Abstract
Objective: Human papillomavirus (HPV) vaccination was introduced in Japan in April 2013, as a national immunization program for girls aged 12–16 years, after an initial introduction in 2010 as a public-aid program for girls aged 13–16 years. The Yuri-Honjo district had the highest vaccine coverage among women aged 17–51 years in 2017, due to the original public-aid program. The aim of this study was to evaluate the differences in the vaccine types of HPV16/18 infections between 2008–2012 (pre-vaccine era) and 2013–2017 (vaccine era).
Materials and Methods: We evaluated whether HPV vaccination was associated with a decrease in the prevalence of HPV16/18 and high-risk HPV and the incidence of HPV-associated cervical lesions. A total of 1,342 women aged 18–49 years, covering both the pre-vaccine and vaccine eras, who visited Yuri Kumiai General Hospital and underwent HPV genotype tests from June 2008 to December 2017 were compared.
Results: Among women aged 18–24 years with higher vaccine coverage (68.2%), the prevalence of HPV16/18 and high-risk HPV decreased from 36.7% and 69.4%, respectively, in the pre-vaccine era to 5.8% and 50.0%, respectively, in the vaccine era (p=0.00013 and p=0.047, respectively). Among those with cervical intraepithelial neoplasia grade 2− and grade 2+, HPV16/18 prevalence decreased from 30.0% to 2.7% (p=0.0018) and from 81.8% to 36.4% (p=0.030), respectively. In this age group, the rate of HPV16/18 positivity decreased significantly. Among age groups with lower vaccine coverage, HPV prevalence did not significantly differ between the two eras.
Conclusion: The prevalence of HPV16/18 and high-risk HPV significantly decreased in women aged 18–24 years, most of whom were vaccinated. HPV vaccination effectively reduced the prevalence of HPV16/18 infections in the Yuri-Honjo district.
Keywords: cervical cancer, HPV16/18, high risk HPV, HPV vaccine, young women
Introduction
Two prophylactic human papillomavirus (HPV) vaccines are available in Japan. The bivalent vaccine targeting HPV16 and HPV18 (HPV16/18) was approved in October 2009, and the quadrivalent vaccine targeting HPV 6, 11, 16 and 18 was approved in July 2011. Both vaccines have been shown in clinical trials to be highly efficacious in preventing HPV infection and associated diseases1,2,3,4).
HPV vaccination was introduced into the immunization schedule in the Yuri-Honjo district—consisting of the cities of Yuri-Honjo and Nikaho in Akita Prefecture, Japan— in April 2010 for females aged 11–45 years and, as a part of the public aid program in this district, in April 2011 for females aged 13–18 years. Governmental support for nationwide HPV vaccination began in November 2010 for girls aged 13–16 years. Since April 2013, it is routinely government-funded for girls aged 12–16 years. In the Yuri-Honjo district, HPV vaccination was recommended particularly early, and the vaccine was provided to women aged 11–45 years in the first year of its introduction. The early establishment of this program, encouraging vaccination, might have led to the high vaccine coverage among younger women in this area. According to the data provided by the Health Promotion Divisions of the Yuri-Honjo and Nikaho public offices, approximately 80% of girls aged 13–18 years were vaccinated against HPV between fiscal years (FY) 2010 and 2012. Furthermore, 95.5% of all vaccinated women in this area received the third dose of the vaccination. However, some vaccine-related adverse events were reported repeatedly in the spring of 2013; thus, the rate of new vaccinations decreased to 1.5% in FY 2013 and 0.1% in FY 2014. Since June 2013, HPV vaccination was no longer mandatory; therefore, only a few girls have since received the vaccination voluntarily. Thus, the prevention of cervical cancer has been unsuccessful in this area of Japan approximately three years after the introduction of HPV vaccines.
The effectiveness of HPV vaccines is determined by comparing the prevalence of the vaccine-type HPV and/or the decrease in HPV-associated lesions among vaccinated women or a specific age group including unvaccinated women. This effectiveness can typically only be demonstrated 4–6 years after HPV vaccination5,6,7,8). From January 2008, we investigated HPV genotypes in women who showed abnormalities in cervical cytology, and from April 2012, we investigated HPV genotypes in women who had cytologic abnormalities and/or were positive for high risk (HR) HPV, as determined by the HC2 HPV DNA test. We noticed declining trends in HPV prevalence in accordance with the type of vaccine used among women in their early twenties visiting the Yuri Kumiai General Hospital. In 2017, HPV vaccine coverage was higher in women in their early twenties but was offered to women up to the age of 51 years in the Yuri-Honjo District, Akita Prefecture, Japan. Moreover, 3-dose vaccine coverage was provided to most of the vaccinated women. We examined the reduction in the prevalence of the strains HPV16 and 18, which were both targeted using two vaccines within the first seven years of vaccine introduction. The aim of this study was to evaluate the differences in the prevalence of HR HPV and HPV16/18, and the incidence of HPV-associated cervical lesions between 2008–2012 (pre-vaccine era) and 2013–2017 (vaccine era).
Material and Methods
Study design
This observational cohort study involved women with abnormal cytologic findings and/or positive HC2 tests, with the aim of determining the differences in the prevalence of HR HPV and HPV16/18, and the incidence of HPV-associated cervical lesions between the pre-vaccine and vaccine eras.
