Abstract
Background
Studies that evaluate larger numbers of protein biomarkers in patients with coronary microvascular dysfunction (CMD) have not previously been performed, and very little is known concerning the pathogenetic mechanisms leading to CMD.
Our objective was to analyze associations between a broad cardiovascular disease (CVD) protein biomarker assay and CMD, and further explore internal biomarker relations in order to identify possible targets for future treatment interventions.
Methods
In 174 women with angina pectoris and no significant obstructive coronary artery disease (<50% stenosis on invasive coronary angiography), CMD was assessed by transthoracic Doppler echocardiography measuring coronary flow velocity reserve (CFVR). Blood samples were analyzed with a CVD proteomic panel encompassing 92 biomarkers. The relation between biomarkers and CFVR was evaluated by regression analysis, and possible interrelations between significant biomarkers were investigated by principal component analysis (PCA).
Results
Median age (SD) was 64 years (9.8), median CFVR (IQR) was 2.3 (1.9–2.7), and 28% of patients had CFVR < 2.0. Eighteen biomarkers were significantly correlated with CFVR. In PCA, 8 of the biomarkers significantly related to CFVR showed high loadings on principal component 1 (PC1). The component scores of PC1 were significantly related to CFVR (p = 0.002). The majority of the 8 interrelated PC1 biomarkers were related to the pro-inflammatory TNF-α – IL-6 – CRP pathway.
Conclusion
Eighteen protein biomarkers were significantly associated with CMD. Eight biomarkers were interrelated in PCA, and share connection with pro-inflammatory pathways, highlighting a possible important role of inflammation in CMD.
Keywords: Coronary microvascular dysfunction, Biomarkers, Principal component analysis
Abbreviations, alphabetical order
- ACS
acute coronary syndrome
- BMI
body mass index
- CAD
coronary artery disease
- CFVR
coronary flow velocity reserve
- CMD
coronary microvascular dysfunction
- CVD
cardiovascular disease
- LoD
limit of detection
- MI
myocardial infarction
- PC
principal component
- PCA
principal component analysis
- PET
positron emission tomography
Biomarkers
- CCL2
C-C motif ligand 2
- CCL15
C-C motif ligand 15
- CCL16
C-C motif ligand 16
- CHI3L1
Chinitase-3-like protein 1
- CXCL16
C-X-C motif ligand 16
- Gal4
Galectin-4
- GDF15
growth differentiation factor 15
- MMP-3
matrix metalloproteinase 3
- MMP-9
matrix metalloproteinase 9
- NTproBNP
N-terminal prohormone of brain natriuretic peptide
- PGLYRP1
peptidoglycan recognition protein 1
- PON3
paraoxonase 3
- SCGB3A2
secretoglobin family 3a member 2
- suPAR
soluble urokinase-type plasminogen activator receptor
- TNF-α
tumor necrosis factor α
- TNFR1
tumor necrosis factor receptor 1
- tPA
tissue plasminogen activator
- TRAIL
TNF-related apoptosis-inducing ligand
1. Introduction
Coronary microvascular dysfunction (CMD) is the possible cause of symptoms in a substantial proportion of patients with angina and no obstructive coronary artery disease (CAD) [1]. Development of novel simple diagnostic tests and effective treatment in this large patient group is hindered by an incomplete understanding of the pathogenetic process leading to CMD. Compared with macrovascular cardiovascular disease (CVD), protein biomarker studies in CMD have been scarce and a recent CMD White Paper called for more studies of functionally related proteins, which may guide targeted intervention in CMD [2].
We have recently identified 4 protein biomarkers associated with CMD evaluated by rubidium-82 positron emission tomography (PET) in 97 patients [3]. In the present study we analyzed a range of CVD biomarkers with the aim of identifying common internal patterns representing pathophysiologic pathways which may be activated in patients with CMD.
2. Patients and methods
2.1. Enrollment, inclusion, baseline data
Participants with angina-like symptoms and no significant obstructive coronary artery disease (<50% coronary artery stenosis) were enrolled between March 2012 and September 2014, according to the inclusion criteria for the iPOWER study [4] (see Supplemental materials). A subgroup of the iPOWER cohort was randomly selected for protein biomarker evaluation in this study.
2.2. Echocardiographic examination and coronary flow velocity reserve measurements
All patients underwent coronary flow velocity reserve (CFVR) measurement by transthoracic Doppler echocardiography of the left anterior descending artery at rest and during maximum hyperemia induced by dipyridamole infusion (0.84 mg/kg over 6 min) [5]. Left ventricular ejection fraction was analyzed by an experienced echocardiographer using the GE EchoPac v. 112 automated biplane calculation Auto-EF tool. For details regarding echocardiographic examination specifics, please see the Supplemental materials.
2.3. Protein biomarkers
All blood samples were analyzed with the Olink cardiovascular disease panel III (CVD III, Olink Proteomics, Uppsala, Sweden) using Proximity Extension Assay technology, measuring 92 protein biomarkers related to cardiovascular or other diseases by real-time polymerase chain reaction (See Online Fig. 1 for biomarker list. See Supplemental materials for a detailed description of the protein biomarker analysis process) [6].
