Abstract
Objective: The development of hepatocellular carcinoma (HCC) is not uncommon in patients who achieve eradication of the hepatitis C virus through direct-acting antiviral (DAA) treatment. The aim of this study was to identify the patients at high risk for novel HCC development after a sustained virologic response (SVR) by DAA treatment.
Patients and Methods: A total of 518 patients with no history of HCC treatment and who achieved SVR by DAA treatment were evaluated retrospectively. The correlations between HCC development and the patients’ characteristics were evaluated. For patients who underwent gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) or dynamic contrast-enhanced computed tomography, the relationship between the imaging findings and subsequent HCC development was also assessed.
Results: HCC developed newly in 22 patients, and the 1-year and 3-year cumulative HCC rates were 2.0% and 8.5%, respectively. In multivariate analysis, a FIB-4 index >4.0 and a post-treatment α-fetoprotein >4.0 ng/ml were significant risk factors for HCC. In 26 of 118 patients who underwent an MRI before DAA treatment, a non-hypervascular hypo-intense nodule was seen in the hepatobiliary phase, and in 6 of 182 patients who underwent a CT, a non-hypervascular hypo-enhanced nodule was seen in the delayed phase. The sensitivity and specificity of the MRI-positive findings for the subsequent development of HCC were 0.92 and 0.87, respectively, and those of the CT were 0.40 and 0.99, respectively. In multivariate analysis of patients who underwent an MRI, a non-hypervascular hypo-intense nodule was the only factor that was significantly related to HCC development (HR 32.4, p = 0.001).
Conclusion: Gd-EOB-DTPA-enhanced MRI was found to be reliable for risk evaluation of subsequent HCC development in patients after SVR by DAA treatment. Patients with a non-hypervascular hypo-intense nodule need more careful observation for incident HCC.
Keywords: hepatocellular carcinoma (HCC), sustained virologic response (SVR), Gd-EOB-DTPA, MRI, hypo-intense nodule
Introduction
Hepatocellular carcinoma (HCC) is known to occur in patients with underlying chronic liver disease, and hepatitis C virus (HCV) infection is one of the most important causes of HCC1, 2). In patients whose HCV was eradicated by interferon (IFN)-based therapy, the risk of deterioration through hepatic fibrosis and the incidence of HCC are reduced, and the prognosis is improved2, 3). However, IFN-based therapy is not well suited for use in compromised patients, such as those with advanced age, severe fibrosis, or comorbidities, because of its adverse effects.
Direct-acting antiviral (DAA) treatment, which is currently recommended in therapeutic guidelines worldwide4,5,6), has good effectiveness and an excellent safety profile. Sustained virologic response (SVR) has been achieved in patients who were not candidates for IFN-based therapy for various reasons. Even in patients with advanced age, severe fibrosis, or a history of HCC treatment, and who are therefore known to be at high risk for hepatocarcinogenesis, SVR was attained in at least 90% of cases7,8,9,10,11).
However, the development of HCC after DAA treatment is not rare in real-world practice. Since there have been reports of the rapid growth of HCC after DAA treatment12,13,14), careful follow-up is required.
Numerous patients, including those at high risk for HCC, achieve SVR, so identifying the characteristics of patients at high risk for HCC is very useful for surveillance after SVR with DAA treatment.
Patients with no prior history of HCC who achieved SVR with DAA treatment were retrospectively reviewed to identify the risk factors for novel HCC development after SVR.
Materials and Methods
Patients
Beginning in September 2014, patients with detectable serum HCV-RNA levels with chronic hepatitis or compensated liver cirrhosis and no HCC received anti-HCV treatment with DAAs. Viral eradication was defined as achieving SVR 12 weeks after the end of treatment (SVR12).
Patients who had no history of HCC treatment and achieved SVR12 were enrolled in this study. Patients with a positive serum hepatitis B surface antigen test were excluded from this study. There were no restrictions on the HCV-genotype or the DAA protocol.
Prior to DAA treatment, an abdominal ultrasound (US), gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI), and/or dynamic contrast-enhanced computed tomography (dyCT) were performed in all cases. In patients with a nodular lesion as seen by an US examination, Gd-EOB-DTPA-enhanced MRI (Gd-EOB-MRI) and/or dyCT were performed to confirm the absence of hypervascular active HCC. Patients with a nodular lesion that was non-hypervascular in the arterial phase and was hypo-enhanced in the portal or hepatobiliary phases as seen by Gd-EOB-MRI or dyCT were considered eligible for DAA treatment.