Setting
We monitored HPV genotype patterns for women who showed abnormal cervical cytology results at the outpatient clinic of the Department of Obstetrics and Gynecology, Yuri Kumiai General Hospital, starting in June 2008. As cytology-based cancer-screening programs and the HC2 test were implemented originally through public aid in April 2012 for women aged 20–49 years in the Yuri-Honjo district, we started to investigate the individual HPV genotypes for women who had positive HC2 tests but who showed no signs of cytologic abnormalities. In this area, both HPV vaccination and screening using the HC2 test were introduced particularly early.
The period of 2008–2012 was considered the pre-vaccine era because HPV vaccination was offered to women aged 20 years or older only after 2013, at which point a cancer-screening program was implemented for girls aged ≥20 years. The vaccine era represented the time during which the impact of the HPV vaccination program might be measurable (2013–2017). The age group with the highest vaccination rate would reach its highest age (23 years) in 2017. When 24-year-old women undergo vaccination voluntarily, the rate of vaccination is expected to be higher than that shown in Table 1.
Table 1. Rate of vaccination according to age group in 2017 based on the data obtained from the Health Promotion Division of the Yuri-Honjo and Nikaho public offices.
| Age | Birth year (FY) | Overall (n) | Vaccinated (n) | Vaccination rate (%) | Full dose (n) | Full dose rate (%) |
|---|---|---|---|---|---|---|
| 17 | 2000 | 455 | 47 | 10.3 | 15 | 31.9 |
| 18 | 1999 | 510 | 407 | 79.8 | 383 | 94.1 |
| 19 | 1998 | 518 | 410 | 79.2 | 389 | 94.9 |
| 20 | 1997 | 546 | 454 | 83.2 | 433 | 95.4 |
| 21 | 1996 | 500 | 438 | 87.6 | 419 | 95.7 |
| 22 | 1995 | 536 | 416 | 77.6 | 408 | 98.1 |
| 23 | 1994 | 547 | 374 | 68.4 | 355 | 94.9 |
| 24 | 1993 | 527 | 12 | 2.3 | 11 | 91.7 |
| 18–24 | 1999–93 | 3,684 | 2,511 | 68.2 | 2,398 | 95.5 |
| 25–29 | 1992–88 | 2,337 | 58 | 2.5 | ||
| 30–34 | 1987–83 | 2,402 | 45 | 1.9 | ||
| 35–39 | 1982–78 | 2,686 | 34 | 1.3 | ||
| 40–44 | 1977–73 | 3,132 | 43 | 1.4 | ||
| 45–49 | 1972–68 | 3,023 | 36 | 1.2 | ||
Human papillomavirus vaccination rates were estimated using the results of previous vaccinations among women aged 17–49 years receiving at least one vaccine dose in Yuri-Honjo distinct, Akita prefecture, Japan (2017). Data were based on the reports from the original public aid program in this area. The human papillomavirus vaccination rates were higher in women aged 18–23 years.
Study subjects
In this study, we targeted women who visited our clinic due to abnormal cytologic findings, treatment of cervical diseases, or other gynecological reasons, or who had positive HC2 test results on routine examination. Women were considered eligible for enrollment if they were 18–49 years of age, visited our clinic from June 2008 through December 2017, provided informed consent, had cervical lesions or abnormal cytologic and/or positive HC2 test results, and underwent testing to determine the HPV genotype.
Definitions
In this study, we considered HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 as HR HPV. Cervical lesions were diagnosed by examination of tissue biopsy specimens obtained via colposcopy and/or surgically in a limited number of cases.
Study procedures
Outcome of interest
The impact of HPV vaccination was evaluated based on differences in the prevalence of HR HPV and HPV16/18, and the incidence of HPV-associated cervical lesions between the pre-vaccine and vaccine eras.
Data collection
Only samples collected during the study period at our clinic were considered for analysis.
Statistical analysis
Descriptive analyses were conducted; all categorical data were presented as frequencies and proportions. Chi squared tests for trends were used to analyze the age-related rates of HR HPV, HPV16/18, and cervical lesions. P-values < 0.05 were considered statistically significant.
Ethics
This study was approved by the ethical review board of the Yuri Kumiai General Hospital. Informed consent was obtained from all participants.
Results
There were 1,817 women aged 18–97 years with abnormal cytologic results and/or positive HC2 results who underwent HPV genotyping in our clinic with the Linear Array HPV Genotyping Assay (Roche Diagnostics)9, 10) in the period 2008–2017. We excluded 475 (26.1%) women who were older than 49 years. In total, 1,342 women aged 18–49 years residing in the catchment area from June 2008 through December 2017 were included in this study: 554 (41.3%) in the pre-vaccine era and 788 (58.7%) in the vaccine era.
In 2017, the age distribution of HPV vaccination rates, which was calculated based on the past vaccination data provided by the public office in the Yuri-Honjo district, is shown in Table 1. Among women aged 17–24 years, the vaccine coverage up to the third dose was 95.5%. The vaccination rates among women aged 18–23 years were particularly high; in 2017 79.8%, 79.2%, 83.2%, 87.6%, 77.6%, 68.4%, and 2.3% of women aged 18, 19, 20, 21, 22, 23, and 24 years, respectively, were vaccinated against HPV. For women aged 18–23 years, in whom the vaccination rate was particularly high, the vaccination rate was 79.2% (2,499/3,157). For women aged 18–24 years, the vaccination rate was 68.2% (2,511/3,684). However, among women aged 25–49 years, the HPV vaccination rates ranged between 1.2–2.5% as of 2017.