2.4. Statistical analyses
Baseline patient characteristics were analyzed for possible associations with CFVR both as a continuous variable using linear regression and dichotomized (<2.5 and ≥2.5) using t-tests and χ2-tests for continuous and categorical baseline variables, respectively.
The relation between each of the 92 protein biomarkers and the primary outcome, CFVR, was assessed using linear regression analysis after plotting each biomarker against CFVR for possible non-linear associations. Model assumptions were assessed graphically for all biomarkers and were best satisfied with log-2 transformed biomarker values (covariate) and log-2 transformed CFVR values (outcome) [3]. Family-wise error correction procedures (e.g. Bonferroni) or false discovery rate correction were not possible to utilize due to the large number of protein biomarkers and relatively small patient cohort (n = 174).
In an attempt to explore internal patterns within the group of protein biomarkers significantly associated with CFVR, a scaled principal component analysis (PCA) was performed. Details of the procedure are described in the Supplemental materials. Briefly, PCA is a statistical tool for data exploration and simplification which enables reduction of a larger number of variables into a smaller number of summary components, while still retaining as much of the variance from the original variables as possible [7]. In recent years PCA has been employed in various fields of health research, e.g. for data exploration purposes and development of clinical risk scores [8,9]. The PCA procedure may be subdivided into 3 steps [10,11]:
-
i.
Component extraction and selection: Significant protein biomarker internal correlations were examined by creating a Pearson correlation matrix followed by calculation of Kaiser-Meyer-Olkin measures and Bartlett's test statistic for sphericity. The cut-off eigenvalue level was set conservatively at 1.2 to avoid false discoveries. To simplify component interpretation, no component rotation was employed.
-
ii.
Interpretation of component loading structure: We determined a threshold for component/variable loading >0.25 as meaningful for interpretation i.e. a specific variable was considered to be associated with a specific component if the loading was above this threshold [12].
-
iii.
Computation and utilization of component scores: The loading coefficients were utilized to compute a separate component score for each patient and each extracted component. The component scores were then used to perform both univariate and multivariate adjusted regression analyses for association between components and CFVR, adjusting for the known risk factors for low CFVR which were found to be significantly associated with CFVR in the present study: Age, non-obstructive atherosclerosis in CAG, hypertension, diabetes mellitus, smoking, body mass index (BMI) and total cholesterol [2].
To obtain a preliminary overview of the internal relations between all protein biomarkers, an initial PCA including all 92 biomarkers was also performed; however, due to the high number of protein biomarkers relative to the cohort size the results were uninterpretable and did not fulfill methodological requirements of PCA. Further analyses and post-estimations including all biomarkers were consequently not conducted.
We assessed the predictive capability of the principal components identified by fitting a logistic regression model to estimate the likelihood of CMD defined as a binary classifier, with CFVR<2.0 designating CMD. A basic model including age and all baseline variables significantly related to CFVR was compared to a model adding any significant principle components, and the difference in diagnostic ability was evaluated using comparative receiver operating characteristics. Statin use was additionally included in the logistic regression model to assess potential anti-inflammatory effects related to statins.
Further, for the principal components which were significantly related to CFVR after multivariate adjustment, each individual biomarker was also separately compared to the basic model, along with all biomarkers which were related to CFVR in linear regression with p < 0.01.
All analyses were performed using STATA/IC 13.1 (StataCorp LP, College Station, TX, USA).
2.5. Ethics
The study was performed in accordance with the Helsinki Declaration and was approved by the Danish Regional Committee on Biomedical Research Ethics (H-3-2012-005). All participants gave written informed consent, after receiving oral and written information about the study.
3. Results
3.1. Study population
The study population consisted of 174 women with angina, median age was 64 years (SD 9.8), median CFVR was 2.3 (IQR 1.9–2.7), and 28% of patients had CFVR < 2.0. Patients with low CFVR more often had diffuse atherosclerosis in the baseline coronary angiogram (p < 0.001) and had more hypertension (p = 0.01) than patients with higher CFVR, while no other baseline characteristics were associated with CFVR (p > 0.05) (Online Fig. 2).
3.2. Biomarkers
95% of the measured proteins were detected in all 174 samples (See Supplemental Materials). In univariate regression analysis, 18 of the 92 protein biomarkers were significantly related to CFVR (Table 1).
Table 1.
Protein biomarkers significantly associated with CFVR.
| Biomarker | R | p Value | Biomarker | R | p Value |
|---|---|---|---|---|---|
| CCL15 | −0.16 | 0.034 | NTproBNP | −0.16 | 0.036 |
| CCL16 | −0.15 | 0.046 | PGLYRP1 | −0.20 | 0.009 |
| CHI3L1 | −0.19 | 0.011 | PON3 | 0.16 | 0.037 |
| CHIT1 | −0.18 | 0.020 | SCGB3A | −0.16 | 0.034 |
| CXCL16 | −0.17 | 0.029 | ST2 | −0.16 | 0.038 |
| GDF15 | −0.25 | 0.001 | suPAR | −0.23 | 0.003 |
| Gal4 | −0.17 | 0.026 | TNFR1 | −0.15 | 0.047 |
| MMP3 | −0.15 | 0.045 | TNFRSF10C | −0.19 | 0.012 |
| MMP9 | −0.24 | 0.002 | tPA | −0.16 | 0.036 |
Regression analysis results for the relation between significant biomarkers CFVR value. R = Pearson correlation coefficient.