Before the initiation of DAA treatment, the patients’ characteristics, hematological data, biochemical data, and virologic data were recorded. Serum alpha-fetoprotein (AFP) data and Mac-2 binding protein glycosylation isomer (M2BPGi) data, a novel serological glyco-biomarker for liver fibrosis that was approved in 2015 in Japan15), were obtained around 12 weeks after the end of DAA treatment.
This study was approved by the Bioethics Committee for Clinical Research A of Jichi Medical University Hospital (A17-154) and was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Follow-up and diagnosis of HCC
All cases were followed up regularly every 3–6 months with blood examinations and an US, Gd-EOB-MRI, or dyCT. When a nodular lesion was detected by imaging or a tumor marker (AFP or protein induced by vitamin K absence or antagonist-II (PIVKA-II)) level was elevated, additional Gd-EOB-MRI and/or dyCT were performed. The diagnosis of HCC was defined by the typical radiographic findings of a tumor, showing early-phase enhancement followed by late-phase washout of contrast agent on Gd-EOB-MRI and/or dyCT.
Evaluation of risk for HCC
The start date of follow-up was defined as the date on which DAA treatment ended. The end of follow-up was defined as an HCC occurrence, the start of other DAA treatment, or the date of the latest hospital visit.
The relationships between the occurrence of classical hypervascular HCC and the patients’ variables were examined by univariate and multivariate analyses using Cox’s proportional hazard model. The variables included patient age, sex, clinical diagnosis (chronic hepatitis or cirrhosis), HCV-genotype, quantitative serum HCV-RNA levels, platelet count (PLT), aspartate aminotransferase (AST) levels, and alanine aminotransferase (ALT) levels before starting DAA treatment, and AFP levels and M2BPGi levels 12 weeks after finishing DAA treatment. The FIB-4 index, which was developed as a biomarker for hepatic fibrosis, was calculated using the following formula: FIB-4 index = (age [year] × AST [U/L]) / ((PLT [109/L]) × (ALT [U/L])1/2)16), and was added to the variables.
In patients who underwent Gd-EOB-MRI or dyCT within 12 months of starting DAA treatment, the presence or absence of a non-hypervascular hypo-enhanced nodule was recorded. The imaging findings were then added to the variables, and their relationship to the development of classical hypervascular HCC was analyzed.
Gd-EOB-DTPA-enhanced MRI
Gd-EOB-MRI was performed by using three 1.5T systems (Achieva dStream®, Philips, Best, The Netherlands; MAGNETOM Avanto®, Siemens, Forchheim, Germany; MAGNETOM Symphony®, Siemens, Forchheim, Germany) and a 3.0T system (MAGNETOM Skyra®, Siemens, Forchheim, Germany). Unenhanced, arterial, portal venous, late, and hepatobiliary phase images were obtained. The time at which to begin the dynamic arterial phase imaging was found using the MR fluoroscopic bolus detection technique. The arterial phase scan was started when contrast was visualized in the left ventricle, after bolus injection of 10 ml Gd-EOB-DTPA (Primovist®, Bayer-Schering Pharma, Osaka, Japan) at a rate of 1 mL/s, using a T1-weighted three-dimensional (3D) gradient-echo (GRE) sequence in a single breath hold. A 30 mL saline flush was administered at 3 mL/s after the Gd-EOB-DTPA injection. The portal venous phase scan and the late phase scan were started 45 s and 150 s, respectively, after the end of the arterial phase scan. The hepatobiliary phase scan was started 20 min after the contrast material was injected.