Comparison of the prevalence of HR HPV and HPV16/18 in the pre-vaccination and vaccination eras
There were significant differences in the prevalence of HR HPV among women aged 18–24 years in the pre-vaccine and vaccine eras (Table 2). The prevalence of HR HPV significantly decreased from 69.4% during the pre-vaccine era to 50.0% in the vaccine era (p=0.047). Overall, there was a 28.0% ([69.4%–50.0%]/69.4%) decline in HR HPV prevalence among women aged 18–24 years. There was no significant decrease in HR HPV prevalence among women over 24 between the pre-vaccine and vaccine eras.
Table 2. Prevalence by age group of HR HPV in women aged 18–49 years with abnormal cytologic findings and/or a positive HC2 test in the pre-vaccine and vaccine eras.
| Pre-vaccine era (2008–2012) | Vaccine era (2013–2017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group at diagnosis | Birth year (FY) | n | HR HPV positive case | Positive (%) | Age group at diagnosis | Birth year (FY) | n | HR HPV positive case | Positive (%) | |
| –24 y | 1984–1994 | 49 | 34 | 69.4% | –24 y | 1989–1999 | 52 | 26 | 50.0% | p=0.047 |
| 25–29 y | 1979–1987 | 82 | 44 | 53.7% | 25–29 y | 1984–1992 | 113 | 63 | 55.8% | ns |
| 30–34 y | 1974–1982 | 93 | 48 | 51.6% | 30–34 y | 1979–1987 | 176 | 74 | 42.0% | ns |
| 35–39 y | 1969–1977 | 118 | 46 | 39.0% | 35–39 y | 1974–1982 | 171 | 75 | 43.9% | ns |
| 40–44 y | 1964–1972 | 115 | 35 | 30.4% | 40–44 y | 1969–1977 | 148 | 55 | 37.2% | ns |
| 45–49 y | 1959–1967 | 97 | 27 | 27.8% | 45–49 y | 1964–1972 | 128 | 34 | 26.6% | ns |
| Total | 554 | 234 | 788 | 327 | ||||||
Data include vaccinated and non-vaccinated women. There was a significant difference in the prevalence of HR HPV among women aged 18–24 years in the two periods (p=0.047). HPV: human papillomavirus; HR HPV: high risk HPV; HC2: Hybrid capture 2.
Additionally, the prevalence of HPV16/18 significantly decreased from 36.7% in the pre-vaccine era to 5.8% in the vaccine era (p=0.00013) among women aged 18–24 years (Table 3). Overall, there was an 84.2% ([36.7%–5.8%]/36.7%) decline in HPV16/18 among women aged 18–24 years. No significant decrease was observed in the prevalence of HPV16/18 among women over 24 between the pre-vaccine and vaccine eras.
Table 3. Prevalence by age group of HPV16 and 18 in women aged 18–49 years with abnormal cytologic findings and/or a positive HC2 test, in the pre-vaccine and vaccine eras.
| Pre-vaccine era (2008–2012) | Vaccine era (2013–2017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group at diagnosis | Birth year (FY) | n | HPV16/18 positive case | Positive (%) | Age group at diagnosis | Birth year (FY) | n | HPV16/18 positive case | Positive (%) | |
| –24 y | 1984–1994 | 49 | 18 | 36.7% | –24 y | 1989–1999 | 52 | 3 | 5.8% | p=0.00013 |
| 25–29 y | 1979–1987 | 82 | 13 | 15.9% | 25–29 y | 1984–1992 | 113 | 20 | 17.7% | ns |
| 30–34 y | 1974–1982 | 93 | 13 | 14.0% | 30–34 y | 1979–1987 | 176 | 30 | 17.0% | ns |
| 35–39 y | 1969–1977 | 118 | 18 | 15.3% | 35–39 y | 1974–1982 | 171 | 19 | 11.1% | ns |
| 40–44 y | 1964–1972 | 115 | 12 | 10.4% | 40–44 y | 1969–1977 | 148 | 8 | 5.4% | ns |
| 45–49 y | 1959–1967 | 97 | 9 | 9.3% | 45–49 y | 1964–1972 | 128 | 5 | 3.9% | ns |
| Total | 554 | 83 | 788 | 85 | ||||||
Data include vaccinated and non-vaccinated women. There was a significant difference in the prevalence of HPV16/18 among women aged 18–24 years in both periods (p=0.00013).
