Abstract
Purpose
To report a case of contact lens-related infectious keratitis caused by the Gram-negative plant pathogen Rhizobium radiobacter.
Observations
A 26-year old lady with history of contact lens use presented with three weeks history of right eye redness and pain, with the left eye also being involved in the past week. Slit lamp examination of the right eye demonstrated multiple faint subepithelial and stromal infiltrates with no overlying epithelial defect, and no anterior chamber activity. Anterior segment optical coherence tomography demonstrated multiple hyper-reflective foci scattered at various depths of the corneal stroma. Corneal scrapings grew Rhizobium radiobacter, and culture-directed antibiotic therapy with topical gentamicin and levofloxacin resulted in slow clinical improvement of the R. radiobacter keratitis without visual sequelae.
Conclusions and importance
We have described the clinical features, microbial susceptibilities, and response to treatment in a case of R. radiobacter infectious keratitis.
R. radiobacter has recently emerged as a source for several ocular and systemic infections and was identified in a series of polymicrobial keratitis cases. Our case report of monomicrobial R. radiobacter keratitis adds to the sparse literature on this uncommon but potentially sight-threatening infection.
Keywords: Rhizobium radiobacter, Infectious keratitis, Contact lens, Atypical bacterial keratitis
1. Introduction
Bacterial keratitis is one of the most commonly encountered sight-threatening conditions in ophthalmology. In developed regions, contact lens-related bacterial keratitis accounts for most of these infections. Estimates of the incidence of contact lens-related bacterial keratitis range from as low as one in 5000 per year of wear for rigid contact lenses to as high as one in 500 per year for extended wear soft contact lenses.1, 2, 3 For the past several decades a small number of bacterial pathogens have been recognized as the etiologic agents for most contact lens-related keratitis cases, including Pseudomonas aeruginosa, Staphylococcus aureus and commensal species of Staphylococcus and Streptococcus.4,5 Less commonly, corneal infections are caused by atypical organisms including environmental bacteria and fungi that may evade microbiological diagnosis. Mixed microbial infections may also occur with atypical organisms that have been associated with a poor visual prognosis due to the difficulty in addressing various antibiotic susceptibilities.6
Here we report a case of atypical bacterial keratitis caused by the Gram-negative plant pathogen Rhizobium radiobacter, which has previously been found to cause several human infections and has recently been identified as a cause of bacterial keratitis.
2. Case report
A 26-year-old Chinese female with a history of monthly disposable soft contact lens wear and poor lens hygiene (used whilst bathing and occasional swimming) presented to our hospital with a one-week history of left eye redness, pain and photophobia. This was preceded by right eye redness and pain for three weeks that was symptomatically improving without treatment.
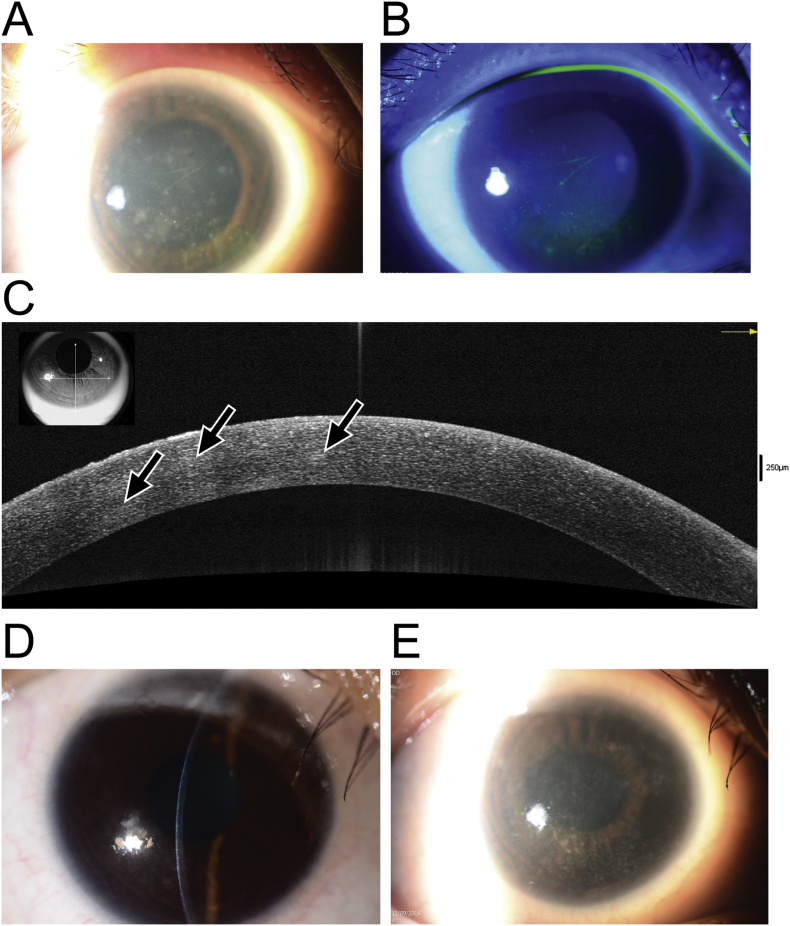
