Abstract
Background
Data on participant recruitment into diabetes prevention trials are limited in low- and middle-income countries (LMICs). We aimed to provide a detailed analysis of participant recruitment into a community-based diabetes prevention trial in India.
Methods
The Kerala Diabetes Prevention Program was conducted in 60 polling areas (electoral divisions) of the Neyyatinkara taluk (subdistrict) in Trivandrum district, Kerala state. Individuals (age 30–60 years) were screened with the Indian Diabetes Risk Score (IDRS) at their homes followed by an oral glucose tolerance test (OGTT) at community-based clinics. Individuals at high-risk of developing diabetes (IDRS score ≥60 and without diabetes on the OGTT) were recruited.
Results
A total of 1007 participants (47.2% women) were recruited over nine months. Pilot testing, personal contact and telephone reminders from community volunteers, and gender matching of staff were effective recruitment strategies. The major recruitment challenges were: (1) during home visits, one-third of potential participants could not be contacted, as they were away for work; and (2) men participated less frequently in the OGTT screening than women (75.2% vs. 84.2%). For non-participation, lack of time (42.0%) was most commonly cited followed by ‘I am already feeling healthy’ (30.0%), personal reasons (24.0%) and ‘no benefit to me or my family’ (4.0%). An average of 17 h were spent to recruit one participant with a cost of US$23. The initial stage of screening and recruitment demanded higher time and costs.
Conclusions
This study provides valuable information for future researchers planning to implement community-based diabetes prevention trials in India or other LMICs.
Trial registration
Australia and New Zealand Clinical Trials Registry: ACTRN12611000262909.
Keywords: Diabetes, Recruitment, Challenges, Costs, Staff time, India
1. Introduction
Type 2 diabetes is a major public health issue worldwide, affecting an estimated 425 million people, and of which more than 75% live in low- and middle-income countries (LMICs) like India [1]. Furthermore, diabetes impacts negatively on the health and wellbeing of individuals and their families, and also has broader social and economic effects on the wider community as well as health care systems [1]. Therefore, prevention of type 2 diabetes has become extremely important.
A number of randomized controlled trials (RCTs) have been conducted to examine the effects of lifestyle interventions (i.e. promoting physical activity, healthy dietary habits and weight loss) or medications to prevent type 2 diabetes among high-risk individuals [2]. While the results from these trials are encouraging with both lifestyle interventions and medications being effective and cost-effective [2,3], little is known about the recruitment challenges and the modes of participant recruitment into these trials. Recent systematic reviews have emphasized the need for better reporting of participant recruitment in diabetes prevention trials to improve the translation of findings into policy and practice, thereby enhancing the public health impact of those findings [4,5]. Furthermore, reporting on participant recruitment will enable future researchers to devise strategies to achieve better recruitment outcomes [6].
The Kerala Diabetes Prevention Program (K-DPP) was a cluster-RCT of a peer-support lifestyle intervention program conducted among individuals at high-risk of developing diabetes in India. High-risk individuals were identified on the basis of a diabetes risk score who either had normal glucose tolerance or prediabetes. The details of the study design, development of the intervention program, baseline characteristics, and trial outcome and implementation results have been previously reported [[7], [8], [9], [10], [11]]. In the present study, we aimed to provide a detailed analysis of participant recruitment into K-DPP in terms of: (1) effective strategies for participant recruitment; (2) challenges; (3) costs and staff time; and (4) reasons for non-participation in the trial.
2. Methods
2.1. Study design
The details of the K-DPP study design have been published previously [7]. Briefly, K-DPP was a two-year cluster-RCT implemented in 60 polling areas (electoral divisions) of the Neyyattinkara taluk (sub-district) in Trivandrum district of Kerala state in India. The 60 polling areas were randomly allocated (1:1) to an intervention group (received a group-based peer-support lifestyle intervention program for 12 months) or a control group (received a booklet on lifestyle modification) by an independent statistician using a computer-generated randomization sequence.
