Abstract
A keloid (KD) is a benign dermal fibrotic tumor. Treatment of KDs is challenging and the recurrence rate is high; thus, there is an unmet need to explore new target sites and new treatment methods. As a tumor-associated cytokine, autocrine motility factor (AMF) can effectively stimulate the random and directional movement of cells. We first found that AMF was overexpressed in keloid fibroblasts (KFs) and the proliferation and migration of KFs were promoted by AMF stimulation. After treatment with Y-27632, RhoA kinase inhibitor, the proliferation and migration capacity of KFs declined significantly, and type I collagen protein, active RhoA and ROCK1 also were downregulated. In addition, a KD transplantation model was established under the skin of nude mice, with KD intramural injection AMF siRNA, we found that the weight of the KD was smaller than in the control group (P < 0.05), KD tissue sections stained by HE and Masson showed that fibers became loose and the blood vessels were visibly reduced. In conclusion, AMF siRNA is expected to be a novel strategy to treat KD by inhibiting signaling pathway of RhoA/ROCK1.
keywords: Autocrine motility factor, Keloid, RhoA, SIRNA, Treatment
Introduction
Keloid (KD) is a common pathological scar caused by skin aberrant wound healing that usually grows exceeding original skin trauma and without self-limitation. The main characteristics of KD include excessive histologic proliferation and deposition,1, 2, 3 “crab feet” appearance in clinic, and accompanying itching and pain. Severe cases can be followed by ulcers and even scar cancer.4, 5 KDs can seriously affect a patient's appearance and quality of life.6, 7 Currently, the treatment methods for KD include surgery, radiotherapy, laser therapy, and glucocorticoid.5 Because the pathogenesis of KD is not clear, the treatment effects are often not ideal and easily recur8; therefore, it is imperative to explore the pathogenesis of KD and find new treatment strategies.
Autocrine motility factor (AMF), a tumor-associated cytokine, is a polypeptide produced and secreted by tumor cells.9 Many studies demonstrated that AMF was overexpressed in serum of many malignant tumors, such as melanoma, liver cancer, gastric cancer, and breast cancer.10, 11, 12 AMF can be combined with a receptor transmembrane glycoprotein, AMFR, in the cell membrane to stimulate cell randomization and directional movement,13 and also can promote the proliferation and metastasis of tumor cells and the formation of new blood vessels.14
The migration of cells is related to the reconstruction of the cytokine skeleton, and studies indicated that RhoA was involved in the reconstruction of actin skeleton in the cell.15 RhoA/ROCK1 signal is involved in the regulation of angiogenesis, and the combination of the activated RhoA and its effector molecule ROCK1 can induce cell migration.16 Activated RhoA also can promote the synthesis of stress fibers.17 Y-27632, RhoA kinase inhibitor, can inhibit the activation translocation of Rho through covalent modification for Rho, and then a series of biological behaviors were mediated by the Rho/ROCK signaling pathway.18
The RNA interference (RNAi) technique is to pair double-stranded RNA (dsRNA) with homologous mRNA through base complementary pairing principles, induced nuclease degrading mRNA at binding site, and quickly blocking the corresponding gene expression.19 Its high specificity, efficiency, selectivity, transmissibility, and heritability, as well as high stability, make RNAi technology a powerful tool for gene silencing.20, 21, 22, 23
In this study, we found that AMF was overexpressed in human KD tissue, and can promote the proliferation and migration of KD fibroblasts and the formation of collagen in KD tissue. We injected the lentivirus loading AMF siRNA into the subcutaneous KD tissue of the nude mice, and found that it can effectively inhibit the growth of KD. In conclusion, AMF can be used as a new target for KD treatment, and AMF siRNA is expected to be a new strategy for treatment of KD through inhibiting the RhoA/ROCK1 signaling pathway.
Materials and methods
Samples and ethical approval of the study protocol
KD samples (10 cases, 16–36 years old), hypertrophic scar (HS) samples (10 cases, 21–48 years old) and foreskin (FS) samples (10 cases, 7–19 years old) were obtained from the Second Affiliated Hospital of Chongqing Medical University. The samples had no infection or ulcer (Supplementary Material, table), and informed consents were signed. This study was approved by ethical committee of the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China).