Biomarker abbreviations alphabetically: CCL15 = C-C motif ligand 15, CCL16 = C-C motif ligand 16, CHI3L1 = Chinitase-3-like protein 1, CHIT1 = chitotriosidase, CXCL16 = C-X-C motif ligand 16, GDF15 = growth differentiation factor 15, Gal4 = Galectin-4, MMP3 = matrix metalloproteinase 3, MMP9 = matrix metalloproteinase 9, NTproBNP = N-terminal prohormone of brain natriuretic peptide, PGLYRP1 = peptidoglycan recognition protein 1, PON3 = paraoxonase 3, SCGB3A = secretoglobin family 3a member 2, TNFR1 = tumor necrosis factor receptor 1.
3.3. PCA
Evaluation of the data structure generally supported the use of PCA: The Pearson correlation matrix showed a large proportion of significant correlations between the selected 18 biomarkers, and roughly half of the correlation coefficients were >0.5 (Online Fig. 3). The Kaiser-Meyer-Olkin measures of sampling adequacy were high (Online Fig. 4), and Bartlett's test for sphericity was highly significant (chi2 < 0.001). In the initial PCA output, 3 components had eigenvalues >1.2, supporting selection of 3 components for further loading structure interpretation (Table 2).
Table 2.
Principal component analysis loading matrix.
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| CCL15 | 0.244 | −0.258 | −0.016 |
| CCL16 | 0.258 | −0.227 | 0.054 |
| CHI3L1 | 0.218 | −0.088 | −0.275 |
| CHIT1 | 0.142 | −0.043 | 0.100 |
| CXCL16 | 0.291 | −0.001 | 0.193 |
| GDF15 | 0.277 | −0.172 | −0.135 |
| Gal4 | 0.228 | −0.257 | −0.050 |
| MMP3 | 0.247 | 0.017 | 0.280 |
| MMP9 | 0.224 | 0.433 | −0.290 |
| NTproBNP | 0.151 | −0.285 | 0.120 |
| PGLYRP1 | 0.253 | 0.314 | −0.288 |
| PON3 | 0.133 | 0.224 | 0.686 |
| SCGB3A | 0.150 | 0.347 | 0.188 |
| ST2 | 0.252 | −0.115 | 0.194 |
| TNFR1 | 0.305 | −0.038 | −0.023 |
| TNFRSF10C | 0.254 | 0.258 | −0.020 |
| suPAR | 0.294 | 0.249 | −0.156 |
| tPA | 0.211 | −0.301 | −0.148 |
| Eigenvalue | 9.18 | 1.47 | 1.21 |
| Total variance, % | 51.04 | 8.18 | 6.73 |
| Accumulative variance, % | 51.04 | 59.22 | 65.95 |
Upper part: Loading scores for the 3 retained principal components. Lower part: Eigenvalues and corresponding variance for the 3 components.
PC = principal component. Biomarker abbreviations as for Table 1. Bold highlights loadings >0.25.
The component loading structure was examined in a loading matrix to assess biomarker loading patterns on the 3 selected components. Differential loading (i.e. one variable loading significantly to more than one component) was minimal (Table 2). The first component (PC1) had loadings >0.25 for 8 biomarkers: C-C motif ligand 16 (CCL16), C-X-C motif ligand 16 (CXCL16), growth differentiation factor 15 (GDF15), peptidoglycan recognition protein 1 (PGLYRP1), ST2, tumor necrosis factor receptor-1 (TNFR1), soluble urokinase-type plasminogen activator receptor (suPAR) and tumor necrosis factor receptor superfamily member 10C (TNFRSF10C); the second component (PC2) for 8 biomarkers: C-C motif ligand 15 (CCL15), galectin-4 (Gal4), MMP-9, N-terminal prohormone of brain natriuretic peptide (NTproBNP), PGLYRP1, secretoglobin family 3a member 2 (SCGB3A2), TNFRSF10C and tissue plasminogen activator (tPA); and the third component (PC3) for 5 biomarkers: Chinitase-3-like protein 1 (CHI3L1), matrix metalloproteinase 3 (MMP-3), MMP-9, PGLYRP1 and paraoxonase 3 (PON3). Total variance explained by the 3 components was 66%.
The aggregate component scores calculated from the component loading coefficients for each patient were significantly associated with CFVR for PC1 (p = 0.002) and PC3 (p = 0.009) in univariate regression analysis, while PC2 showed no association with CFVR (p = 0.91). After multivariate adjustment for CMD risk factors (Online Fig. 5) component 1 was still significantly related to CFVR (p = 0.04), while component 3 was not (p = 0.14).