2.2. Screening and recruitment of participants
A total of 80 individuals (50 males and 30 females), aged 30–60 years, were selected randomly from the electoral roll of each of the 60 polling areas. Details on how we arrived at the number of 80 individuals per polling area are given in the published study protocol [7]. These 80 individuals were then approached at their homes by trained field staff (“home visits”). Individuals who were not willing to participate in the trial were asked about the reason(s) for their decision using a questionnaire. Consenting individuals were screened with the following eligibility criteria: no history of diabetes or other major chronic illness, not pregnant, literate in the local language and not taking drugs known to affect glucose tolerance. We used a two-step screening procedure to recruit participants, which has been detailed elsewhere [12]. Briefly, the first screening involved administration of the Indian Diabetes Risk Score (IDRS). The IDRS is a simple, non-invasive screening tool consisting of four parameters namely, age, family history of diabetes, waist circumference and physical activity [13]. The IDRS has been validated widely in India for detecting people with undiagnosed diabetes [14], including in our study area, where a score of ≥60 had a sensitivity of 85.7%, specificity of 59.4% and accuracy of 80% [15]. The second screening step consisted of an oral glucose tolerance test (OGTT) among those with an IDRS score ≥60 at community-based clinics. Clinics were organized during weekends in the local community itself using community buildings e.g., schools, community halls and library halls. Individuals diagnosed with diabetes based on the American Diabetes Association (ADA) criteria [16] were excluded from the study, and were referred to healthcare facilities for treatment and care. The remaining individuals were recruited to the trial.
2.3. Strategies employed for recruiting participants
The strategies employed for recruiting participants for the trial are given in Table 1.
Table 1.
Strategies employed for recruiting participants.
| Recruitment strategy | Description |
|---|---|
| Pilot study | We pilot tested the recruitment protocol in two polling areas in 2012–13 [7]. During the pilot, study packs (invitation letter from the Indian principal investigator, consent form, study information sheet and the schedule of upcoming home visit) were mailed to 160 potential participants (@80 per polling area), at least a week in advance of the scheduled home visit. Due to incorrect home addresses, nearly half of the posted packs were returned or did not reach the intended individuals. Therefore, to locate the households of potential participants, the study team decided to engage trained volunteers from the local community. Additionally, the pilot study showed that in order to achieve a gender balance in the study sample, it is essential to screen more men than women for the following reasons. Compared to women, men were less likely to have IDRS score ≥60 and to attend for the OGTT screening, and more likely to have diabetes on the OGTT. Thus, of the 80 individuals who were selected randomly from each polling area, 50 were men and 30 were women. |
| Engaging community leaders and local resource persons | We approached the elected representatives (referred as community leaders hereafter) of the 60 polling areas, and requested they identify a community volunteer (referred as local resource persons (LRPs) hereafter) for their polling areas. LRPs were female community health workers, educated up to secondary school or higher, and they have a good rapport with the people in the local community. LRPs were formally recruited to the K-DPP recruitment team. During home visits, LRPs accompanied the field staff and helped them in locating the correct home address of potential participants, gave participants a telephone reminder on the day before their scheduled clinic date and assisted in organizing clinics. |
| Gender matching of staff | Experience from our previous studies in the study region [[17], [18], [19]] have shown that men are generally less likely to be available at home in the morning hours, owing to work commitments. Therefore, we gender matched our recruitment staff i.e. male staff to contact male participants and female staff to contact female participants, so that male staff could contact male participants during late evening hours after they had return from work. |
| Follow-up clinics | Participants who were not able to attend clinics in the first instance were invited to attend follow-up clinics. Each follow-up clinic was conducted for participants from two to three neighborhoods within close proximity. |
2.4. Recruitment team
The recruitment team consisted of 76 members including one project manager, one project assistant, one field assistant, 10 field staff (5 males and 5 females), 3 phlebotomists and 60 LRPs. The recruitment team met on a regular basis to discuss the challenges and difficulties faced, and how to tackle the issues raised. In addition, they tracked the recruitment progress, and prepared recruitment reports to share with the study investigators. The project manager was available by phone to clarify any doubts or issues the field staff might be facing at the time of recruitment.