Tissue immunofluorescent staining
The human KD tissues, HS tissues, and FS tissues were taken out of liquid nitrogen and placed in frozen section, fixed with acetone for 30 min, washed with PBS for 3 min, blocked with 10% goat serum for 30 min at room temperature, and excess serum removed. Next, the tissues were incubated with primary antibodies against AMF monoclonal antibody (1:100, Abcam) at 4 °C overnight, and washed with PBS 3 times for 10 min each. Slices were incubated FITC-conjugated secondary antibodies (1:200, Abcam) for 1 h at 37 °C in a wet box away from light, then washed with PBS 3 times for 5 min each. The slices were examined using an inverted fluorescence microscope (Olympus, IX53, Japan).
Primary cell culture and animal model
Fibroblasts from KD, HS, and FS were isolated and cultured as described previously.24 Culturing was maintained in DMEM (Gibco, USA) containing FBS (10% v/v), penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37 °C in a humidified incubator in an atmosphere of 5% CO2. Cells were subcultured with 0.25% trypsin when reaching 80%–90% confluence and used at passages 3 to 8 for experiments. The cell numbers for all the experiments were determined using a hemocytometer.
All nude mice were pathogen free and allowed access to food and water ad libitum. Animal experiments were carried out with the permission of the Animal Ethical Commission of Chongqing Medical University. The KD tissues were cut into small pieces (∼5 mm × 5 mm × 5 mm). The nude mice were anesthetized with 1% pentobarbital, and the lower right shoulder blade skin was sterilized with alcohol and a 5-mm incision made. KD tissue was implanted under the skin, then the incision was sewn up. KD-bearing nude mice were allowed to grow under standard conditions for 16 days after KD tissue implantation and used for subsequent experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
To detect the expression of AMF mRNA in keloid fibroblasts (KFs), hypertrophic scar fibroblasts (HFs), and normal epidermal fibroblasts (NFs), total RNA was extracted using Trizol reagent (TaKaRa, Japan), and qRT-PCR was employed using a Prime Script Reverse-Transcription Reagent Kit (Ta Ka Ra, Japan) according to the manufacturer's instructions with the amplifying primer pair for AMF (5′- GCGTCTGGTATGTCTCCAACA-3′ and 5′-TGGTCTCCTGGGTAGTAAAGGTC-3′), and GAPDH (5′-CTTTGGTATCGTGGAAGGACTC-3′ and 5′-GTAGAGGCAGGGATGATGTTCT-3′) served as an internal reference. The complementary DNA was amplified with the SYBR Premix Ex TaqTM Kit (Ta Ka Ra). The PCR amplification cycles were programmed for 3 min at 95 °C, followed by 40 cycles at 95 °C for 10 s and 30 s at 55 °C. The change in the expression of the target gene (AMF) was calculated relative to the mean critical threshold (CT) values of the GAPDH gene.
SDS-PAGE and western blotting analysis
Western blotting analysis was employed as described previously.9 KFs, HFs, and NFs were digested and collected, the protein lysates were separated either on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) separating gels and 4% stacking gels, and subsequently transferred to polyvinylidene difluoride (PVDF) membrane. After blocking with 5% defatted milk in PBS containing 0.1% PBS-T, the membranes were incubated overnight at 4 °C with primary antibodies (antibodies against AMF, Abcam, USA) and the corresponding horseradish peroxidase-conjugated secondary antibodies (Abcam, USA) at room temperature for 1 h. The protein bands were detected by enhanced chemiluminescence reagent. β-actin was used as internal control.
Cell proliferation assay
The stimulation proliferation effect of AMF on KFs was assessed by the CCK-8 assay. KFs were seeded into 96-well plates in triplicate at a density of 4000 cells and cultured overnight. To evaluate the stimulation effect of different concentrations (0, 1, 10, 20, and 30 ng/mL) of AMF (Genway, USA) on KFs, the control group received no stimulation, and other groups were stimulated by 50 μL corresponding AMF solution for 24 h. In addition, to evaluate the stimulation effect of the same concentration of AMF at different time points (0, 12, 24, 48, and 72 h) on KFs, each group was stimulated with 50 μL 10 ng/mL AMF. Twenty-four hours after stimulation, 10 μL of CCK-8 reagent (Dojindo, Kumamoto, Japan) was added into each well and incubated at 37 °C for 2 h. The optical density was read at 450 nm in a microplate reader (Thermo Fisher Scientific, USA).