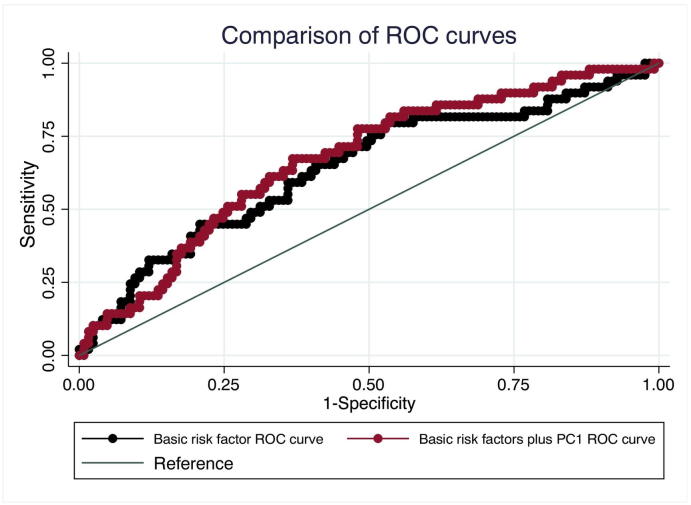
Addition of PC1 scores to a multiple logistic regression model for predicting CFVR < 2.0 did not significantly improve area under the curve (0.67 vs 0.64, p = 0.22) (Fig. 1). Further addition of statin use to the logistic regression model neither increased nor decreased the area under the curve significantly (0.64 vs. 0.67, p = 0.24).
Fig. 1.
Comparison of ROC curves
Addition of PC1 to the ROC plot increased predictive power (red curve), but this effect was not significant (p = 0.22). PC1 = principal component 1.
Individual addition of the PC1 related biomarkers and MMP-9 to the basic model did not significantly improve the area under the curve (Online Fig. 6).
4. Discussion
In this study investigating the relationship between a large number of CVD protein biomarkers and CMD, 18 biomarkers were significantly related to CFVR. We identified two principal components significantly associated with CFVR. PC1 accounted for 51% of biomarker variance and was significantly related to CFVR after multiple adjustment.
4.1. Principal component 1 protein biomarkers and inflammation
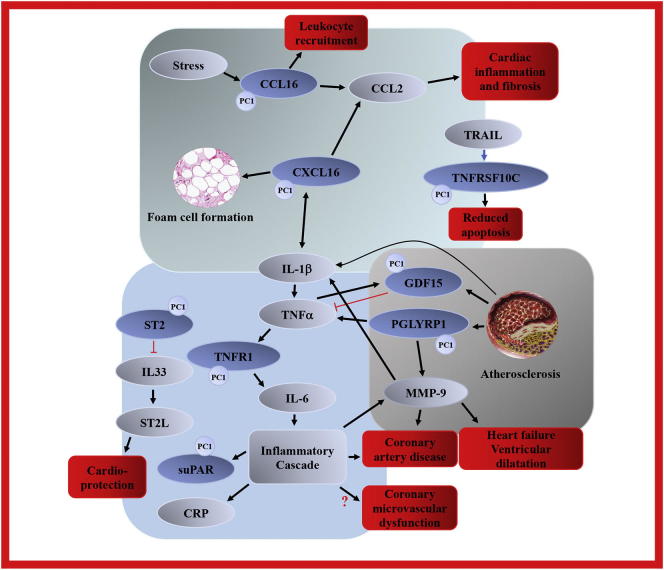
The 6 PC1 biomarkers CCL16, CXCL16, PGLYRP1, TNFR1, GDF15 and TNFRSF10C all have direct or indirect associations with the interleukin IL-1β – TNF-α – IL-6 – CRP inflammatory pathway and the pro-inflammatory hypothesis of atherosclerotic development. This is noteworthy in the light of past findings in CMD research which also highlight this specific pathway [13,14]. In Fig. 2, an overview of functional relationships between PC1 biomarkers and related proteins are outlined graphically.
Fig. 2.
Principal component 1 biomarker overview
Protein biomarker interactions and downstream pathological effects. See “Discussion” for details and references.
Principal component 1 biomarkers in grey, highlighted with “PC1” marker. Other proteins and effectors in dark blue. Downstream effects in red. MMP-9 was not part of PC1, but was independently related to CFVR after multivariate adjustment.
Black arrows indicate a stimulation or upregulation, red truncated connectors indicate inhibition. Red question mark indicates a possible connection.
CCL2 = C-C motif ligand 2, CRP = c-reactive protein, IL-1β = interleukin 1β, IL-6 = interleukin 6, IL-33 = interleukin 33, ST2L = transmembrane ST2, TNFα = tumor necrosis factor α, TRAIL = TNF-related apoptosis-inducing ligand. All other abbreviations as in Table 1.
The chemokine C-C motif ligand 16 (CCL16) is upregulated in stress conditions, and elicits its effects both as a direct chemoattractant for monocytes and lymphocytes, and by activating the more well described chemokine C-C motif ligand 2 (CCL2) which has been implicated in development of cardiac fibrosis, inflammation and hypertension in animal models [15]. The related chemokine CXC ligand 16 (CXCL16) also induces CCL2 release, but more importantly CXCL16 is an important inflammatory mediator in the IL-1β/TNF-α inflammatory pathway in patients with atherosclerotic heart disease [16]. In CAD patients blockage of IL-1β signaling directly inhibited CXCL16 release from peripheral blood monocytes [17]. CXCL16 is expressed in atherosclerotic lesions in both humans and animal models and has also been proposed as a mediator between inflammation and dyslipidemia in the atherosclerotic process due to increased foam cell formation resulting from CXCL16 stimulation [18].
