it is increasingly important to have a glaucoma-specific database (or multiple databases) that are easily accessible for centralized information storage and retrieval. The National Center for Microscopy and Imaging Research (NCMIR, Director Mark Ellisman) supports the Cell Image Library (http://cellimagelibrary.org/), which is currently the only database that handles massive cell image datasets. This database, or one similar to it, would be a way of sharing “gold standard” archival datasets. In this scenario, groups could store information in the database that would be accessible from a web interface after release, e.g., after publication of the relevant paper(s).
8. Experiments and questions beyond five years
Some of the questions or ideas that were generated will probably take more than five years to address.
RGC labeling in vivo.
While mutant mice are available, in which RGC somata synthetize a fluorescent reporter protein, it would be desirable to have a method for labeling only a small population of RGCs and their axons, in which the entire cell was labelled all the way to its termination in the brain, i.e., a system comparable to the “Brainbow” mouse (Weissman and Pan, 2015). Current work by several laboratories is in this direction (El-Danaf and Huberman, 2015; Qiao and Sanes, 2015). There should in principle be ways to do this in mice using the Thy-1 promoter because it is usually is active only in a few RGCs. There may also be genetic solutions, e.g., to express the coding sequences of a reporter gene under control of a ubiquitous promoter in a transgenic mouse strain. Animals from that strain could then be bred with transgenic mice in which Cre recombinase is under control of a tamoxifen-inducible promoter. A small amount of tamoxifen might be then appropriate to generate the mosaic induction of CRE.
Implantable IOP sensors and IOP control.
The development of implantable IOP sensors for long term monitoring and control of IOP would allow informed correlation studies of IOP, blood flow, ONH glial, scleral fibroblast and RGC axon and RGC somal responses (Downs et al., 2011). We know remarkably little about the actual magnitude and fluctuation of IOP in individual eyes and how it interacts with other components of ONH susceptibility in individual animals and patients. An implantable IOP sensor that would gather data at sufficiently high frequency and sensitivity, and was not subject to significant long-term drift, would allow a better understanding of how IOP affects disease progression. One of the issues with trying to compartmentalize the disease into various susceptibility phenotypes is that, because we cannot effectively control for IOP, which has been shown to be an important risk factor at all levels of IOP, we cannot isolate and study non-IOP risk factors. A powerful approach to telemetric IOP measurement would ideally incorporate both IOP characterization and IOP control. Experiments could then be designed to assess the effects of pressure - sustained, transient, spikes, prolonged, etc. - on RGC axons, ONH glia, scleral fibroblasts and other constituent cells. In parallel experiments, the magnitude and character of IOP insult could be controlled (i.e. kept consistent across all groups) while other determinants of ONH physiology such as blood pressure, CSF pressure and/or the presence and character of inflammatory mediators could then be varied. This point is also discussed in (Stowell et al., In preparation).
The role of ageing.
Age is an important risk factor for both the prevalence and progression of glaucoma at all stages of the neuropathy. The effects of aging on all components of the visual system, including the biomechanical properties of relevant ocular tissues, need to be determined.
Recruitment of scientists.
If the field fails to continuously attract new scientists, the open questions will not be answered, nor will the appropriate techniques be developed. It is important to identify ways to increase overall scientific interest in glaucoma and to ensure that funds for glaucoma research are effectively raised.
Discussion Leaders:
Claude Burgoyne, Devers Eye Institute, Portland, OR, USA
C. Ross Ethier, Georgia Institute of Technology/Emory University, Atlanta, GA, USA
Ernst R. Tamm, University of Regensburg, Regensburg, Germany
Scribe:
Cheri Stowell, Devers Eye Institute, Portland, OR, USA
Discussion Participants:
John E. Dowling, Harvard Medical School, Boston, MA, USA
Crawford Downs, University of Alabama, Birmingham, AL, USA
Mark H. Ellisman, University of California, San Diego, CA, USA
Steven Fisher, University of California, Santa Barbara, CA, USA
Brad Fortune, Devers Eye Institute, Portland, OR, USA
Marcus Fruttiger, University College London, London, UK
Tatjana Jakobs, Harvard Medical School, Boston, MA, USA
Geoffrey Lewis, University of California, Santa Barbara, CA, USA
Claire H. Mitchell, University of Pennsylvania, Philadelphia, PA, USA
John Morrison, Oregon Health and Science University, Portland, OR,
Sansar C. Sharma, New York Medical College, Valhalla, NY, USA
Ian Sigal, University of Pittsburgh, Pittsburgh, PA, USA
Michael Sofroniew, University of California, Los Angeles, CA, USA
Lin Wang, Devers Eye Institute, Portland, OR, USA
Janey Wiggs, Massachusetts Eye and Ear, Boston, MA, USA
Samuel Wu, Baylor College of Medicine, Houston, TX, USA
Richard H. Masland, Harvard Medical School, Boston, MA, USA
References
- Anderson DR, 1969. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch. Ophthalmol. 82, 800–814. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Allingham RR, Johnson DH, 2008a. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115, 763–768. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR, 2008b. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest. Ophthalmol. Vis. Sci. 49, 5412–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV, 2014. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 24, 39–73. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B, 2011. The single-molecule mechanics of the latent TGF-beta1 complex. Curr. Biol. 21, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, Pan J, Archibald ML, LeVatte TL, Kelly ME, Tremblay F, 2002. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest. Ophthalmol. Vis. Sci. 43, 2969–2976. [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group, 1998a. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 126, 487–497. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group, 1998b. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am. J. Ophthalmol. 126, 498–505. [DOI] [PubMed] [Google Scholar]
- Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA, 2010. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp. Eye Res. 91, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA, 2012. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp. Eye Res. 99, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone-Kimball E, Nguyen C, Oglesby EN, Pease ME, Steinhart MR, Quigley HA, 2013. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis. 19, 2023–2039. [PMC free article] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y, 2012. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 60, 13–28. [DOI] [PubMed] [Google Scholar]
- Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, Kim DS, Svoboda K, 2014. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PloS one 9, e108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T, 2003. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest. Ophthalmol. Vis. Sci. 44, 5151–5162. [DOI] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N, 2014. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. U S A 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF, 2008. Mechanical environment of the optic nerve head in glaucoma. Optom. Vis. Sci. 85, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V, 2011. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci 52, 7365–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Huberman AD, 2015. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J. Neurosci. 35, 2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberer F, Kroisamer JS, Baumann B, Zotter S, Schmidt-Erfurth U, Hitzenberger CK, Pircher M, 2014. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed. Opt. Express 5, 439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberer F, Rechenmacher M, Haindl R, Baumann B, Hitzenberger CK, Pircher M, 2015. Imaging of retinal vasculature using adaptive optics SLO/OCT. Biomed. Opt. Express 6, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaasterland D, Tanishima T, Kuwabara T, 1978. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nervehead during development of glaucomatous cupping. Invest. Ophthalmol. Vis. Sci. 17, 838–846. [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A, 2007. Transcriptional regulation of scar gene expression in primary astrocytes. Glia 55, 1145–1155. [DOI] [PubMed] [Google Scholar]
- Guo L, Tsatourian V, Luong V, Podoleanu AG, Jackson DA, Fitzke FW, Cordeiro MF, 2005. En face optical coherence tomography: a new method to analyse structural changes of the optic nerve head in rat glaucoma. Br. J. Ophthalmol. 89, 1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC, 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV, 2008. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 28, 7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, 2015. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 47, 54–65. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW, 2007. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 179, 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, John SW, 2014. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol. Dis. 71, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SW, 2013. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J. Neuroinflammation 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW, 2012. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J. Clin. Invest. 122, 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XR, Knighton RW, Feuer WJ, Qiao J, 2016. Retinal nerve fiber layer reflectometry must consider directional reflectance. Biomed. Opt. Express 7, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XR, Knighton RW, Zhou Y, Zhao XP, 2013. Reflectance speckle of retinal nerve fiber layer reveals axonal activity. Invest. Ophthalmol. Vis. Sci. 54, 2616–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XR, Zhou Y, Knighton RW, Kong W, Feuer WJ, 2012. Wavelength-dependent change of retinal nerve fiber layer reflectance in glaucomatous retinas. Invest. Ophthalmol. Vis. Sci. 53, 5869–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JO, 1987. The lamina cribrosa in the eyes of rats, hamsters, gerbils and guinea pigs. Acta Anat (Basel) 128, 55–62. [DOI] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR, 1998. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 39, 951–962. [PubMed] [Google Scholar]
- Johnson DH, Tschumper RC, 1987. Human trabecular meshwork organ culture: A new method. Invest. Ophthalmol. Vis. Sci. 28, 945. [PubMed] [Google Scholar]
- Johnson EC, Cepurna WO, Choi D, Choe TE, Morrison JC, 2015. Radiation pretreatment does not protect the rat optic nerve from elevated intraocular pressure-induced injury. Invest. Ophthalmol. Vis. Sci. 56, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC, 2000. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 41,431–442. [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC, 2007. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 48, 3161–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Morrison JC, Farrell S, Deppmeier L, Moore CG, McGinty MR, 1996. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp. Eye Res. 62, 663–674. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Gordon MO, 2002. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120, 701–713; discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA, 2014. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 128, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B, 2014. Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J. Cell Biol. 207, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaoglu OP, Cense B, Jonnal RS, Wang Q, Lee S, Gao W, Miller DT, 2011. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Res. 51, 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnt H, 1879. Zur Kenntniss des Sehnerven und der Netzhaut. Graefes Arch. Clin. Exp. Ophthalmol. 25, 179–288. [Google Scholar]
- Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Lariviere R, 2005. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J. Hypertens. 23, 1895–1903. [DOI] [PubMed] [Google Scholar]
- Lei Y, Rajabi S, Pedrigi RM, Overby DR, Read AT, Ethier CR, 2011. In vitro models for glaucoma research: effects of hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 52, 6329–6339. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, 2003. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch. Ophthalmol. 121, 48–56. [DOI] [PubMed] [Google Scholar]
- Li Y, Li D, Ying X, Khaw PT, Raisman G, 2015. An energy theory of glaucoma. Glia 63, 1537–1552. [DOI] [PubMed] [Google Scholar]
- Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ, 2001. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103, 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S, 1989. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur. J. Neurosci. 1,27–33. [DOI] [PubMed] [Google Scholar]
- Malone P, Miao H, Parker A, Juarez S, Hernandez MR, 2007. Pressure induces loss of gap junction communication and redistribution of connexin 43 in astrocytes. Glia 55, 1085–1098. [DOI] [PubMed] [Google Scholar]
- May CA, Lütjen-Drecoll E, 2002. Morphology of the murine optic nerve. Invest. Ophthalmol. Vis. Sci. 43, 2206–2212. [PubMed] [Google Scholar]
- Morrison JC, L'Hernault NL, Jerdan JA, Quigley HA, 1989. Ultrastructural location of extracellular matrix components in the optic nerve head. Arch. Ophthalmol. 107, 123–129. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA, 2013. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest. Ophthalmol. Vis. Sci. 54, 1767–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR, 2011. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp. Eye Res. 93, 4–12. [DOI] [PubMed] [Google Scholar]
- Pazos M, Yang H, Gardiner SK, Cepurna WO, Johnson EC, Morrison JC, Burgoyne CF, 2015a. Expansions of the neurovascular scleral canal and contained optic nerve occur early in the hypertonic saline rat experimental glaucoma model. Exp. Eye Res. 145, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Yang H, Gardiner SK, Cepurna WO, Johnson EC, Morrison JC, Burgoyne CF, 2015b. Rat optic nerve head anatomy within 3D histomorphometric reconstructions of normal control eyes. Exp. Eye Res. 139, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR, 1999. Transforming growth factor beta isoforms in human optic nerve heads. Br. J. Ophthalmol. 83, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER, 1991. Very Slow Retrograde and Wallerian Degeneration in the CNS of C57BL/Ola Mice. Eur. J. Neurosci. 3, 102–105. [DOI] [PubMed] [Google Scholar]
- Prasse M, Rauscher FG, Wiedemann P, Reichenbach A, Francke M, 2013. Optical properties of retinal tissue and the potential of adaptive optics to visualize retinal ganglion cells in vivo. Cell Tissue Res. 353, 269–278. [DOI] [PubMed] [Google Scholar]
- Qiao M, Sanes JR, 2015. Genetic Method for Labeling Electrically Coupled Cells: Application to Retina. Front. Mol. Neurosci. 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, 1980. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest. Ophthalmol. Vis. Sci. 19, 137–152. [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, Maumenee AE, 1981. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 99, 635–649. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Brown AE, Morrison JD, Drance SM, 1990. The size and shape of the optic disc in normal human eyes. Arch. Ophthalmol. 108, 51–57. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Pitha IF, Welsbie DS, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Berlinicke CA, Mitchell KL, Kim J, Jefferys JJ, Kimball EC, 2015. Losartan Treatment Protects Retinal Ganglion Cells and Alters Scleral Remodeling in Experimental Glaucoma. PloS one 10, e0141137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, 2015. Latent TGF-beta-binding proteins. Matrix Biol. 47, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K, 2010. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J. Neurosci. 30, 5843–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW, 2006. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C, 2006. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR, 2009. Biomechanics of the optic nerve head. Exp. Eye Res. 88, 799–807. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Ethier CR, 2005. Factors influencing optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci. 46, 4189–4199. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, 2009. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV, 2010. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell C, Burgoyne C, Tamm ER, Ethier CR, The Lasker/IRFF Initiative on Astrocytes and Glauomatous Neurodegeneration Participants. In preparation. Biomechanical aspects of axonal damage in glaucoma: a brief review. Exp Eye Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Lye-Barthel M, Masland RH, Jakobs TC, 2009. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 516, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER, Dowling JE, 2016. Editorial. Astrocytes and Glaucomatous Neurodegeneration. The Lasker/IRRF Initiative for Innovation in Vision Science. Exp. Eye Res. in press. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators, 2000. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 130, 429–440. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Zhang N, Wang C, Herrler T, Li Q, 2015. An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell. Mol. Life Sci. 72, 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Pan YA, 2015. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics 199, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW, 2012. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ, 2011. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol. Vis. 17, 1745–1758. [PMC free article] [PubMed] [Google Scholar]