On presentation her best-corrected visual acuity was 20/30 in the right eye 20/40 in the left. Slit lamp examination of the right eye disclosed multiple faint subepithelial and stromal infiltrates without significant corneal edema or anterior chamber reaction, few punctate epithelial erosions and a pseudo-dendritic staining lesion. (Fig. 1A and B). The left eye had a central stromal infiltrate associated with a 3.5 × 2.5 mm epithelial defect and anterior chamber cells 1 + with mild conjunctival injection. No radial perineuritis was noted. Bilateral peripheral corneal vascularization consistent with contact lens over-wear was seen. Posterior segment examination of both eyes was unremarkable.
Fig. 1.
A. Clinical photograph showing Rhizobium radiobacter keratitis at presentation, with multiple foci of feathery subepithelial and stromal infiltrates. B. Fluorescein staining with cobalt blue illumination of the infection at presentation showing punctate epithelial erosions but no epithelial defect. C. Anterior segment optical coherence tomography (AS-OCT) demonstrating the presence of multiple mildly hyperreflective foci throughout the anterior, middle, and deep stroma (arrows). D and E. Clinical photographs showing stromal scar formation following two weeks of antibiotic therapy.
Anterior segment optical coherence tomography of the right eye demonstrated multiple hyper-reflective foci corresponding to the stromal infiltrates, which occurred from the subepithelial region down the deep stromal and predescemetic layers (Fig. 1C). Corneal scrapings of both eyes were taken for microscopy and cultures and the patient was commenced on empirical antibiotic therapy with hourly cefazolin 50 mg/mL and gentamicin 14 mg/mL (compounded by Singapore General Hospital pharmacy services). In view of the significant complaints of severe ocular pain, with the atypical appearance of the infiltrates and concomitant history of contact lens wear, this prompted suspicion of possible Acanthamoeba infection which is common in the local setting. Accordingly, hourly topical chlorhexidine 0.02% (compounded by Singapore General Hospital pharmacy services) was added to the initial treatment regime in the first 24 hours before culture results were obtained.
Corneal scrape cultures from the right eye grew Rhizobium radiobacter on brain heart infusion broth with sensitivity to cefepime (MIC 4 μg/mL), ciprofloxacin (MIC 0.25 μg/mL) and gentamicin (MIC 4 μg/mL). No microbial growth was observed on blood, chocolate or Sabaraud agar, non-nutrient agar with E. coli overlay, or thioglycolate broth, and fungal microscopy was negative. Cultures from the contact lens of the right eye returned mixed bacterial growth with Bacillus spp., Elizabethkingia spp., Serratia marcescens, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. No growth was seen on cultures for the left eye scraping, while the left contact lens returned Bacillus spp., Serratia marcescens, Stenotrophomonas maltophilia, Elizabethkingia spp., and Pseudomonas aeruginosa. Bacterial identification was performed using MALDI-TOF mass spectrometry fingerprinting (MALDI Biotyper, Bruker, MA, USA).