2.5. Data analysis
Data were summarized using frequencies and percentages. The recruitment costs were calculated across the following categories: home visits, community-based clinics, training sessions for the field staff and LRPs and administrative costs. Personnel costs were based on the actual salary (or remuneration) paid to the recruitment staff, and were estimated for the time they spent on various recruitment activities. Non-personnel costs (IDRS printing charges, OGTT costs, training sessions for the field staff and LRPs, travel costs, rent for clinic venues, communication costs and administrative costs) were estimated based on the actual expenditure for those items. The cost figures were obtained from the finance registers. The cost estimates in Indian Rupees (INR) were converted to US$ using an exchange rate of INR 58.6 = 1US$ for the year 2013 [20]. All analyses were performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, Washington, USA).
2.6. Ethics approval
K-DPP was approved by the Health Ministry Screening Committee of the Government of India, and ethics committees of the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCT/IEC-333/May 2011), Trivandrum, India, and Monash University (CF11/0457–2011000194) and The University of Melbourne (1441736) in Australia. Written informed consent was obtained from all study participants.
3. Results
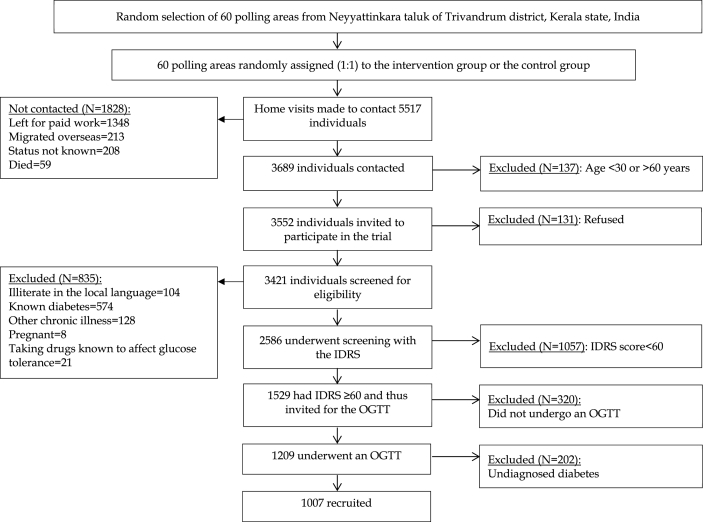
The screening and recruitment took place over a period of nine months from January 26, 2013 to October 27, 2013. Fig. 1 shows the screening and recruitment flow chart. Home visits were made to a total of 5517 individuals, of which 3689 (66.9%) were contacted. The reasons for not being able to contact 1828 individuals were: left for paid work at the time of home visit (73.7%), migrated overseas (11.6%), status of the individual not known (11.4%) and died (3.2%). Of those contacted, 137 (3.7%) were aged <30 or >60 years, and were therefore excluded. The remaining 3552 individuals (age 30–60 years) were invited to participate, of which 3421 (96.3%) consented.
Fig. 1.
K-DPP screening and recruitment flow chart.
Of 3421 consenting individuals, 835 (24.4%) did not satisfy the eligibility criteria, and the remaining 2586 were screened with the IDRS. Of these, 1529 (59.1%) had a score of ≥60, and therefore invited to undergo OGTT at community-based clinics. Males were less likely to have IDRS score ≥60 compared to females (49.8% vs. 78.6%). A total of 1209 (79.1% of those invited) individuals attended community-based clinics and underwent an OGTT. Of the initial non-attendees (n = 333), 26 expressed willingness to attend follow-up clinics but only 13 (50%) of those attended in reality. A lower proportion of males (75.2%) attended clinics compared to females (84.2%). After excluding 202 individuals (18.7% males; 14.4% females) with diabetes on the OGTT, the remaining 1007 (46.9% females) were recruited. The baseline characteristics of participants have been reported elsewhere [14].