Comparison of the prevalence of HR HPV excluding HPV16/18, multiple infections, and HPV genotypes among women aged 18–24 years in the pre-vaccination and vaccination eras
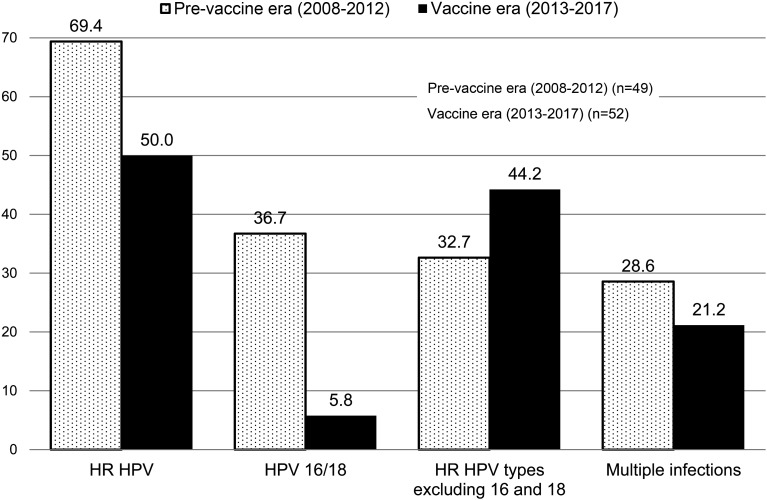
We evaluated whether the significant decrease in the prevalence of HR HPV was associated with decreased prevalence of HPV16/18 among women aged 18–24 years. The prevalence of HR HPV, excluding HPV16/18, was 32.7% and 44.2% in the pre-vaccine and vaccine eras, respectively (p=0.23). Additionally, 28.6% and 21.2% of women had multiple HPV infections in the pre-vaccine and vaccine eras, respectively, resulting in a 25.9% ([28.6%–21.2%]/28.6%) decrease in the vaccine era (p=0.38) (Figure 1).
Figure 1.
Differences in prevalence rates of HR HPV, HPV16 and 18, HR HPV excluding HPV16 and 18, and multiple infections associated with HR HPV among women aged 18–24 years between the pre-vaccine and vaccine eras.
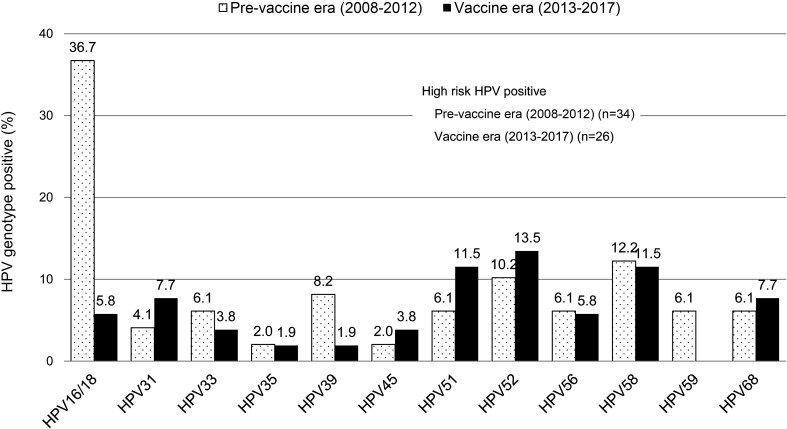
Regarding the various HPV genotypes, the most frequently detected types were HPV16/18 (36.7%) in the pre-vaccine era, and HPV52 (13.5%) in the vaccine era, followed by HPV51 (11.5%), HPV58 (11.5%), HPV31 (7.7%), HPV68 (7.7%) and HPV16/18 (5.8%). There were no significant differences in the prevalence of HR HPV types between the pre-vaccine and vaccine eras, apart from the prevalence of the vaccine-targeted HPV types 16/18 (Figure 2).
Figure 2.
Differences in the prevalence of HR HPV genotypes among women aged 18–24 years between the pre-vaccine and vaccine eras.
Comparison of the incidence of cervical lesions caused by HR HPV and HPV16/18 in the pre-vaccine and vaccine eras
Among patients with low-grade squamous cervical intraepithelial neoplasia (CIN2−), the prevalence of HR HPV and HPV16/18 decreased from 73.3% and 30.0% in the pre-vaccine era to 54.1% and 2.7% in the vaccine era among women aged 18–24 years, respectively (p=0.10 and p=0.0018, respectively). These decreased values represented a 26.2% ([73.3%–54.1%]/73.3%) and 91.0% ([30.0%–2.7%]/30.0%) decline for HR HPV and HPV16/18, respectively. No significant decrease was observed in women over the age of 24 (p>0.05).
Women aged 18–24 years with high-grade squamous cervical intraepithelial neoplasia (CIN2+) all yielded positive results for HR HPV in the pre-vaccine and vaccine eras. The prevalence of HPV16/18 significantly decreased from 81.8% to 36.4% (p=0.030), resulting in a 55.5% ([81.8%–36.4%]/81.8%) decline in the vaccine era among women in this age group (Table 4). Five women had histologically-confirmed cervical lesions, one with CIN2− and four with CIN2+, who tested positive for HPV16/18 in the vaccine era. None of them had received HPV vaccination retrospectively. No significant difference was observed in the prevalence of HR HPV and HPV16/18 in women over 24 years (p>0.05) in the pre-vaccine and vaccine eras.
Table 4. Differences in the prevalence of HR HPV and HPV16 and 18 in cervical lesions (cervical intraepithelial neoplasia grades 2− and 2+) in women aged up to 49 years between the pre-vaccine and vaccine eras.