Peptidoglycan is a major constituent of most bacteria's cell wall, and peptidoglycan recognition proteins (PGLYRPs) play an important role both as direct bactericides and as inflammatory mediators. Presence of PGLYRP1 has been demonstrated in human coronary atheroma, stimulating production of the pro-inflammatory cytokines interleukin-1/-6, TNF-α, CCL2 and metalloproteinases such as MMP-9 [19]. Increased levels of PGLYRP1 were associated with both increased coronary artery calcium and plaque burden in the Dallas Heart Study of >3000 community dwelling individuals [20]. In a subsequent follow-up study PGLYRP1 levels robustly predicted a 3-fold increase in atherosclerotic CVD events, and this association was strongest in patients with elevated hs-CRP levels. Accordingly, PGLYRP1 has been proposed as a possible link between subclinical inflammation, immuno-modulation and early development of atherosclerosis.
While TNF-α itself was not featured in the present biomarker panel due to technical restrictions in the applied assay, its main effector receptor TNF receptor 1 (TNFR1) was significantly associated with CFVR and showed high loading on PC1. Activation of TNFR1 produces extensive downstream pro-inflammatory effects e.g. activation of IL-6 cascade and nuclear factor kappa B [21]. Downstream harmful inflammatory effects of TNFR1 stimulation have long been implicated in CAD development through alteration of vascular cell function [22]. TNFR1 activation is also suggested to play a more short-acting role in the event of acute coronary syndrome plaque instability, and elevated levels of TNF-α and TNFR1 have been shown to predict coronary events in 1464 patients with chronic systolic heart failure [23]. A range of pathways associated with TNF-α, TNFR1, IL-6 and IL-1β are being investigated for possible therapeutic inhibition which may improve outcomes in different groups of CVD patients [22,24], e.g. the TNF-α inhibitors adalimumab and infliximab [25]. It has recently been demonstrated in the CANTOS trial that monoclonal antibodies targeting interleukin-1β, an upstream regulator of TNF-α, reduced first occurrence of major cardiovascular events (myocardial infarction (MI), stroke and CV death) compared with placebo in patients with previous MI [26].
TNF receptor superfamily 10C (TNFRSF10C) is also known as decoy receptor 1, and TNF-related apoptosis-inducing ligand (TRAIL) is the primary ligand for TNFRSF10C. The main known effect of TRAIL is to induce apoptosis via binding to death receptors, but binding of TRAIL to TNFRSF10C conversely attenuates the apoptotic actions of TRAIL via sequestering [27]. The exact role of TNFRSF10C is largely unknown, but it may be speculated that a general inflammatory activation in CMD patients also upregulates balancing proteins such as TNFRSF10C, which in turn may moderate the cascade responses of the pro-inflammatory pathways.
Growth differentiation factor 15 (GDF15) is a further inflammatory biomarker which showed loading on PC1. It has been intensively studied in recent years as a strong and robust predictor of all-cause mortality, and adverse cancer and CVD outcomes [28]. GDF15 synthesis is stimulated by TNF-α, but at the same time it may also serve as a paracrine negative feedback inhibitor of TNF-α release which moderates the TNF-α cascade. GDF15 is highly expressed by macrophages in atherosclerotic lesions, and GDF15 has shown promise as a new, robust predictor for adverse CVD outcomes in large observational cohorts and clinical trials such as STABILITY and PLATO [29,30].
Past biomarker studies in patients with CMD have also implicated TNF-α, IL-6 and hs-CRP as possible pathogenetic determinants: A study of 86 obese patients found IL-6 and TNF-α to be significantly associated with CMD (p < 0.03 for both) [14], and in a small population of cardiac syndrome X patients hs-CRP was significantly associated with CMD (p = 0.001) [13].
4.1.1. ST2
ST2 is also known as interleukin-1 receptor-like 1 and exists in a membrane bound receptor form (ST2L) and a soluble form (sST2); the latter was measured in the present study and found to load on PC1. The ligand for both forms is interleukin-33 (IL-33), and in a number of experimental studies myocardial mechanical stress and hypoxia have been shown to upregulate the expression of both IL-33 and ST2L, resulting in cardioprotective effects such as reduction of cardiomyocyte hypertrophy, prevention of hypoxia-related apoptosis and reduced mortality; the soluble form sST2, on the other hand, sequesters IL-33 thereby decreasing activation of membrane bound ST2L and consequently reducing the given cardioprotective effects (Fig. 2) [31]. While IL-33 significantly reduced atherosclerotic plaque size in experimental models of atherosclerosis, addition of sST2 directly counteracted this effect and resulted in increased plaque size [32]. These findings have motivated numerous studies examining the potential of sST2 as a risk marker in patients with heart failure, acute coronary syndrome (ACS) and stable CAD [33,34], persistently demonstrating sST2 as a robust prognostic biomarker of CVD outcomes. Therapeutic trials with sST2-inhibiting antibodies have been shown to reduce graft versus host disease severity and mortality, while sST2-inhibition remains to be investigated in CVD.