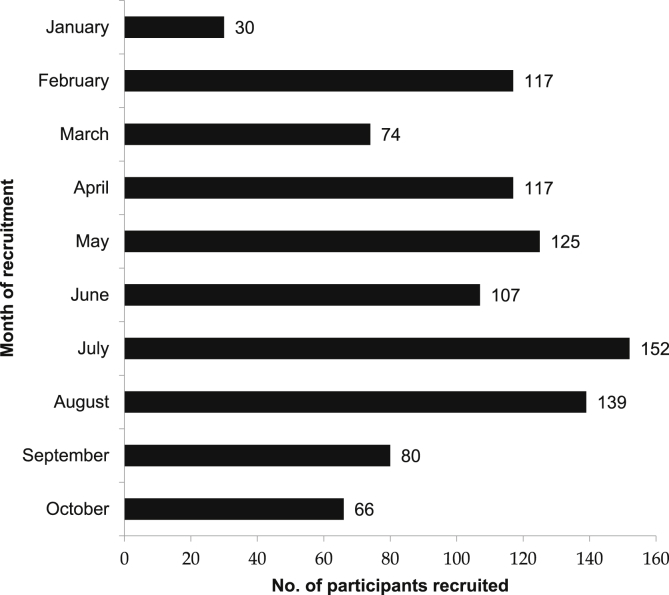
Fig. 2 shows the number of participants recruited each month during the 9-month recruitment period. During most of the months (excluding first and last month with less number of recruitment days), 100 or more participants were recruited, except during local festival seasons (March and September) where lower number of participants (74–80) were recruited.
Fig. 2.
K-DPP recruitment progress.
Table 2 shows the staff time spent for recruitment. A team of 76 staff members spent a total of 16,864 h over nine months to recruit 1007 participants. Thus, on average, 17 h were spent to recruit one participant. Of the total staff hours (16,864 h), home visits demanded a large proportion (77.5%) followed by training sessions for the field staff and LRPs (12.2%) and conducting community-based clinics (10.3%).
Table 2.
Staff time for recruitment.
| Recruitment staff | Task (s) related to recruitment | Total hours |
|---|---|---|
| Project manager (n = 1) | To meet community leaders | 42 |
| To train the field staff | 8 | |
| To train LRPs | 20 | |
| To manage community-based clinics | 264 | |
| Project assistant (n = 1) | To assist the project manager with recruitment-related activities | 334 |
| To make phone calls to the field staff and LRPs for attending training sessions | 58 | |
| Field assistant (n = 1) | To manage community-based clinics | 240 |
| Field staff (n = 10) | To make home visits, and screen the potential participants with the eligibility criteria and the IDRS | 12,378 |
| To attend the training sessions | 144 | |
| LRPs (n = 60) | To assist the field staff in identifying the home address of potential participants | 600 |
| To attend the training sessions | 1800 | |
| To give telephone reminders to participants to attend for the OGTT | 32 | |
| Phlebotomists (n = 3) | To collect blood samples for the OGTT at community-based clinics | 945 |
| Total | 16,864 |
IDRS, Indian Diabetes Risk Score; LRP, local resource person; OGTT, oral glucose tolerance test.
Table 3 shows the screening and recruitment costs. In total, US$23,525 were spent for recruiting participants, which translates to an average of US$23 to recruit one participant. The largest cost contributor was home visits (59.9%), followed by community-based clinics (30.7%), administrative costs (6.6%), and training sessions for the field staff and LRPs (2.9%). Personnel costs (US$14,931) were nearly two-third (63.5%) of the total costs.
Table 3.