Dynamic contrast-enhanced CT
DyCT was performed using a 64-slice CT (Sensation 64®, Siemens, Forchheim, Germany), a 128-slice CT (Somatom Definition AS+®, Siemens, Forchheim, Germany), or a 128-slice dual-source CT (Somatom Definition Flash®, Siemens, Forchheim, Germany). Unenhanced, early arterial, late arterial, and delayed equilibrium phase images were obtained. Almost all of the patients were given 540 mg of iodine per kilogram of body weight, with 300, 320, 350, or 370 mgI/mL of nonionic contrast medium and at a maximum dose of 150 mL per patient. The warmed contrast medium was administered intravenously with a mechanical power injector at 2.7–5 mL/s with a fixed injection duration of 30 seconds. The bolus-tracking technique was used to automatically start the early arterial phase scan after the injection of contrast material. Early arterial phase imaging was started just after the trigger threshold (100 HU elevation) was reached at the abdominal aorta. The late arterial phase CT scan was started immediately after the end of the early arterial phase scan. The delayed equilibrium phase CT scan was started 120 s after the start of the contrast material injection.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) and EZR (Saitama Medical Center, Jichi Medical University, Japan)17), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
In comparisons between groups, nominal variables were evaluated by Fisher’s exact probability test, and continuous variables were evaluated by the Mann-Whitney U-test.
The risk factors associated with HCC occurrence after SVR were analyzed by Cox’s proportional hazard regression. Before performing regression analysis, the cut-off values of continuous variables were decided by the Youden index through a receiver operating characteristic curve analysis.
The odds ratios and their 95% confidence intervals (CIs) are shown. Kaplan-Meier survival curves were compared using log-rank tests. p<0.05 was considered significant.
Results
Evaluation of all cases
Between September 2014 and August 2017, 658 patients with HCV infection started DAA treatment. Eight patients discontinued the treatment and 7 dropped out of follow-up; the remaining 643 patients completed the treatment and the subsequent 12-week follow-up. Of the 601 who achieved SVR12, 518, excluding 79 with a history of HCC treatment and 4 who tested positive for HBs antigen, were enrolled in this study.
The median follow-up period was 2.0 years (range: 0.1–3.9 years). In 22 of the 518 patients, a new, classical hypervascular HCC developed during the follow-up period. The HCC occurrence rates at 1 year, 2 years, and 3 years were 2.0%, 3.5%, and 8.5%, respectively.
The overall characteristics of the patients and a comparison according to HCC occurrence after DAA treatment are shown in Table 1. On the whole, more of the patients were male (286/518, 55.2%), the median age was 66 years, and 135 (26.1%) were clinically diagnosed as having compensated liver cirrhosis. In comparing patients with and without subsequent HCC development, being an elderly patient, having higher levels of pre-treatment AST and of ALT, having a higher score on the FIB-4 index, having a lower pre-treatment platelet count, and having higher levels of post-treatment AFP and of M2BPGi were significantly associated with HCC (Table 1).
Table 1. Characteristics of patients as related to HCC occurrence.
| Overall n=518 |
HCC occurrence – n=496 |
HCC occurrence + n=22 |
p value | |
|---|---|---|---|---|
| Sex (Male/Female) | 286/232 | 273/223 | 12/10 | 1.0 |
| Age (years) | 66 (26–88) | 65.5(26–88) | 72 (59–83) | 0.0008 |
| Diagnosis CH/LC | 383/135 | 369/127 | 14/8 | 0.32 |
| HCV-RNA (LogIU/mL) | 6.2 (2.2–7.3) | 6.2 (2.2–7.3) | 6.1 (4.5–7.0) | 0.77 |
| Genotype 1/2 | 39360/158 | 345/151 | 15/7 | 1.0 |
| AST (U/L) | 43 (11–610) | 42 (11–610) | 63.5 (21–177) | 0.006 |
| ALT (U/L) | 43 (8–749) | 41 (8–749) | 49.5 (21–193) | 0.19 |
| PLT (× 104/µL) | 14.5 (2.9–34.8) | 14.5 (2.9–34.8) | 10.7 (5.7–20.5) | 0.0004 |
| FIB-4 index | 3.25 (0.38–32.0) | 3.18 (0.37–32.0) | 6.01 (2.53–14.4) | 0.00007 |
| Post-AFP (ng/mL) | 3 (1–68) | 3 (1–29) | 5 (2.3–68) | 0.0001 |
| Post-M2BPGi (COI) | 1.16 (0.13–15.3) | 1.14 (0.13–15.3) | 2.29 (0.69–7.95) | 0.0005 |
CH: chronic hepatitis; LC: liver cirrhosis; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PLT: platelet count; AFP: α-fetoprotein; M2BPGi: Mac-2 binding protein glycosylation isomer.