In view of these data the treatment was changed on day four of treatment to hourly levofloxacin 1.5% (Santen Pharmaceuticals, Osaka, Japan), hourly cefazolin, and two-hourly chlorhexidine to both eyes during waking hours and ciprofloxacin 0.3% ointment (Alcon Laboratories, Texas, USA) overnight. With this therapy moderate symptomatic and clinical improvement was noted within a week, with shrinkage of the right eye stromal infiltrates and reduction in the size of the epithelial defect. The left eye ulcer had become consolidated with early scar formation. Antibiotics were tapered to three-hourly by two weeks and the patient converted to levofloxacin 1.5% monotherapy for both eyes at four weeks. Chlorhexidine drops were maintained until four weeks in view of the high initial clinical suspicion of Acanthamoeba. At this time the right eye was quiet with small stromal opacities (Fig. 1 D and E) while the left eye had a central anterior to mid stromal scar. The best corrected visual acuity at this most recent follow-up was 20/20 on the right and 20/25 on the left.
3. Discussion
Here we present the atypical clinical features of a contact lens-related keratitis secondary to monomicrobial Rhizobium infection, which may mimic that of Acanthamoeba infection or may even be mistaken for viral infections such as HSV keratitis. Our case report is distinct from a previous publication of a single case of pre-existing fungal keratitis with secondary R. radiobacter infection, only isolated after four weeks of pre-treatment, which made it difficult to characterize its clinical presentation and signs.
Human infection by the plant pathogen R. radiobacter (previously Agrobacterium radiobacter) has been appreciated since the late 1980s, with clinical syndromes including bacteremia, urinary tract infections, cerebral abscesses, pericarditis and endocarditis, and peritonitis.7, 8, 9, 10, 11, 12, 13, 14 R. radiobacter is an aerobic, motile, oxidase-positive and non-spore-forming Gram-negative bacillus that is unique among rhizobia in that it causes disease in humans. A noticeable trend in these infections is the tendency of R. radiobacter to adhere to implanted devices.15 As a plant pathogen, Rhizobium colonizes plant roots using well characterized biofilm adherence mechanisms16 which may also be employed during human infections.15 A series of case reports have also implicated R. radiobacter in ocular infections, including endophthalmitis17, 18, 19, 20 and conjunctivitis.21 More recently, Barker and colleagues published the first report of infectious keratitis involving R. radiobacter.22 In their series three out of four cases were contact lens wearers, with contact lens cultures returning a similar complement of environmental bacterial flora to those reported here, including Serratia, Stenotrophomonas, and Pseudomonas species, among others. Similar to that case series, our patient's clinical picture was that of an atypical infectious keratitis, although in their series R. radiobacter was isolated with several other bacteria from the corneal scrapes, or following pre-treatment for a fungal keratitis for several weeks prior to isolation of R. radiobacter.