Screening and recruitment costs.
| Categories | Inputs | Total cost (US$) | Percent of total cost |
|---|---|---|---|
| Training sessions (four for field staff and five for LRPs) | 672 | 2.9% | |
| Personnel costs | 161 | ||
| Travel, food and logistics costs | 450 | ||
| Phone call costs | 61 | ||
| Home visits (to contact 5517 potential participants) | 14,081 | 59.9% | |
| Personnel costs | 13,626 | ||
| IDRS printing charges | 66 | ||
| Phone call costs | 389 | ||
| Community-based clinics (n = 63) | 7223 | 30.7% | |
| Personnel costs | 1144 | ||
| OGTT costs | 3817 | ||
| Travel costs | 2123 | ||
| Rent for clinic venues | 87 | ||
| Phone call costs | 52 | ||
| Administrative costs | 1549 | 6.6% | |
| Total costs | 23,525 | 100% |
LRP, local resource person; IDRS, Indian Diabetes Risk Score; OGTT, oral glucose tolerance test. Rent was charged only for some of the venues. Costs in Indian Rupees (INR) were converted to US$ using an exchange rate of INR58.6=US$1 for the year 2013
Of the 131 individuals who refused to participate, 81 (61.8%) did not provide a specific reason for their decision. Among those providing a reason (n = 50), lack of time (42.0%, n = 21) was most commonly cited followed by ‘I am feeling healthy and therefore not in need of any intervention’ (30.0%, n = 15), personal reasons (24.0%, n = 12) and ‘no benefit to me or my family’ (4.0%, n = 2).
4. Discussion
K-DPP is the first RCT from a LMIC to evaluate the effectiveness of a peer-support lifestyle intervention delivered primarily by lay peer leaders for the prevention of type 2 diabetes. This paper provides valuable information on participant recruitment for future researchers planning to implement community-based diabetes prevention trials in India or other LMICs.
4.1. Effective recruitment strategies
Appropriate recruitment strategies is the key to delivering an effective intervention, achieving better outcomes and generalizability of the study findings. In our trial, we could reach the recruitment target within the expected time frame through learnings from the pilot study, community engagement and gender matching of staff. Consequently, we could start and complete the intervention without any undue delay. Furthermore, learnings from the pilot study were instrumental in achieving a gender balance in the study sample, thereby advancing the generalizability of the study findings to both sexes. We also believe that the good rapport that LRPs have with the people in the community may not only have enhanced the recruitment but also improved the attendance in intervention sessions [21].
4.2. Recruitment challenges
There were some challenges during recruitment. First, one-third of individuals, for whom initial home visits were made, could not be contacted. The main reason was that many of these individuals were not available at home due to work commitments. Screening at worksites may be a more effective strategy for reaching such individuals, however, these individuals will generally be employed at many different workplaces and often at some distance from the community where they live. Another important reason was that a substantial number of individuals had migrated overseas (either temporarily or permanently), mainly to Gulf countries. According to the latest Kerala Migration Survey, 19% of households in Kerala had emigrants in 2014, and they were predominantly men (86%) [22]. The other challenge was that during festive seasons it was difficult to recruit participants. During the month of Ramadan, Muslim participants were not able to attend clinics for the OGTT in a fasting state, hence, we had to postpone some clinics. Also, during Onam festival (local festival) in September, we had to stop recruitment for at least two weeks as it was difficult to mobilize people to the clinics. Finally, the participation rate for the OGTT was lower among males as compared to females (75.2% vs. 84.2%). Since we anticipated this based on pilot study findings, for the main trial, we conducted clinics only during weekends so that working men could attend. Although, this strategy improved the attendance in men (from 50% in the pilot study to 75.2% in the main trial), the rate was still lower than in women. One possible reason for this is that manual laborers go for paid work even during weekends and they leave for work early in the morning, and therefore they cannot stay at the clinic for 2 h for the OGTT. In our previous research, men had a much higher response rate (∼96%) for a one-time blood test (i.e. fasting plasma glucose) [19]. Therefore, it might be surmised that the lengthy nature of the OGTT may have dissuaded some men from attending, particularly manual laborers.