The cut-off indices (COIs), which were determined for univariate and multivariate analyses, of age, viral load, AST, ALT, PLT count, the FIB-4 index, AFP, and M2BPGi were 65 years, 6.0 LogIU/mL, 34 U/L, 42 U/L, 12.5 × 104/µL, 4.0, 4.0 ng/mL, and 1.6 COI, respectively.
In univariate analysis, age, AST, ALT, PLT count, the FIB-4 index, AFP, and M2BPGi were found to be factors related to HCC development. Since age, AST, ALT, and PLT count are components of the formula for the FIB-4 index, multivariate analysis was performed using the FIB-4 index, AFP, and M2BPGi as the variables. It was found that a high pre-treatment FIB-4 index and high post-treatment AFP levels were significantly associated with the development of HCC (Table 2).
Table 2. Results of univariate and multivariate analyses for variables associated with the development of HCC.
| n=518 | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex | Female | 1 | |||
| Male | 1.02 (0.44–2.37) | 0.96 | |||
| Age (years) | ≤64 | 1 | |||
| >64 | 5.18 (1.53–17.5) | 0.008 | |||
| CH/LC | CH | 1 | |||
| LC | 1.26 (0.52–3.02) | 0.61 | |||
| HCV-RNA (LogIU/mL) | ≤6.0 | 1 | |||
| >6.0 | 0.64 (0.28–1.49) | 0.31 | |||
| HCV-genotype | 1 | 1 | |||
| 2 | 1.11 (0.42–2.91) | 0.84 | |||
| AST (U/L) | ≤34 | 1 | |||
| >34 | 9.18 (1.23–68.5) | 0.03 | |||
| ALT (U/L) | ≤42 | 1 | |||
| >42 | 3.07 (1.13–8.33) | 0.03 | |||
| PLT (× 104/µL) | ≥12.5 | 1 | |||
| <12.5 | 3.77 (1.47–9.68) | 0.006 | |||
| FIB-4 index | ≤4.0 | 1 | 1 | ||
| >4.0 | 9.00 (2.64–30.4) | 0.0004 | 4.84 (1.26–18.6) | 0.02 | |
| Post-AFP (ng/mL) | ≤4.0 | 1 | 1 | ||
| >4.0 | 4.66 (1.90–11.4) | 0.0008 | 2.91 (1.15–7.38) | 0.02 | |
| Post-M2BPGi (COI) | ≤1.6 | 1 | 1 | ||
| >1.6 | 4.33 (1.76–10.7) | 0.001 | 1.57 (0.58–4.27) | 0.37 | |
CH: chronic hepatitis; LC: liver cirrhosis; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PLT: platelet count; AFP: α-fetoprotein; M2BPGi: Mac-2 binding protein glycosylation isomer; HR: hazard ratio; CI: confidence interval.
Evaluation of patients who underwent imaging examinations
Gd-EOB-MRI was performed 2.8 ± 3.1 (average ± standard deviation) months before DAA treatment began in 118 patients, and dyCT was performed 3.2 ± 2.6 months before DAA treatment began in 182 patients. Forty-one patients underwent both an MRI and a CT with 3.0 ± 2.2 month intervals.
A nodule that was not hypervascular in the arterial phase and exhibited hypo-intensity in the hepatobiliary phase as seen by Gd-EOB-MRI was defined as a non-hypervascular hypo-intense nodule, regardless of signal intensity in T1-weighted or T2-weighted images. Similarly, a nodule that was not hypervascular in the arterial phase and was detected as a hypo-enhanced lesion in the delayed equilibrium phase by dyCT was defined as a non-hypervascular hypo-enhanced nodule.
The rate of detecting a non-hypervascular hypo-enhanced nodule by Gd-EOB-MRI was 22.0% (26/118), while the rate of detecting a non-hypervascular hypo-enhanced nodule by dyCT was 3.3% (6/182).
Of the patients who underwent Gd-EOB-MRI, 13 subsequently developed HCC, and a non-hypervascular nodule was detected in 12 of these 13 patients. New HCC developed in only one patient who did not have positive findings by prior Gd-EOB-MRI. Of the patients who underwent dyCT, 10 subsequently developed HCC, and 4 of them had a non-hypervascular hypo-enhanced nodule as seen by dyCT.
The sensitivity and specificity of Gd-EOB-MRI for subsequent HCC development were 0.92 and 0.87, respectively, and those of dyCT were 0.40 and 0.99, respectively (Table 3).