Transwell invasion assay
The KFs invasion assay was employed with Transwell chamber (Corning, USA) with Matrigel, and Matrigel transwell chambers were inserted in 24-well plates. After stimulation with different concentrations of AMF (0, 10, and 20 ng/mL), 100 μL corresponding KFs suspension (1 × 106/mL) was added into the upper chamber, 600 μL DMEM containing 10% FBS was added into the lower chamber, and then the suspensions were incubated at 37 °C in a humidified incubator in an atmosphere of 5% CO2 for 36 h. After fixation with formaldehyde for 15 min and staining with crystal violet, the numbers of invaded cells were counted in 5 randomly selected fields under microscope.
The effect of AMF on collagen I and RhoA/ROCK1 signal in human KFs
To clarify the effect of AMF on collagen and RhoA/ROCK1 signal in human KFs, KFs were seeded into a 60-mm petri dish, the cells in exponential phase were divided into three groups: control, 10 ng/mL, and 20 ng/mL. The control group was added to PBS, the other groups were added to 1 mL corresponding concentration AMF. The KFs were treated for 36 h and collected. Western blotting was used to detect collagen I, total RhoA, active RhoA, and ROCK1 in each group.
Inhibiting the effect of RhoA/ROCK1 signaling pathway on the biological behavior of KFs induced by AMF
The role of RhoA/ROCK1 signaling pathway in the proliferation, migration, synthesis of collagen I, total RhoA, active RhoA, and ROCK1 of KFs was explored. The cells were seeded at a certain concentration into 96-well plates, Transwell chamber, and 60-mm Petri dishes. The KFs were divided four groups: control, AMF, Y-27632, and AMF + Y-27632 group. KFs in the control group were cultured with DMEM; KFs in AMF group were treated with DMEM containing 10 ng/mL AMF for 24 h; KFs in the Y-27632 group were treated with DMEM containing Y-27632 for 1 h; KFs in the AMF + Y-27632 group were pretreated by Y-27632 for 1 h, and then treated with DMEM containing 10 ng/mL AMF for 24 h. Each group was washed with PBS three times for 3 min each; then, CCK-8 was used to detect the proliferation, Transwell assay was used to detect the cell migration, and western blotting was employed to detect type I collagen protein, total RhoA, active RhoA, and ROCK1.
Therapeutic effect in vivo
To evaluate the therapeutic effect of AMF siRNA on KD in nude mice, the KD-bearing mice were distributed to two groups at random (n = 6): AMF siRNA group and PBS control group. In the AMF siRNA group, 50 μL AMF siRNA with 0.8 μg/mL was injected into KD tissue, and the PBS control group received an injection of 50 μL PBS. The aforementioned treatment was once every 3 days, and after 25 days, all mice were killed and KD tissue was excised, the general morphology was observed, and weight was measured. The paraffin tissue sections were stained with HE and Masson, respectively.
Statistical analyses