4.1.2. Soluble urokinase-type plasminogen activator receptor (suPAR)
SuPAR loaded on PC1, and was also highly significantly related to CFVR as a separate biomarker (p = 0.003). Increased suPAR levels reflect low grade inflammation and have been linked to the early stages of atherosclerotic development and subclinical organ damage [35]. SuPAR has previously been linked to CMD assessed by intracoronary Doppler flow measurement of CMD in 66 patients with non-obstructive coronary atherosclerosis, where a higher level of suPAR remained significantly associated with a lower CFVR after multivariate adjustment (p = 0.04) [36]. SuPAR level was further related to the presence of CAD and CVD outcomes in >3000 patients with suspected CAD, and addition of suPAR improved the C-statistic significantly compared to a model based on traditional risk factors [37].
4.1.3. Common features of PC1 related biomarkers
Most of the 8 protein biomarkers with high loadings on PC1 are related to inflammatory pathways, either as upstream regulators, downstream protein products expressing a pro-inflammatory state, or parallel and moderating messenger pathways (Fig. 2). A majority of the biomarkers have connection with the important IL-1β - TNF-α – IL-6 – CRP inflammatory activation pathway, and inflammatory biomarkers such as GDF15, ST2 and suPAR are increasingly discussed as promising future risk stratification biomarkers in both stable CAD, ACS and heart failure. The concept of prolonged low-grade subclinical inflammation as a pivotal component in the development of CVD is not new [38], but in recent years evidence supporting the inflammatory hypothesis has been gathering [39]. This is further supported by biomarker studies which have helped introduce potential upstream proteins available for targeted treatment interventions. Several studies are evaluating different immunomodulation strategies in CVD [25,26], yet no such interventional trials have been undertaken in the field of CMD. The current study suggests that the well-known association between inflammation and macrovascular CVD may also pertain to presence of CMD, i.e. inflammation may be a common pathogenetic factor in both macro- and microvascular disease.
Addition of PC1 to a standard risk factor based prediction model did not significantly improve the area under the curve. Consequently, the PCA results are not readily translatable to diagnostic implementation, and should be interpreted as hypothesis generating. Lack of enhancement of predictive power after adding a biomarker to standard risk factors has also been described for a majority of CVD biomarkers in past, larger studies. This finding has been attributed to a possible role of biomarkers as mediating or co-incident factors, rather than entities representing independent increases in patient risk [40,41].
4.2. Other protein biomarkers
MMP-9 had a strong association with CFVR (p = 0.002) and is also activated by PGLYRP1 which was part of PC1. Matrix metalloproteinases (MMPs) are important in normal degradation of collagen in the extracellular matrix and migration of neutrophil granulocytes across the basement membrane. Following stress such as MI, MMPs are activated by pro-inflammatory cytokines resulting in increased breakdown of the cardiomyocyte collagen skeleton which otherwise ensures normal structure, thereby contributing to post-ischemic ventricular dilatation [42]. MMP-9 has been studied in relation to development of heart failure, where MMP-9 levels were associated with increased left ventricular diastolic dimensions and wall thickness in 699 patients with previous MI [43]. MMP-9 has also shown potential as a general CAD risk marker, e.g. predicting CV events in >1000 patients with baseline CAD [44]. MMP-9's direct involvement in heart failure remodeling and cytokine activation has given rise to interventional trials with the nonspecific MMP inhibitor doxycycline, effectively blocking MMP-9 activities and attenuating myocardial fibrosis and adverse LV remodeling [45].
In a previous, smaller study by our group investigating possible associations between the CVD III biomarker panel and CMD evaluated with PET, 4 biomarkers were significantly associated with CMD, 3 of which (GDF15, tPA, Gal4) were also significant in the present study [3]. Inter-study differences may conceivably be ascribed to smaller population size, different examination modalities and random variation. Few other studies have investigated biomarkers in small CMD populations: One study found increased activation of circulating progenitor cells related to CMD in 123 women [46], while a study by Bozcali et al. in 115 cardiac syndrome X patients found higher levels of the inflammation and fibrosis biomarker galectin-3 (Gal3) compared with healthy controls [47]. In the present study, there was no significant relation between Gal3 and CFVR.
4.3. Study strengths and limitations
To date only few studies have examined protein biomarkers in CMD, and they have mainly evaluated individual biomarkers in small patient populations. The present study is the first to evaluate a large-scale biomarker panel in relation to CMD. Further, we attempt to uncover new internal patterns of CMD-related biomarkers through the utilization of PCA statistics, which is an apt approach for identification of underlying patterns in large data sets.
Although the present study is fairly large in the context of CMD research, the main limitation is nonetheless the relatively small population sample size in relation to the wide range of biomarkers represented in the used assay. As noted, strict adjustment of p-values for multiple comparisons was not possible due to population size (risk of type II error), and this in turn naturally introduces a risk of type I error in the initial separate regression analyses. Accordingly, our findings should be interpreted as hypothesis generating; however, the use of PCA and subsequent review of available evidence support the notion that a considerable proportion of the identified correlations are not mere chance findings, since 18 out of 92 biomarkers were related to CFVR, and the identified PC1 biomarkers share roles in common inflammatory pathways.