| CIN2– | CIN2+ | ||||||
|---|---|---|---|---|---|---|---|
| 2008–2012 | 2013–2017 | 2008–2012 | 2013–2017 | ||||
| −24 y | n=30 | n=37 | n=11 | n=11 | |||
| HR | 0.733 | 0.541 | ns | 1.000 | 1.000 | ||
| HPV16/18 | 0.300 | 0.027 | p=0.0018 | 0.818 | 0.364 | p=0.030 | |
| 25−29 y | n=29 | n=55 | n=22 | n=18 | |||
| HR | 0.655 | 0.745 | ns | 0.955 | 0.944 | ns | |
| HPV16/18 | 0.103 | 0.164 | ns | 0.455 | 0.500 | ns | |
| 30−34 y | n=47 | n=72 | n=17 | n=30 | |||
| HR | 0.702 | 0.556 | ns | 0.882 | 1.000 | ns | |
| HPV16/18 | 0.191 | 0.167 | ns | 0.412 | 0.367 | ns | |
| 35−39 y | n=44 | n=64 | n=27 | n=39 | |||
| HR | 0.432 | 0.531 | ns | 0.926 | 0.923 | ns | |
| HPV16/18 | 0.068 | 0.047 | ns | 0.444 | 0.462 | ns | |
| 40−44 y | n=51 | n=58 | n=19 | n=25 | |||
| HR | 0.314 | 0.466 | ns | 0.895 | 0.960 | ns | |
| HPV16/18 | 0.078 | 0.052 | ns | 0.368 | 0.240 | ns | |
| 45−49 y | n=49 | n=52 | n=10 | n=13 | |||
| HR | 0.347 | 0.250 | ns | 0.900 | 1.000 | ns | |
| HPV16/18 | 0.102 | 0.019 | ns | 0.400 | 0.385 | ns | |
There were significant differences in the prevalence of HPV16/18 among women aged 18–24 years with cervical intraepithelial neoplasia grades 2− (CIN2−) and 2+ (CIN2+) in both periods.
Discussion
We evaluated whether the introduction of two HPV vaccines was associated with a decline in the prevalence of HPV16/18 and the incidence of HPV-associated cervical lesions in the pre-vaccine and vaccine eras. Also, we examined the impact of HPV vaccination from April 2010 through June 2013 in the Yuri-Honjo district of Akita Prefecture, Japan. During this period, the vaccine coverage extended to women aged 17–51 years until December 2017 and the HPV vaccination rate was 79.2% in girls aged 18–23 years who benefited from the original public aid program in this district. However, in Japan, the average rate of HPV vaccination with at least one dose was 70% among girls aged 13–16 years, prior to the release of reports detailing adverse events associated with HPV vaccination11). We found that the third dose was administered to 95.5% of those vaccinated for HPV, but this value declined after June 2013.
In the first seven years following the introduction of HPV vaccination, we found a decreased prevalence of HPV16/18 among younger women who visited our clinic voluntarily and underwent HPV genotyping and/or HC2 testing. We believe that the initial decline in HPV16/18 prevalence among 18–24-year-olds was associated with the introduction of intensive HPV vaccination in this area from April 2010 to June 2013. In 2017, the vaccine coverage was extended to include women aged 17–51 years in the study area, although it was lower in age groups other than 18–23 years. We compared the overall prevalence of vaccine-type HPV16/18 and HR HPV among women aged 18–49 years in the pre-vaccine and vaccine eras, since only three women aged 50 years or older were vaccinated. We further assessed the differences in multiple infections, the distribution of HPV genotypes, and the incidence of HPV-associated cervical lesions between the pre-vaccine and vaccine eras.
There was a 28.0% decrease in the prevalence of HR HPV and an 84.2% decrease in the prevalence of HPV16/18 among women aged 18–24 years in the vaccine era. There were no significant decreases among women older than 24 years in either era. Markowitz et al. similarly analyzed HPV prevalence in the pre-vaccine and vaccine eras among women aged 14–59 years within 4 years of vaccine introduction and reported that the HR vaccine-type prevalence significantly decreased, with a 50% decline among vaccinated participants aged 14–19 years. This decrease was observed despite low rates (34.1%) for the administration of at least one dose of the vaccine. Among other age groups who were not vaccinated, the prevalence did not differ significantly between the pre-vaccine and vaccine eras5). Furthermore, within six years of vaccine introduction, Markowitz et al. showed that HR vaccine-type prevalence decreased by 61% and 31% among vaccinated females aged 14–19 and 20–24 years, respectively. The vaccination rates for females aged 14–19 years who received three doses and those aged 20–24 years who received one dose were 34.6% and 18.1%, respectively6). Thus, the effectiveness of vaccination was higher in younger women who also received a greater number of vaccinations. Although we analyzed the HPV genotypes for women with abnormal cytologic findings and/or positive HC2 tests only, our results were similar to those previously reported, as we observed a significant decrease in the prevalence of HPV16/18 (HR vaccine-type) among highly vaccinated women aged 18–24 years, with no significant decreases among those aged over 24 years between the eras. The current vaccine coverage extended to those aged up to 51 years in this area, but the vaccination rates were lower, ranging between 1.2–2.5%, among those aged 25–49 years. We hypothesized a more substantial decline in HPV prevalence among women aged up to 24 years in this area, since the vaccine coverage was high in this age group, the majority of those vaccinated had received the third dose, and seven years had passed since the initiation of HPV vaccination in this population.