Table 3. Predictability of a non-hypervascular hypo-enhanced nodule for subsequent HCC occurrence.
| (a) Distribution of cases that underwent Gd-EOB-DTPA-enhanced MRI. | |||||||
| n=118 | HCC occurrence+ | HCC occurrence− | |||||
| nHHN + on MRI | 12 | 14 | |||||
| nHHN − on MRI | 1 | 91 | |||||
| (b) Distribution of cases that underwent dynamic CT. | |||||||
| n=182 | HCC occurrence+ | HCC occurrence− | |||||
| nHHN + on CT | 4 | 2 | |||||
| nHHN − on CT | 6 | 170 | |||||
| (c) Performance of positive findings by MRI and CT for HCC occurrence. | |||||||
| Sensitivity | Specificity | PPV | NPV | LR+ | LR− | ||
| nHHN + on MRI | 0.92 | 0.87 | 0.46 | 0.99 | 6.9 | 0.09 | |
| nHHN + on CT | 0.40 | 0.99 | 0.67 | 0.97 | 34 | 0.61 | |
HCC: hepatocellular carcinoma; nHHN: non-hypervascular hypo-enhanced nodule; MRI: magnetic resonance imaging; CT: computed tomography; PPV: positive predictive value; NPV: negative predictive value; LR+: positive likelihood ratio; LR–: negative likelihood ratio.
Seven of 41 patients who underwent both Gd-EOB-MRI and dyCT developed HCC. Gd-EOB-MRI showed a non-hypervascular hypo-intense nodule in all cases, while dyCT showed a non-hypervascular hypo-enhanced nodule in 4 of the 7 patients.
In 118 patients who underwent Gd-EOB-MRI, multivariate analysis was performed with “presence or absence of non-hypervascular hypo-intense nodule” as an additional variable.
The presence of a non-hypervascular hypo-intense nodule was highly associated with subsequent HCC development (OR 32.4, p=0.001), while the other variables were not significant (Table 4).
Table 4. Multivariate analysis of variables associated with the occurrence of HCC in patients who underwent Gd-EOB-DTPA-enhanced MRI before DAA treatment.
| n=118 | Multivariate analysis | ||
|---|---|---|---|
| Variable | HR (95% CI) | p value | |
| FIB-4 index | ≤ 4.0 | 1 | |
| > 4.0 | 3.20 (0.34–29.8) | 0.31 | |
| Post-AFP (ng/mL) | ≤ 4.0 | 1 | |
| > 4.0 | 2.32 (0.60–9.01) | 0.22 | |
| Post-M2BPGi (COI) | ≤ 1.6 | 1 | |
| > 1.6 | 0.63 (0.15–2.69) | 0.53 | |
| Non-hypervascular hypo-intense nodule | negative | 1 | |
| positive | 32.4 (3.92–268) | 0.001 | |
AFP: α-fetoprotein; M2BPGi: Mac-2 binding protein glycosylation isomer; HR: hazard ratio; CI: confidence interval; Gd-EOB-DTPA: gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid; MRI: magnetic resonance imaging; DAA: direct-acting antiviral.
From a Kaplan-Meyer analysis, the cumulative 3-year HCC development rates of patients with and without a non-hypervascular hypo-intense nodule were 75.6% and 1.1%, respectively (p<0.0001) (Figure 1).
Figure 1.
Cumulative HCC rates after achieving SVR according to Gd-EOB-DTPA-enhanced MRI findings showing non-hypervascular hypo-intense nodules (nHHNs) in the hepatobiliary phase. The dashed line indicates HCC incidence in patients with nHHNs, and the solid line indicates HCC incidence in patients without nHHNs. The incidence of HCC is significantly higher in patients with nHHNs than in patients without nHHNs (p<0.0001, log-rank test). HCC: hepatocellular carcinoma; SVR: sustained virologic response; Gd-EOB-DTPA: gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid; MRI: magnetic resonance imaging.
The median diameter of the hypo-intense nodules was 7 mm as obtained by Gd-EOB-MRI before DAA treatment. The median diameter of the HCC in patients who underwent an MRI before DAA treatment was 13 mm (range: 10–19 mm), and the median diameter of the HCC in patients who did not receive an MRI before DAA treatment was 19 mm (range: 13–40 mm).