Data are shown as mean ± standard deviation, and were analyzed using SPSS 19.0 software. One-way ANOVA and Student's t-test were utilized for statistical evaluation, SNK-q test was used for further comparison. Differences were considered significant at P < 0.05.
Results
The expression of AMF
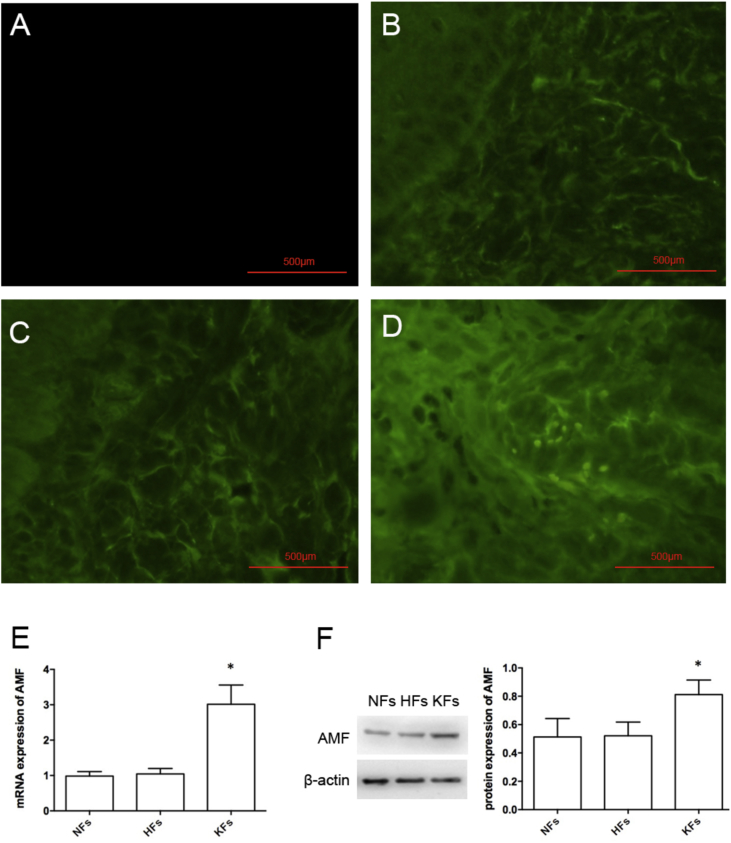
The expression of AMF in normal epidermal tissue, hypertrophic scar, and KD were detected by tissue immunofluorescence staining, and the results showed that PBS-replaced primary antibody negative control group had no stain (Fig. 1A), and the stain was weak both in normal epidermal tissue and hypertrophic scar (Fig. 1B and C); however, the stain in KD was stronger than in other groups (Fig. 1D). In addition, the mRNA and protein of AMF in NFs, HFs, and KFs were detected by qRT-PCR and western blotting, respectively, and the difference in the expression of mRNA and protein of AMF in NFs and HFs groups was not statistically significant (P > 0.05); however, the expression of mRNA and protein of AMF in KFs groups was higher than in other groups, and the difference showed statistical significance (P < 0.05) (Fig. 1E and F).
Figure 1.
The expression of AMF. The tissue immunofluorescence staining in the PBS negative control group (A), normal epidermal tissue (B), hypertrophic scar (C) and KD (D). Scale bars: 500 μm. The mRNA (E) and protein (F) expression of NFs, HFs, and KFs. *P < 0.05.
AMF stimulation promoted proliferation and migration of KFs, synthesis of type I collagen, and upregulation of RhoA/ROCK1 signaling pathway
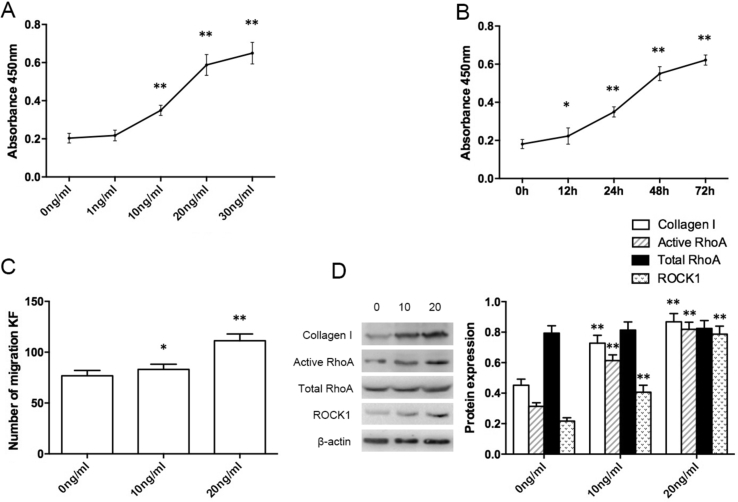
After stimulation with different concentrations of AMF, the proliferation of KFs was detected by CCK-8. The absorbance value was increased in the 1 ng/mL group, but compared with control group, the difference was not statistically significant (P > 0.05), in the 10 ng/mL group, the absorbance value was significantly increased (P < 0.01), with increasing of concentration (20 and 30 ng/mL) of AMF, the absorbance value increased more significantly (Fig. 2A). In addition, the proliferation of KFs was observed at 0, 12, 24, 48, and 72 h after using 10 ng/mL AMF to stimulate KFs. The results showed that with the extension of time, the absorbance value increased, and compared with the control group, the absorbance value in the 12 h group was increased and the difference was statistically significant (P < 0.05) (Fig. 2B). The results of migration assay showed that the number of KFs under transwell chamber in AMF stimulation groups was significantly increased, and with increasing of concentration of AMF, the number of KFs was increased, and the difference was statistically significant (Fig. 2C). After stimulating with different concentrations of AMF for 36 h, compared with blank control group without AMF stimulation, the total RhoA protein in the 10 ng/mL and 20 ng/mL groups was not statistically significant (P > 0.05), but the expression of active RhoA and its downstream signal molecule ROCK1 were significantly increased (P < 0.01), and increased with increasing concentration of AMF (Fig. 2D).