In Australia, Tabrizi et al. reported that, in women aged 18–24 years who had received a Papanicolaou (Pap) screening test, the prevalence of HPV16/18 and HR HPV were significantly lower in the post-vaccine than in the pre-vaccine implementation group. At least 86% of women received one dose of the vaccination in the post-vaccine implementation group. The authors also reported that the prevalence of HR HPV and vaccine-type HPV significantly decreased by 25.7% and 77.3%, respectively, in the post-vaccine compared to the pre-vaccine implementation group7). We found similar rates of HPV vaccine coverage to the study conducted in Australia; we found 28.0% and 84.2% decreases in the prevalence of HR HPV and HPV16/18 among women aged 18–24 years in the vaccine era. Among women with high HPV vaccine coverage, the prevalence of HPV16/18 was significantly lower than expected because of herd immunity acquired by HPV vaccination of all females in the vaccine era, as previous reports have shown7, 12,13,14). Furthermore, cross-protection against HPV types related to the vaccine-type HPV may be associated with decreased prevalence in HR HPV1, 12, 15, 16). Our investigation detected three women aged 18–24 years who tested positive for HPV16/18 in the vaccine era, none of whom had ever been vaccinated. In clinical studies, HPV vaccines have demonstrated close to 100% protection against vaccine-type infection and associated diseases2, 17,18,19). Our results also demonstrated the high protective effect of these vaccines, especially among young women in the vaccine era. To determine whether the decline in HPV16/18 was associated with the decreased prevalence of HR HPV, we evaluated the individual changes in the prevalence of HPV16/18 and HR HPV, excluding HPV16/18, between the two eras. Only women aged 18–24 years in the vaccine era experienced significant decreases (84.2%) in the prevalence of HPV16/18; there were no significant differences in the prevalence of HR HPV, excluding HPV16/18, in the pre-vaccine and vaccine eras (32.7% vs. 44.2%). These results indicate that, in the vaccine era, a more substantial decline in HPV16/18 reduced the overall prevalence of HR HPV among the 18–24 age group (28.0%). Onuki et al. investigated Japanese women with normal cytologic findings and showed that the prevalence of both oncogenic HPV types and HPV16/18 were highest in women aged 15–24 years, decreasing with age thereafter20). Inoue et al. also demonstrated that the age-related prevalence of HR HPV was highest in women aged 15–19 years, with a second peak in women aged 20–24 years, followed by a gradual decline in the aging population21). In another study of healthy Japanese women aged 20–25 years, HPV16/18 (10.5%) were the most frequently detected types of HPV22). These studies support the consensus that HPV vaccines are more useful if given before young women become sexually active. Among our participants in the pre-vaccine era, age-related HPV prevalence was similar to that reported in previous studies. While the prevalence of HR HPV types decreased with age among women in the pre-vaccine era and women aged 25–49 years in the vaccine era, only women aged 18–24 years in the vaccine era experienced significant decreases in the prevalence of both HPV16/18 and HR HPV. Therefore, these results indicate that the great decline in the HPV16/18 infection rate has influenced the decrease in the overall prevalence of HR HPV in the highly vaccinated group aged 18–24 years. However, among vaccinated women, there has been conflicting information regarding decreases12, 14) and increases13, 23) in the prevalence of non-vaccine type HPV. In the vaccinated group aged 18–24 years, the rate of multiple HPV infections decreased from 28.6% to 21.2% between the pre-vaccine and vaccine eras, reflecting a decline of 25.9%, which was not statistically significant. Nielsen et al. demonstrated that multiple HPV infections were highly prevalent, and that HR HPV infection was common, with HPV 16 more predominant in younger than older women24). The same study suggested that the prevalence of multiple HPV infections was significantly higher and the overall prevalence of HPV16/18 were the highest among younger women in Japan20, 21, 25). We speculated that the decrease in prevalence of vaccine types HPV16/18, though not statistically significant, has made an important contribution to the reduction in multiple infections. In Australia, after excluding HPV16/18, no significant difference was observed in the prevalence of HR HPV between the two periods. HPV52 was the type most commonly identified, followed by HPV31 and HPV58 in all women aged 18–24 years who underwent Pap smear screening, with vaccine coverage as high as 86% for the first dose in the vaccine era7). Interestingly, we found that after high-coverage vaccination, HPV52 was the type most prevalent, followed by HPV58, HPV51 and HPV31, which is comparable with the results presented in the Australian study. Cameron et al. reported a non-significant increase in HPV51 in the vaccine era with a significant decrease in HPV16/1812).