All HCCs in patients who underwent an MRI before DAA treatment were curatively treated with RFA, while HCCs in some of the patients who did not receive a prior MRI were treated with either a liver resection or transcatheter arterial chemoembolization (TACE), depending on the tumor size or number.
Discussion
Anti-HCV treatment has shifted from IFN-based to IFN-free DAA treatment, and SVR has become possible in many cases. However, the development of HCC after SVR is not rare. It has been reported that IFN-based treatment reduces the risk of hepatic carcinogenesis2, 3), but it is still not clear whether there is a similar effect with DAA treatment14, 18). DAA treatment provides a high SVR rate even in patients with fibrosis or who are elderly and are thus considered to be at a high risk of developing HCC. Therefore, appropriate follow-up is crucial for identifying those at high risk of developing HCC after achieving SVR with DAA treatment in the real world.
In this study, the focus was on the novel development of HCC after SVR. The relationship between carcinogenesis and various clinical findings in patients who had no history of HCC and achieved SVR after DAA treatment were examined retrospectively. New HCC developed in 22 of 518 patients, and the 3-year cumulative HCC rate was 8.5% for all cases.
A previous study reported that the risk factors for HCC development after IFN-based treatment were advanced fibrosis, old age, being male, and having diabetes2, 19,20,21). In recent years, higher levels of post-treatment AFP and of M2BPGi were found to be associated with HCC development after viral eradication with DAA treatment22, 23).
In the present cohort, advanced age, a low PLT count, high levels of transaminase, a high FIB-4 index, and high levels of post-treatment AFP and of M2BPGi were significant in univariate analysis. In multivariate analysis, a high FIB-4 index and high levels of post-treatment AFP were significant risk factors for novel HCC development. The present finding that the FIB-4 index is a risk factor for HCC development supports prior reports that hepatic fibrosis is as well.
The high AFP value after virus eradication might have been due to the persistent inflammation caused by hepatic diseases rather than hepatitis virus infection or the presence of precancerous lesions. However, it is important to note that the threshold of AFP in the present study was 4 ng/dL, which is considerably lower than the normal upper limit for a general HCC biomarker.
Furthermore, in the present study, the relationship between the imaging findings before DAA treatment and hepatic carcinogenesis was examined. The sensitivity of Gd-EOB-MRI for subsequent HCC development based on the presence of a hypo-enhanced nodule was 0.92, higher than that of dyCT (0.40).
In 26 of the 118 cases that underwent Gd-EOB-MRI, a hypo-intense nodule in the hepatobiliary phase without arterial hypervascularity was detected. Novel HCC developed in 12 of the 26 cases with such a nodule, while it developed in only one of 92 cases without that nodule. In multivariate analysis, the presence of a non-hypervascular hypo-intense nodule was associated with an over 30-fold higher risk of HCC occurrence, while the FIB-4 index and the AFP and M2BPGi values were not significant. Analysis using the presence of a non-hypervascular hypo-enhanced nodule as seen by dyCT yielded similar results (OR 14.2, p=0.0007). However, Gd-EOB-MRI was considered to be more useful for identifying high-risk cases and screening for HCC because of its high sensitivity.
Toyoda et al. reported that there was a correlation between non-hypervascular hypo-intense nodules and the levels of M2BPGi or of AFP24). In the current study, the presence of a non-hypervascular hypo-intense nodule was also related to a high FIB-4 index and high levels of AFP and of M2BPGi. High levels of these markers might represent the presence of a non-hypervascular hypo-intense nodule. Blood examinations are useful because they are less invasive and can be checked repeatedly. However, although an MRI is slightly burdensome, the presence of a non-hypervascular hypo-intense nodule showed the strongest association with hepatic carcinogenesis, with a higher hazard ratio than the other variables, and it was found to directly indicate the risk of HCC developing. In the current study, a patient with a non-hypervascular hypo-intense nodule and normal laboratory findings subsequently developed HCC (Figure 2).
Figure 2.