The method used for inducing maximum hyperemia by dipyridamole infusion mainly evaluates non-endothelium dependent vasodilatation. Results are not comparable to coronary vascular function assessed by e.g. acetylcholine provocation.
All studied patients were female, and it is therefore unknown whether the results would be different in a cohort including male angina patients.
4.4. Conclusion
We identified 18 protein biomarkers significantly associated with impaired microvascular dilatation in women with angina but no significant CAD. We further identified 8 biomarkers with common loading on a principal component significantly associated with CMD after multiple adjustment, most of which were related to the TNF-α – IL-6 pro-inflammatory pathway.
Funding
This work was supported by the Danish Heart Foundation (grant 11-10-R87-B-A3628–22687).
Disclosures
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100370.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mygind N.D., Michelsen M.M., Pena A., Frestad D., Dose N., Aziz A. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J. Am. Heart Assoc. 2015;5(3) doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noel Bairey Merz C., Pepine C.J., Walsh M.N., Fleg J.L. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder J., Zethner-Moller R., Bové K.B., Mygind N.D., Hasbak P., Michelsen M.M. Protein biomarkers and coronary microvascular dilatation assessed by rubidium-82 PET in women with angina pectoris and no obstructive coronary artery disease. Atherosclerosis. 2018 Jun 19;275:319–327. doi: 10.1016/j.atherosclerosis.2018.06.864. [DOI] [PubMed] [Google Scholar]
- 4.Prescott E., Abildstrøm S.Z., Aziz A., Merz N.B., Gustafsson I., Halcox J. Improving diagnosis and treatment of women with angina pectoris and microvascular disease: the iPOWER study design and rationale. Am. Heart J. 2014;167(4):452–458. doi: 10.1016/j.ahj.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Sicari R., Nihoyannopoulos P., Evangelista A., Kasprzak J., Lancellotti P., Poldermans D. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur. J. Echocardiogr. 2008 Feb 19;9(4):415–437. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 6.Assarsson E., Lundberg M., Holmquist G., Björkesten J., Thorsen S.B., Ekman D. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman G., Streiner D. 3rd ed. Jama. pmph; 2009. Biostatistics: The Bare Essentials. [Google Scholar]
- 8.Luke J.N., Brown A.D., Brazionis L., O'Dea K., Best J.D., McDermott R.A. Exploring clinical predictors of cardiovascular disease in a central Australian aboriginal cohort. Eur. J. Prev. Cardiol. 2013;20(2):246–253. doi: 10.1177/2047487312437713. [DOI] [PubMed] [Google Scholar]
- 9.Goodman E., Dolan L.M., Morrison J.A., Daniels S.R. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005;111(15):1970–1977. doi: 10.1161/01.CIR.0000161957.34198.2B. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani A. The application of principal component analysis to drug discovery and biomedical data. Drug Discov. Today. 2017;22(7):1069–1076. doi: 10.1016/j.drudis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Nunnally J., Bernstein I. McGraw-Hill; New York: 1994. Psychometric Theory. Vol. 3; p. 701. [Google Scholar]
- 12.Stevens JP. Applied multivariate statistics for the social sciences. Routledge. 2009.663 p.
- 13.Recio-Mayoral A., Rimoldi O.E., Camici P.G., Kaski J.C. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc. Imaging. 2013 Jun;6(6):660–667. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Tona F., Serra R., Di Ascenzo L., Osto E., Scarda A., Fabris R. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr. Metab. Cardiovasc. Dis. 2014 Apr;24(4):447–453. doi: 10.1016/j.numecd.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Shen J.Z., Morgan J., Tesch G.H., Fuller P.J., Young M.J. CCL2-dependent macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in male mice. Endocrinology. 2014;155(3):1057–1066. doi: 10.1210/en.2013-1772. [DOI] [PubMed] [Google Scholar]
- 16.Xing J., Liu Y., Chen T. Correlations of chemokine CXCL16 and TNF-α with coronary atherosclerotic heart disease. Exp. Ther. Med. 2018;15(1):773–776. doi: 10.3892/etm.2017.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C., Halvorsen B., Otterdal K., Wæhre T., Yndestad A., Fevang B. High levels and inflammatory effects of soluble CXC ligand 16 (CXCL16) in coronary artery disease: Down-regulatory effects of statins. Cardiovasc. Res. 2008;79(1):195–203. doi: 10.1093/cvr/cvn071. [DOI] [PubMed] [Google Scholar]
- 18.Wuttge D.M., Zhou X., Sheikine Y., Wågsäter D., Stemme V., Hedin U. CXCL16/SR-PSOX is an interferon-γ-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2004;24(4):750–755. doi: 10.1161/01.ATV.0000124102.11472.36. [DOI] [PubMed] [Google Scholar]