4.3. Staff time and recruitment costs
An average of 17 h were spent to recruit one participant with a cost of US$23. Home visits demanded a substantial proportion of the staff time (77.5%) and recruitment costs (59.9%), emphasizing the importance of the initial screening stage in the recruitment process, which is line with findings from other diabetes prevention trials [23]. The recruitment cost (US$23 per participant) was much lower than that in other diabetes prevention trials. For example, in the Indian Diabetes Prevention Programme [24], the cost of identifying one participant was US$117 (year 2006) and in the United States Diabetes Prevention Program it was US$139 (year 2000) [25]. These numbers are, however, not strictly comparable with ours, given the difference in the way high-risk individuals were defined across these studies. Nevertheless, it is worth mentioning that to recruit participants, we used a diabetes risk score in a stepwise screening approach, which reduced the number of OGTTs required, thereby possibly reducing the overall recruitment costs [26]. In a program setting, we anticipate that our recruitment costs would be even lower, as community health workers and healthcare providers will screen and identify high-risk individuals as part of the routine healthcare service [27].
4.4. Reasons for non-participation in the trial
A large proportion of potential participants did not provide a reason for choosing not to participate. Of those who did, lack of time was the most frequently cited reason. Flexibility in the delivery of intervention may address this barrier to a certain extent. For example, as an alternative to personal contact sessions, mobile phones could be used to deliver the intervention, given the widespread use of mobile phones in India and other LMICs, and its proven feasibility and effectiveness in diabetes prevention [28]. Interventions could also be delivered at worksites. ‘I am feeling healthy and therefore not in need of any intervention’ also emerged as a significant reason. This probably reflects the low awareness about diabetes, low risk perception and low self-efficacy to make lifestyle changes among high-risk individuals in India [29]. Moreover, the risk of acquiring diabetes is not a major factor in the food decision-making process in Indian households [30], and the cultural norms and certain misconceptions negatively influence women to engage in physical activity [31]. Other Indian investigators have pointed out that the “hidden” nature of prediabetes is a big challenge to sensitize people to their risk and to the benefits of making lifestyle changes for diabetes prevention.
4.5. Strengths and limitations
This study adds to the very limited body of literature on participant recruitment into diabetes prevention trials in LMICs. To our knowledge, K-DPP is the first diabetes prevention trial from a LMIC to provide data on reasons for non-participation. The limitation was that we did not ask the non-attendees for the OGTT screening the reason (s) for their decision. Future research, preferably a qualitative research, should try and elicit the barriers for attending the OGTT screening, particularly among men.
To conclude, the K-DPP recruitment experience offers important lessons for researchers planning to design and implement diabetes prevention trials in community settings in India or other LMICs: (1) pilot testing the recruitment protocol may help to identify some of the challenges and difficulties, thereby providing an opportunity to revise the recruitment approaches; (2) engaging the community in recruitment is valuable. We were able to engage the community and create ownership in the community leaders and LRPs who effectively promoted recruitment; (3) gender matching of staff may be required to enhance the reach of men; (4) local festive seasons should be accounted for in the recruitment schedule as delays might be expected; (5) the initial stage of recruitment demanded more staff time and costs compared to later stages, and this should be considered when budgeting the trial expenses; (6) a step-wise screening procedure involving a diabetes risk score reduced the number of OGTTs required, thereby reducing possibility reducing the overall recruitment costs; and (7) it is important to raise awareness among people about prediabetes and diabetes, and the role of lifestyle changes in diabetes prevention through health education programs.
Conflicts of interest
Authors have nothing to disclose.