Since prophylactic HPV vaccines result in highly effective prevention of vaccine-type HPV, it is predicted that the decreased prevalence of HR HPV, including vaccine-type HPV, contributes to the overall decline in abnormal cytologic findings and HPV-associated cervical lesions. In fact, a systematic review showed that the detection rate of abnormal cytologic findings and cervical lesions was significantly decreased in all countries that introduced national HPV vaccination programs26). As HPV DNA testing is still unavailable for mass screening in Japan, cervical lesions caused by HPV infection are usually diagnosed via cytology. Ozawa et al. reported that HPV vaccination significantly reduced the incidence of abnormal cytologic findings in vaccinated women aged 20–24 years in Miyagi, Japan, with a 52.1% decline in abnormal cytologic findings compared to unvaccinated women of the same age27). This was the first report on the beneficial effects of the HPV vaccine in the prevention of cervical abnormalities after the implementation of a national immunization program in Japan. They demonstrated the effects of HPV vaccination, showing a decrease in the rate of abnormal cytologic findings in vaccinated women, but failed to mention the impact on the prevalence of vaccine-type HPV and HR HPV among these women. HPV vaccines could significantly reduce the number of young women infected with HPV; furthermore, our data suggest that they could significantly decrease the prevalence of HR HPV mainly caused by HPV16/18 in young women. We calculated HPV prevalence among women with HPV-associated cervical lesions in the pre-vaccine and vaccine eras by age group for HPV16/18 and HR HPV. Women with CIN2− in the vaccinated group aged 18–24 years showed a reduction in the prevalence of HR HPV (26.2%) in the vaccine era, although the difference did not reach statistical significance. Furthermore, we found there was a significant decline (91.0%) in HPV16/18 prevalence, which might have played an important role in decreasing the overall prevalence of HR HPV infections in the vaccinated age group. Although women with CIN2+ cervical lesions showed no difference in the prevalence of HR HPV between the two eras, a significant decline (55.5%) in HPV16/18 prevalence was observed in those aged 18–24 years in the vaccine era. Our data suggested that HR HPV caused all the CIN2+ lesions in this group, but HPV vaccination was effective in preventing HPV16/18-related CIN2+. Since the number of women with CIN2+ cervical lesions was small, these results should be interpreted with caution. In the vaccine era five women had histologically-confirmed cervical lesions, one with CIN2− and four with CIN2+, were positive for HPV16/18, and none of them had received HPV vaccination. In the USA, Hariri et al. reported that 48-months after the first vaccination, the estimated vaccine effectiveness for the prevention of HPV16/18-related CIN2+ was 72% in women aged 18–39 years who received ≥1 dose28). Additionally, Herweijer et al. showed that effectiveness against CIN2+ was 75%, 46%, and 22%, for those receiving at least one HPV vaccination dose before the age of 17 years, and between the ages of 17–19 and 20–29 years, respectively29). They concluded that the HPV vaccine was effective in preventing CIN2+ lesions among girls who were younger when receiving the first dose. Similarly, four years after the implementation of HPV vaccination, it conferred statistically significant protection against cervical abnormalities, including low-grade lesions in women younger at the first dose; vaccine effectiveness also increased according to the number of doses received30). In Denmark, after HPV vaccination, the incidence of cytological atypia decreased significantly in women younger than 18 years (33.4% decrease) and in those aged 18–20 years (12.6% decrease)31). These studies showed that HPV vaccination at younger ages, receipt of all three doses, and high vaccine coverage were more effective in preventing HPV16/18-related cervical lesions. In the study area, girls aged 13–18 years at vaccination had high completion rates of the third dose of the vaccine (79.2%); thus, it is expected that the number of women with cervical lesions will greatly decrease with higher effectiveness of HPV vaccines26, 29,30,31). Furthermore, data regarding cross-protection against HPV31, 33, 45, and 52 have suggested added protection against cervical lesions resulting from HPV types which cannot be vaccinated against15,16,17,18).
Our study has several limitations. Since the participants are women who had some gynecological abnormalities and visited our clinic, we cannot directly demonstrate HPV vaccine effectiveness among vaccinated women. As the individual vaccination status of reported cases is unknown, we cannot mention the vaccination rate of women aged 18–24 years in the vaccine era including unvaccinated women. However, our data clearly suggested that in young women aged 18–24 years in the vaccine era, HPV vaccines contributed to the tremendous decreases in HPV16/18 infection and HPV16/18-related cervical lesions. HPV16/18 infection is a significant risk factor for the progression of cervical cancer in young women32), is highly prevalent among younger women, and more dangerous than other oncogenic types33, 34). Currently, HPV vaccines are essential for minimizing the early occurrence of cervical cancer in younger women, as they result in a substantially decreased incidence of HPV16/18 infection.
Conclusion
We found a substantial and significant decrease in the prevalence of HPV16/18 among highly vaccinated young women, irrespective of vaccination status; this decrease was also confirmed among cases with CIN2−. Only unvaccinated women were infected with HPV16/18 in the vaccine era. These data suggest that HPV vaccination in Japan was effective in reducing the prevalence of HPV16/18, which have the highest oncogenic potential for cervical cancer in young women. Since the vaccine is more effective in girls who had not engaged in sexual activity and were not infected with HPV16/18, efforts should be made to prevent HPV16/18 infection by resuming the vaccination program especially targeting this age group.
Conflict of Interest
The authors have no conflicts of interest.
Acknowledgment
We would like to thank Kenji Kikuchi, MD and Naoki Kon, MD for assisting with the introduction of HPV vaccination and HPV DNA testing in the Yuri-Honjo district of Akita prefecture, and Naoko Kimura, MD for help regarding statistical management.