- 19.Wang J.E., JØrgensen P.F., Almlöf M., Thiemermann C., Foster S.J., Aasen A.O. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect. Immun. 2000;68(7):3965–3970. doi: 10.1128/iai.68.7.3965-3970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohatgi A., Ayers C.R., Khera A., McGuire D.K., Das S.R., Matulevicius S. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: observations from the Dallas Heart Study. Atherosclerosis. 2009;203(2):569–575. doi: 10.1016/j.atherosclerosis.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Cabal-Hierro L., Lazo P.S. Signal transduction by tumor necrosis factor receptors. Cell. Signal. 2012;24(6):1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Kleinbongard P., Heusch G., Schulz R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010;127(3):295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Nymo S.H., Hulthe J., Ueland T., McMurray J., Wikstrand J., Askevold E.T. Inflammatory cytokines in chronic heart failure: Interleukin-8 is associated with adverse outcome. Results from CORONA. Eur. J. Heart Fail. 2014;16(1):68–75. doi: 10.1093/eurjhf/hft125. [DOI] [PubMed] [Google Scholar]
- 24.Ridker P.M. From C-reactive protein to Interleukin-6 to Interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ. Res. 2016;118(1):145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker P.M., Lüscher T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014;35(27):1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017; NEJMoa1707914. [DOI] [PubMed]
- 27.Bisgin A., Terzioglu E., Aydin C., Yoldas B., Yazisiz V., Balci N. TRAIL death receptor-4, decoy receptor-1 and decoy receptor-2 expression on CD8+ T cells correlate with the disease severity in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2010;11 doi: 10.1186/1471-2474-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollert K.C., Kempf T., Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 2017;63(1):140–151. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 29.Hagström E., Held C., Stewart R.A.H., Aylward P.E., Budaj A., Cannon C.P. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin. Chem. 2017;63(1):325–333. doi: 10.1373/clinchem.2016.260570. [DOI] [PubMed] [Google Scholar]
- 30.Hagström E., James S.K., Bertilsson M., Becker R.C., Himmelmann A., Husted S. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur. Heart J. 2016;37(16):1325–1333. doi: 10.1093/eurheartj/ehv491. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg E.O., Shimpo M., De Keulenaer G.W., MacGillivray C., ichi Tominaga S., Solomon S.D. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Hear Fail. 2009; nov (6): 684–91. [DOI] [PubMed]
- 33.Dieplinger B., Egger M., Haltmayer M., Kleber M.E., Scharnagl H., Silbernagel G. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the ludwigshafen risk and cardiovascular health study. Clin. Chem. 2014;60(3):530–540. doi: 10.1373/clinchem.2013.209858. [DOI] [PubMed] [Google Scholar]
- 34.Richards A.M., Di Somma S., Mueller T. ST2 in stable and unstable ischemic heart diseases. Am. J. Cardiol. 2015;115(7):48B–58B. doi: 10.1016/j.amjcard.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Lyngbæk S., Sehestedt T., Marott J.L., Hansen T.W., Olsen M.H., Andersen O. CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int. J. Cardiol. 2013;167(3):781–785. doi: 10.1016/j.ijcard.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Mekonnen G., Corban M.T., Hung O.Y., Eshtehardi P., Eapen D.J., Al-Kassem H. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis. 2015 Mar;239(1):55–60. doi: 10.1016/j.atherosclerosis.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Eapen D.J., Manocha P., Ghasemzadeh N., Patel R.S., Al Kassem H., Hammadah M. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J. Am. Heart Assoc. 2014;3(5) doi: 10.1161/JAHA.114.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 39.Geovanini G.R., Libby P. Atherosclerosis and inflammation: overview and updates. Clin. Sci. 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 40.Yeboah J., Mcclelland R.L., Polonsky T.S., Burke G.L., Sibley C.T., Leary D.O. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate risk individuals. The multi-ethnic study of atherosclerosis. JAMA August. 2012;229624(3088):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macnamara J., Eapen D.J., Quyyumi A., Sperling L. Novel biomarkers for cardiovascular risk assessment: current status and future directions. Futur. Cardiol. 2015;11(5):597–613. doi: 10.2217/fca.15.39. [DOI] [PubMed] [Google Scholar]
- 42.Braunwal E. Biomarkers in heart failure management. Curr. Opin. Cardiol. 2008;23(2):127–133. doi: 10.1097/HCO.0b013e3282f43039. [DOI] [PubMed] [Google Scholar]
- 43.Sundström J., Evans J.C., Benjamin E.J., Levy D., Larson M.G., Sawyer D.B. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham heart study. Circulation. 2004;109(23):2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 44.Blankenberg S., Rupprecht H.J., Poirier O., Bickel C., Smieja M., Hafner G. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 45.Cerisano G., Buonamici P., Valenti R., Sciagrà R., Raspanti S., Santini A. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur. Heart J. 2014;35(3):184–191. doi: 10.1093/eurheartj/eht420. [DOI] [PubMed] [Google Scholar]
- 46.Mekonnen G., Hayek S.S., Mehta P.K., Li Q., Mahar E., Mou L. Circulating progenitor cells and coronary microvascular dysfunction: results from the NHLBI-sponsored Women's ischemia syndrome evaluation–coronary vascular dysfunction study (WISE-CVD) Atherosclerosis. 2016 Oct;253:111–117. doi: 10.1016/j.atherosclerosis.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozcali E., Polat V., Aciksari G., Opan S., Bayrak I.H., Paker N. Serum concentrations of galectin-3 in patients with cardiac syndrome X. Atherosclerosis. 2014 Nov;237(1):259–263. doi: 10.1016/j.atherosclerosis.2014.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material