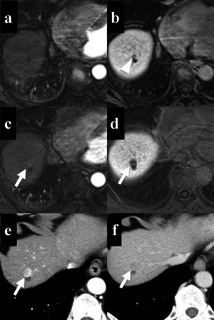
MRI and CT findings in a patient who developed HCC after SVR. The patient was a 64-year-old male, and the platelet count, FIB-4 index, post-treatment AFP levels, and post-treatment M2BPGi levels were 185,000 /µL, 2.75, 3 ng/mL, and 1.11 COI, respectively. Gd-EOB-DTPA-enhanced MRI performed before DAA treatment shows a small nodule next to a cyst in the hepatobiliary phase (arrowhead) (b), while it is undetectable in the arterial phase (a).At 1.2 years after achieving SVR, the nodule has enlarged and shows hypervascularity in the arterial phase (c, e), following hypo-enhancement in the hepatobiliary phase (d) or the portal phase (f) (arrows). (c, d: Gd-EOB-DTPA-enhanced MRI; e, f: dynamic CT). CT: computed tomography; MRI: magnetic resonance imaging; HCC: hepatocellular carcinoma; SVR: sustained virologic response; AFP: α-fetoprotein; M2BPGi: Mac-2 binding protein glycosylation isomer; Gd-EOB-DTPA: gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid; DAA: direct-acting antiviral.
Generally, DAA treatment is not administered to patients with active HCC in Japan. When a nodular lesion is detected in the liver, differentiating between whether it is benign or malignant might be difficult with clinical images alone.
Matsui et al. reported that the intranodular arterial blood supply increased as hepatocarcinogenesis progressed25). Generally, the detection of hypervascularity was visible in well- to moderately-differentiated HCC.
Thus, in many institutions, the finding of hypervascularity in the arterial phase on an enhanced CT or MRI is clinically considered to be the threshold at which to start anti-cancer treatment for hepatic nodular lesions.
In our institution, we checked for nodular lesions before DAA treatment, and patients were not excluded from DAA treatment if a non-hypervascular hypo-enhanced nodule was present.
The median follow-up period was as short as 2.0 years, and most of the HCCs developed from non-hypervascular hypo-intense nodules in cases that underwent Gd-EOB-MRI before DAA treatment. It is highly possible that HCC development in this short follow-up period was the cancerization of a pre-existing precancerous lesion, such as the hypo-intense nodule.
A non-hypervascular hypo-intense nodule in the hepatobiliary phase as seen by Gd-EOB-MRI has been clinically diagnosed as a dysplastic nodule, which has been recognized as a precancerous lesion. Therefore, the occurrence of classical hypervascular HCC from such nodules is not rare26).
This study was not a comparative study, so it could not evaluate whether DAA treatment promoted cancerization of non-hypervascular hypo-intense nodules. However, it was confirmed that the presence of a non-hypervascular hypo-intense nodule was strongly associated with subsequent classical HCC development after SVR by DAA treatment. In cases with a non-hypervascular hypo-intense nodule, the 3-year cumulative incidence of HCC reached 75.6%, although there was bias in that Gd-EOB-MRI was performed in high-risk cases, such as those with advanced fibrosis. Therefore, in monitoring for HCC development after SVR, the use of Gd-EOB-MRI appears to be very useful in assessing the risk of future HCC occurrence.
In the present study, careful follow-up of cases with a nodule led to early diagnosis of HCC and the administration of curative treatment. We believe that Gd-EOB-MRI should be performed at least in cases with patients at an advanced age, with advanced fibrosis, or with a high post-treatment AFP level.
The limitations of this study included the fact that it was a retrospective observational study in a single institution. There was bias in that Gd-EOB-MRI was performed only for some of the patients, so the proposed likelihood of HCC occurrence might not be accurate. In addition, the short follow-up period might have underestimated the factors related to de novo carcinogenesis. Ongoing further follow-up and long-term studies with a larger population are needed to assess the risk factors and establish appropriate HCC surveillance systems after HCV eradication.