Acknowledgement
K-DPP was funded by the National Health and Medical Research Council, Australia (Project Grant ID 1005324). TS was supported by the Victoria India Doctoral Scholarship (VIDS) for his PhD at the University of Melbourne, Australia. TS was also supported by the ASCEND Program, funded by the Fogarty International Centre of the National Institutes of Health (NIH) under Award Number: D43TW008332. We also acknowledge Peers for Progress, a programme of the American Academy of Family Physicians Foundation supported by the Eli Lily and Company Foundation. The contents of this paper are solely the responsibility of the authors and do not reflect the views of NHMRC, NIH, Peers for Progress or the ASCEND Program.
References
- 1.International Diabetes Federation IDF Diabetes Atlas 8th Edition. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html accessed 10/05/2018.
- 2.Haw J.S., Galaviz K.I., Straus A.N., Kowalski A.J., Magee M.J., Weber M.B. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 2017;177:1808–1817. doi: 10.1001/jamainternmed.2017.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts S., Barry E., Craig D., Airoldi M., Bevan G., Greenhalgh T. Preventing type 2 diabetes: systematic review of studies of cost-effectiveness of lifestyle programmes and metformin, with and without screening, for pre-diabetes. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laws R.A., St George A.B., Rychetnik L., Bauman A.E. Diabetes prevention research: a systematic review of external validity in lifestyle interventions. Am. J. Prev. Med. 2012;43:205–214. doi: 10.1016/j.amepre.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Aziz Z., Absetz P., Oldroyd J., Pronk N.P., Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement. Sci. 2015;10:172. doi: 10.1186/s13012-015-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treweek S., Lockhart P., Pitkethly M., Cook J.A., Kjeldstrom M., Johansen M. Methods to improve recruitment to randomised controlled trials: cochrane systematic review and meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathish T., Williams E.D., Pasricha N., Absetz P., Lorgelly P., Wolfe R. Cluster randomised controlled trial of a peer-led lifestyle intervention program: study protocol for the Kerala diabetes prevention program. BMC Public Health. 2013;13:1035. doi: 10.1186/1471-2458-13-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thankappan K.R., Sathish T., Tapp R.J., Shaw J.E., Lotfaliany M., Wolfe R. A peer-support lifestyle intervention for preventing type 2 diabetes in India: a cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz Z., Mathews E., Absetz P., Sathish T., Oldroyd J., Balachandran S. A group-based lifestyle intervention for diabetes prevention in low- and middle-income country: implementation evaluation of the Kerala Diabetes Prevention Program. Implement. Sci. 2018;13:97. doi: 10.1186/s13012-018-0791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews E., Thomas E., Absetz P. Cultural adaptation of a peer-led lifestyle intervention program for diabetes prevention in India: the Kerala diabetes prevention program (K-DPP) BMC Public Health. 2018;17:974. doi: 10.1186/s12889-017-4986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathish T., Oldenburg B., Tapp R.J., Shaw J.E., Wolfe R., Sajitha B. Baseline characteristics of participants in the Kerala Diabetes Prevention Program: a cluster randomized controlled trial of lifestyle intervention in Asian Indians. Diabet. Med. 2017;34:647–653. doi: 10.1111/dme.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sathish T., Shaw J.E., Tapp R.J. Targeted screening for prediabetes and undiagnosed diabetes in a community setting in India. Diabetes Metab. Syndr. 2019;13:1785–1790. doi: 10.1016/j.dsx.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Mohan V., Deepa R., Deepa M., Somannavar S., Datta M. A simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J. Assoc. Phys. India. 2005;53:759–763. [PubMed] [Google Scholar]
- 14.Mohan V., Pradeepa R., Deepa M., Anjana R.M., Unnikrishnan R., Datta M. How to detect the millions of people in India with undiagnosed diabetes cost effectively. Med. Update. 2010;20:93–96. [Google Scholar]
- 15.Sathish T., Kannan S., Sarma S.P., Thankappan K.R. Screening performance of diabetes risk scores among asians and whites in rural Kerala, India. Prev. Chronic Dis. 2013;10:E37. doi: 10.5888/pcd10.120131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Association Diabetes Association Standards of Medical Care in Diabetes - 2018. http://care.diabetesjournals.org/content/41/Supplement_1/S1 accessed 10/05/2018.