Taking our new case and those reported by Barker et al.22 together, several consistencies are apparent. Rhizobium radiobacter keratitis infections appear to be mainly contact lens-related with poor contact lens hygiene as a prominent risk factor. Additionally, the contact lens contamination exists as a mixed bacterial population comprising environmental bacteria. Clinically, the appearance of the corneal infiltrates is highly variable, but does not resemble typical Gram-negative keratitis. Infiltrates have a variable appearance, although only a single case from Baker and colleagues’ series and the case presented here were demonstrated to have R. radiobacter as the sole etiological agent. The intensity of inflammation ranged from dense stromal infiltrates with hypopyon to subepithelial infiltrates without epithelial defects and more limited anterior chamber reaction. It is also important to note that in our patient the eye infected with R. radiobacter was only minimally symptomatic at presentation and appeared to have improved without antibiotic therapy prior to presentation. This contrasts with the large deep stromal infiltrate and hypopyon noted in the monomicrobial infection described by Barker and colleagues, although in their case the infection was complicated by the previous involvement of fungus which was treated for several weeks prior to re-scraping and identification of R. radiobacter. It is also interesting to note that despite the plethora of bacterial species isolated from the contact lenses of our patient, R. radiobacter was only isolated from a corneal scraping. This suggests either a very low initial inoculum was present in the contact lens that prevented detection in the contact lens, or that R. radiobacter was introduced into the eye from direct trauma, with the contact lens wear compromising the epithelial barrier and enabling bacterial penetration into the stroma. Another important finding noted to by Barker et al. is the absence of an epithelial defect at presentation,22 which is a finding shared by our case.
Antibiotic sensitivities noted for the current R. radiobacter infection and that of Barker et al. include fluoroquinolones, cephalosporins, and gentamicin. In each case the infection improved within a month of starting appropriate antibiotic therapy, although in our case the response to treatment appeared to be far slower than that observed in the monomicrobial infection reported by Barker and coworkers. As with other atypical infections in our Asian population of contact lens wearers, poor response to antibiotic therapy may require therapeutic or tectonic keratoplasty to fully treat the condition.23, 24, 25
This interesting case of an atypical bacterial keratitis caused by R. radiobacter serves to provide the clinical presentation of keratitis and raise the awareness amongst clinicians for consideration in patients with atypical presentations and poor response to treatment. An increased surveillance of this plant pathogen may reveal this as an emerging cause of multiple local and systemic infections in humans.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to identification of the patient.
Acknowledgements and disclosures
Funding
No funding or grant support
Conflicts of interest
The authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Liesegang T.J. Contact lens-related microbial keratitis: Part I: Epidemiology. Cornea. 1997;16:125–131. [PubMed] [Google Scholar]
- 2.Cheng K.H., Leung S.L., Hoekman H.W. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F., Keay L., Edwards K. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F., Naduvilath T., Keay L. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green M., Apel A., Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 6.Ahn M., Yoon K.C., Ryu S.K., Cho N.C., You I.C. Clinical aspects and prognosis of mixed microbial (bacterial and fungal) keratitis. Cornea. 2011;30:409–413. doi: 10.1097/ICO.0b013e3181f23704. [DOI] [PubMed] [Google Scholar]
- 7.Edmond M.B., Riddler S.A., Baxter C.M., Wicklund B.M., Pasculle A.W. Agrobacterium radiobacter: a recently recognized opportunistic pathogen. Clin Infect Dis. 1993;16:388–391. doi: 10.1093/clind/16.3.388. [DOI] [PubMed] [Google Scholar]