Conclusion
In this study, a non-hypervascular hypo-intense nodule as seen by Gd-EOB-DTPA-enhanced MRI was strongly associated with liver carcinogenesis. Gd-EOB-DTPA-enhanced MRI was extremely reliable in assessing the potential risk of hepatocarcinogenesis and is highly recommended for cases with fibrosis before and/or after DAA treatment. When a non-hypervascular hypo-intense nodule is present, extremely careful follow-up with a focus on the potential development of HCC is needed.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Poynard T, Yuen MF, Ratziu V. Viral hepatitis C. Lancet 2003; 362: 2095–2100. doi: 10.1016/S0140-6736(03)15109-4 [DOI] [PubMed] [Google Scholar]
- 2.Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer 2015; 121: 2874–2882. doi: 10.1002/cncr.29528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RL, Baack B, Smith BD. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158: 329–337. doi: 10.7326/0003-4819-158-5-201303050-00005 [DOI] [PubMed] [Google Scholar]
- 4.Drafting Committee for Hepatitis Management Guidelines the JS of H. JSH guidelines for the management of hepatitis C virus infection: A 2014 Update for Genotype 1. Hepatol Res 2014; 44: 59–70. doi: 10.1111/hepr.12272 [DOI] [PubMed] [Google Scholar]
- 5.Chung RT, Davis GL, Jensen DM. AASLD/IDSA HCV Guidance PanelHepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62: 932–954. doi: 10.1002/hep.27950 [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.euEASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66: 153–194. doi: 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Kumada H, Suzuki Y, Ikeda K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 2014; 59: 2083–2091. doi: 10.1002/hep.27113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizokami M, Yokosuka O, Takehara T. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015; 15: 645–653. doi: 10.1016/S1473-3099(15)70099-X [DOI] [PubMed] [Google Scholar]
- 9.Omata M, Nishiguchi S, Ueno Y. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat 2014; 21: 762–768. doi: 10.1111/jvh.12312 [DOI] [PubMed] [Google Scholar]
- 10.Kumada H, Chayama K, Rodrigues L., Jr Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology 2015; 62: 1037–1046. doi: 10.1002/hep.27972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumada H, Suzuki Y, Karino Y. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol 2017; 52: 520–533. doi: 10.1007/s00535-016-1285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reig M, Boix L, Bruix J. The impact of direct antiviral agents on the development and recurrence of hepatocellular carcinoma. Liver Int 2017; 37(Suppl 1): 136–139. doi: 10.1111/liv.13321 [DOI] [PubMed] [Google Scholar]
- 13.Kozbial K, Moser S, Schwarzer R. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol 2016; 65: 856–858. doi: 10.1016/j.jhep.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 14.Conti F, Buonfiglioli F, Scuteri A. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016; 65: 727–733. doi: 10.1016/j.jhep.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 15.Kuno A, Ikehara Y, Tanaka Y. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013; 3: 1065. doi: 10.1038/srep01065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N. APRICOT Clinical InvestigatorsDevelopment of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317–1325. doi: 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 17.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li DK, Ren Y, Fierer DS. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 2018; 67: 2244–2253. doi: 10.1002/hep.29707 [DOI] [PubMed] [Google Scholar]
- 19.Arase Y, Kobayashi M, Suzuki F. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 2013; 57: 964–973. doi: 10.1002/hep.26087 [DOI] [PubMed] [Google Scholar]
- 20.Toyoda H, Kumada T, Tada T. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol 2015; 30: 1183–1189. doi: 10.1111/jgh.12915 [DOI] [PubMed] [Google Scholar]
- 21.Chang KC, Tseng PL, Hung HC. P0337 : A polymorphism in IFNL3 is an independent risk factor for development of hepatocellular carcinoma after treatment of HCV infection. J Hepatol 2015; 62: S436. doi: 10.1016/S0168-8278(15)30552-3 [DOI] [PubMed] [Google Scholar]
- 22.Nagata H, Nakagawa M, Asahina Y. Ochanomizu Liver Conference Study GroupEffect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol 2017; 67: 933–939. doi: 10.1016/j.jhep.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 23.Ogawa E, Furusyo N, Nomura H. Kyushu University Liver Disease Study (KULDS) GroupShort-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther 2018; 47: 104–113. doi: 10.1111/apt.14380 [DOI] [PubMed] [Google Scholar]
- 24.Toyoda H, Kumada T, Tada T. Imaging basis of AFP and WFA+M2BP as indicators of the risk of HCC after SVR. J Hepatol 2018; 68: 606–607. doi: 10.1016/j.jhep.2017.08.040 [DOI] [PubMed] [Google Scholar]
- 25.Matsui O, Kobayashi S, Sanada J. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 2011; 36: 264–272. doi: 10.1007/s00261-011-9685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue M, Ogasawara S, Chiba T. Presence of non-hypervascular hypointense nodules on Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2017; 32: 908–915. doi: 10.1111/jgh.13622 [DOI] [PubMed] [Google Scholar]