References
- 1.Paavonen J, Naud P, Salmerón J. HPV PATRICIA Study GroupEfficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301–314. doi: 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 2.FUTURE II Study GroupQuadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915–1927. doi: 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- 3.Descamps D, Hardt K, Spiessens B. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5: 332–340. doi: 10.4161/hv.5.5.7211 [DOI] [PubMed] [Google Scholar]
- 4.Villa LL, Costa RL, Petta CA. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–278. doi: 10.1016/S1470-2045(05)70101-7 [DOI] [PubMed] [Google Scholar]
- 5.Markowitz LE, Hariri S, Lin C. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013; 208: 385–393. doi: 10.1093/infdis/jit192 [DOI] [PubMed] [Google Scholar]
- 6.Markowitz LE, Liu G, Hariri S. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137: e20151968. doi: 10.1542/peds.2015-1968 [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi SN, Brotherton JM, Kaldor JM. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis 2014; 14: 958–966. doi: 10.1016/S1473-3099(14)70841-2 [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J. Long-term cervical cancer prevention strategies across the globe. Gynecol Oncol 2010; 117(Suppl): S11–S14. doi: 10.1016/j.ygyno.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 9.Stevens MP, Garland SM, Tabrizi SN. Human papillomavirus genotyping using a modified linear array detection protocol. J Virol Methods 2006; 135: 124–126. doi: 10.1016/j.jviromet.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Stevens MP, Garland SM, Tabrizi SN. Validation of an automated detection platform for use with the roche linear array human papillomavirus genotyping test. J Clin Microbiol 2008; 46: 3813–3816. doi: 10.1128/JCM.01169-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda Y, Enomoto T, Sekine M. Japan’s failure to vaccinate girls against human papillomavirus. Am J Obstet Gynecol 2015; 212: 405–406. doi: 10.1016/j.ajog.2014.11.037 [DOI] [PubMed] [Google Scholar]
- 12.Cameron RL, Kavanagh K, Pan J. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 2016; 22: 56–64. doi: 10.3201/eid2201.150736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JA, Brown DR, Ding L. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics 2012; 130: e249–e256. doi: 10.1542/peds.2011-3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabrizi SN, Brotherton JM, Kaldor JM. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis 2012; 206: 1645–1651. doi: 10.1093/infdis/jis590 [DOI] [PubMed] [Google Scholar]
- 15.Wheeler CM, Kjaer SK, Sigurdsson K. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis 2009; 199: 936–944. doi: 10.1086/597309 [DOI] [PubMed] [Google Scholar]
- 16.Brown DR, Kjaer SK, Sigurdsson K. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009; 199: 926–935. doi: 10.1086/597307 [DOI] [PubMed] [Google Scholar]
- 17.Harper DM, Franco EL, Wheeler CM. HPV Vaccine Study groupSustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–1255. doi: 10.1016/S0140-6736(06)68439-0 [DOI] [PubMed] [Google Scholar]
- 18.Paavonen J, Jenkins D, Bosch FX. HPV PATRICIA study groupEfficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161–2170. doi: 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 19.Muñoz N, Kjaer SK, Sigurdsson K. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102: 325–339. doi: 10.1093/jnci/djp534 [DOI] [PubMed] [Google Scholar]
- 20.Onuki M, Matsumoto K, Satoh T. Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer. Cancer Sci 2009; 100: 1312–1316. doi: 10.1111/j.1349-7006.2009.01161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue M, Sakaguchi J, Sasagawa T. The evaluation of human papillomavirus DNA testing in primary screening for cervical lesions in a large Japanese population. Int J Gynecol Cancer 2006; 16: 1007–1013. doi: 10.1111/j.1525-1438.2006.00460.x [DOI] [PubMed] [Google Scholar]
- 22.Konno R, Tamura S, Dobbelaere K. Prevalence and type distribution of human papillomavirus in healthy Japanese women aged 20 to 25 years old enrolled in a clinical study. Cancer Sci 2011; 102: 877–882. doi: 10.1111/j.1349-7006.2011.01878.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20–26 years). Hum Vaccin Immunother 2015; 11: 2337–2344. doi: 10.1080/21645515.2015.1066948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen A, Kjaer SK, Munk C. Type-specific HPV infection and multiple HPV types: prevalence and risk factor profile in nearly 12,000 younger and older Danish women. Sex Transm Dis 2008; 35: 276–282. doi: 10.1097/OLQ.0b013e31815ac5c7 [DOI] [PubMed] [Google Scholar]
- 25.Sasagawa T, Maehama T, Ideta K. Fujiko Itoh J-HERS Study GroupPopulation-based study for human papillomavirus (HPV) infection in young women in Japan: A multicenter study by the Japanese human papillomavirus disease education research survey group (J-HERS). J Med Virol 2016; 88: 324–335. doi: 10.1002/jmv.24323 [DOI] [PubMed] [Google Scholar]
- 26.Garland SM, Kjaer SK, Muñoz N. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016; 63: 519–527. doi: 10.1093/cid/ciw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa N, Ito K, Tase T. Beneficial effects of human papillomavirus vaccine for prevention of cervical abnormalities in Miyagi, Japan. Tohoku J Exp Med 2016; 240: 147–151. doi: 10.1620/tjem.240.147 [DOI] [PubMed] [Google Scholar]
- 28.Hariri S, Bennett NM, Niccolai LM. HPV-IMPACT Working GroupReduction in HPV 16/18—associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine 2015; 33: 1608–1613. doi: 10.1016/j.vaccine.2015.01.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herweijer E, Sundström K, Ploner A. Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: A population-based study. Int J Cancer 2016; 138: 2867–2874. doi: 10.1002/ijc.30035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowe E, Pandeya N, Brotherton JM. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348: g1458. doi: 10.1136/bmj.g1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldur-Felskov B, Dehlendorff C, Junge J. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control 2014; 25: 915–922. doi: 10.1007/s10552-014-0392-4 [DOI] [PubMed] [Google Scholar]
- 32.Karube A, Sasaki M, Tanaka H. Human papilloma virus type 16 infection and the early onset of cervical cancer. Biochem Biophys Res Commun 2004; 323: 621–624. doi: 10.1016/j.bbrc.2004.08.142 [DOI] [PubMed] [Google Scholar]
- 33.Khan MJ, Castle PE, Lorincz AT. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97: 1072–1079. doi: 10.1093/jnci/dji187 [DOI] [PubMed] [Google Scholar]
- 34.Vinokurova S, Wentzensen N, Kraus I. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res 2008; 68: 307–313. doi: 10.1158/0008-5472.CAN-07-2754 [DOI] [PubMed] [Google Scholar]