- 17.Sathish T., Kannan S., Sarma P.S., Razum O., Thankappan K.R. Incidence of hypertension and its risk factors in rural Kerala, India: a community-based cohort study. Publ. Health. 2012;126:25–32. doi: 10.1016/j.puhe.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Sathish T., Kannan S., Sarma S.P., Razum O., Sauzet O., Thankappan K.R. Seven-year longitudinal change in risk factors for non-communicable diseases in rural Kerala, India: the WHO STEPS approach. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thankappan K.R., Shah B., Mathur P., Sarma P.S., Srinivas G., Mini G.K. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J. Med. Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 20.International Monetary Fund Exchange Rate Archives by Month. https://www.imf.org/external/np/fin/data/param_rms_mth.aspx Available from. accessed 10/05/2018.
- 21.Waheed W., Hughes-Morley A., Woodham A., Allen G., Bower P. Overcoming barriers to recruiting ethnic minorities to mental health research: a typology of recruitment strategies. BMC Psychiatry. 2015;15:101. doi: 10.1186/s12888-015-0484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Government of Kerala . 2014. Dynamics of Emigration and Remittances in Kerala: Results from the Kerala Migration Survey.http://cds.edu/wp-content/uploads/2015/10/WP463.pdf accessed 10/05/2018. [Google Scholar]
- 23.Ranjani H., Weber M.B., Anjana R.M., Lakshmi N., Venkat Narayan K.M., Mohan V. Recruitment challenges in a diabetes prevention trial in a low- and middle-income setting. Diabetes Res. Clin. Pract. 2015;110:51–59. doi: 10.1016/j.diabres.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran A., Snehalatha C., Yamuna A., Mary S., Ping Z. Cost-effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within-trial results of the Indian Diabetes Prevention Programme (IDPP) Diabetes Care. 2007;30:2548–2552. doi: 10.2337/dc07-0150. [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan V., Goldhaber-Fiebert J.D., Radha V., Gokulakrishnan K. Screening with OGTT alone or in combination with the Indian diabetes risk score or genotyping of TCF7L2 to detect undiagnosed type 2 diabetes in Asian Indians. Indian J. Med. Res. 2011;133:294–299. [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health and Family Welfare, Government of India National Programme for Prevention and Control of Diabetes, Cardiovascular Disease and Stroke. https://mohfw.gov.in/about-us/departments/departments-health-and-family-welfare/national-programme-prevention-and-control-cancer-diabetes-cardiovascular-disease-and accessed 10/05/2018.
- 28.Ramachandran A., Snehalatha C., Ram J., Selvam S., Simon M., Nanditha A. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1:191–198. doi: 10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 29.Daivadanam M., Absetz P., Sathish T., Thankappan K.R., Fisher E.B., Philip N.E. Lifestyle change in Kerala, India: needs assessment and planning for a community-based diabetes prevention trial. BMC Public Health. 2013;13:95. doi: 10.1186/1471-2458-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daivadanam M., Wahlstrom R., Thankappan K.R., Ravindran T.K. Balancing expectations amidst limitations: the dynamics of food decision-making in rural Kerala. BMC Public Health. 2015;15:644. doi: 10.1186/s12889-015-1880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews E., Lakshmi J.K., Ravindran T.K., Pratt M., Thankappan K.R. Perceptions of barriers and facilitators in physical activity participation among women in Thiruvananthapuram City, India. Glob. Health Promot. 2016;23:27–36. doi: 10.1177/1757975915573878. [DOI] [PMC free article] [PubMed] [Google Scholar]