- 8.Dunne W.M., Jr., Tillman J., Murray J.C. Recovery of a strain of Agrobacterium radiobacter with a mucoid phenotype from an immunocompromised child with bacteremia. J Clin Microbiol. 1993;31:2541–2543. doi: 10.1128/jcm.31.9.2541-2543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaya R.A., Edwards M.S. Agrobacterium radiobacter bacteremia in pediatric patients: case report and review. Pediatr Infect Dis J. 2003;22:183–186. doi: 10.1097/01.inf.0000048960.37470.ef. [DOI] [PubMed] [Google Scholar]
- 10.Paphitou N.I., Rolston K.V. Catheter-related bacteremia caused by Agrobacterium radiobacter in a cancer patient: case report and literature review. Infection. 2003;31:421–424. doi: 10.1007/s15010-003-3175-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen C.Y., Hansen K.S., Hansen L.K. Rhizobium radiobacter as an opportunistic pathogen in central venous catheter-associated bloodstream infection: case report and review. J Hosp Infect. 2008;68:203–207. doi: 10.1016/j.jhin.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Rojas L.O., Martinez L.F., Vives C.H., Lopez E.A., Cifuentes M. Cerebral abscess caused by Rhizobium radiobacter: first case report. AIDS. 2012;26:897–898. doi: 10.1097/QAD.0b013e3283528b3f. [DOI] [PubMed] [Google Scholar]
- 13.Badrising S., Bakker L., Lobatto S., van Es A. Peritonitis in a peritoneal dialysis patient due to Rhizobium radiobacter and Moraxella osleonsis: case report and literature review. Perit Dial Int. 2014;34:813–815. doi: 10.3747/pdi.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley P.S., Weaver R.E. Comparison of thirty-seven strains of Vd-3 bacteria with Agrobacterium radiobacter: morphological and physiological observations. J Clin Microbiol. 1977;5:172–177. doi: 10.1128/jcm.5.2.172-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawhney S., Naab T., Oneal P. Rhizobium radiobacter infection in a 27-year-old african American woman with munchausen syndrome. Lab Med. 2016;47:e32–e34. doi: 10.1093/labmed/lmw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heindl J.E., Wang Y., Heckel B.C., Mohari B., Feirer N., Fuqua C. Mechanisms and regulation of surface interactions and biofilm formation in Agrobacterium. Front Plant Sci. 2014;5:176. doi: 10.3389/fpls.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre-Filho Pde T., Ribeiro A.P., Passos E.D., Torigoe M., de Vasconcellos J.P. Endophthalmitis caused by Agrobacterium radiobacter. Scand J Infect Dis. 2003;35:410–411. doi: 10.1080/00365540310012253. [DOI] [PubMed] [Google Scholar]
- 18.Moreau-Gaudry V., Chiquet C., Boisset S. Three cases of post-cataract surgery endophthalmitis due to Rhizobium (Agrobacterium) radiobacter. J Clin Microbiol. 2012;50:1487–1490. doi: 10.1128/JCM.06106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Abdullah A.A., Al-Falah M., Al-Rashaed S., Khandekar R., Arevalo J.F. Endophthalmitis caused by Rhizobium radiobacter after posterior chamber phakic intraocular lens implantation to correct myopia. J Refract Surg. 2015;31:561–563. doi: 10.3928/1081597X-20150728-02. [DOI] [PubMed] [Google Scholar]
- 20.Namdari H., Hamzavi S., Peairs R.R. Rhizobium (Agrobacterium) radiobacter identified as a cause of chronic endophthalmitis subsequent to cataract extraction. J Clin Microbiol. 2003;41:3998–4000. doi: 10.1128/JCM.41.8.3998-4000.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson J.A., Call N.B., Kasworm E.M., Dirks M.S., Turner R.B. Safety and efficacy of topical norfloxacin versus tobramycin in the treatment of external ocular infections. Antimicrob Agents Chemother. 1988;32:1820–1824. doi: 10.1128/aac.32.12.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N.H., Thompson J.M., Mullen M.G. Rhizobium radiobacter: a recently recognized cause of bacterial keratitis. Cornea. 2016;35:679–682. doi: 10.1097/ICO.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 23.Ang M., Mehta J.S., Mantoo S., Tan D. Deep anterior lamellar keratoplasty to treat microsporidial stromal keratitis. Cornea. 2009;28:832–835. doi: 10.1097/ICO.0b013e3181930ddc. [DOI] [PubMed] [Google Scholar]
- 24.Ang M., Mehta J.S., Sng C.C., Htoon H.M., Tan D.T. Indications, outcomes, and risk factors for failure in tectonic keratoplasty. Ophthalmology. 2012;119:1311–1319. doi: 10.1016/j.ophtha.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Ang M., Mehta J.S., Arundhati A., Tan D.T. Anterior lamellar keratoplasty over penetrating keratoplasty for optical, therapeutic, and tectonic indications: a case series. Am J Ophthalmol. 2009;147:697–702 e2. doi: 10.1016/j.ajo.2008.10.002. [DOI] [PubMed] [Google Scholar]