Figure 2.
The effect of the stimulation with different concentrations of AMF at different times on KFs. The absorbance value of KFs after stimulation with different concentration (A) and different time points (B), the migration of KFs (C), and the expression of the protein of collagen I, total RhoA, active RhoA, and ROCK1 (D). *P < 0.05, **P < 0.01.
The effect of inhibition of RhoA/ROCK1 signaling pathway on the biological behavior of KFs induced by AMF
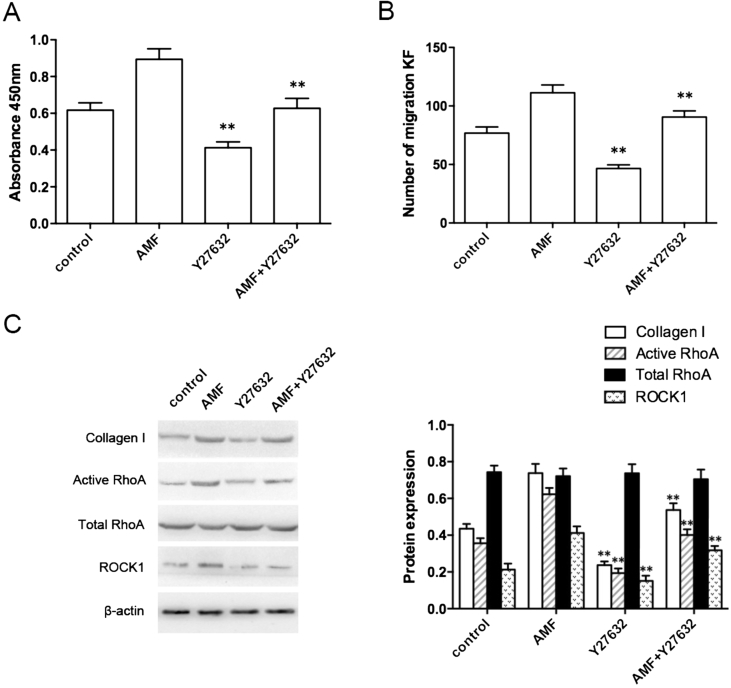
After pretreatment with Y-27632, compared with the control group, the absorbance value (Fig. 3A), the number of migration KFs (Fig. 3B), and the expression of type I collagen, active RhoA, and ROCK1 in the Y-27632 group were sharply decreased (Fig. 3C), and the difference was statistically significant (P < 0.01). Compared with the AMF group, the absorbance value, the number of KFs, and the expression of type I collagen, active RhoA, and ROCK1 in Y-27632 + AMF group were significantly decreased, and the difference was statistically significant (P < 0.01).
Figure 3.
Effect of RhoA/ROCK1 signaling pathway on the biological behavior of KFs challenged with Y-27632. The absorbance value (A), the number of migration KFs (B), and the expression of collagen I, active RhoA, total RhoA, and ROCK1 (C). **P < 0.01.
Therapeutic effect in vivo
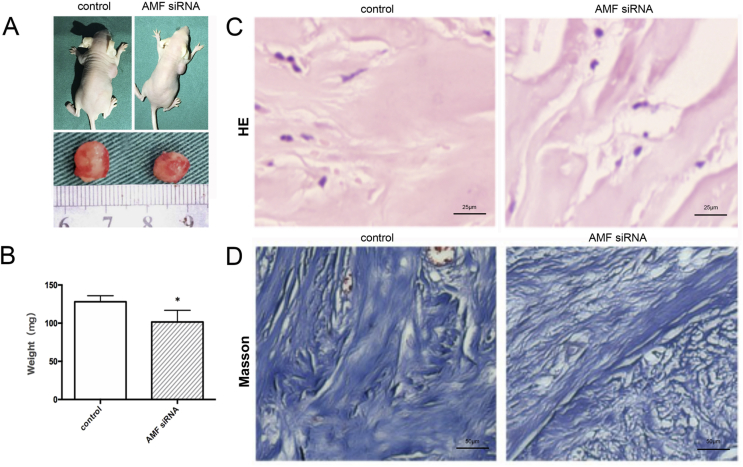
Finally, we tested the in vivo therapeutic effect of AMF siRNA, and the effect of AMF siRNA inhibited KD growth significantly, the nude mice were killed and KD was isolated, and the surface of KD tissue was covered by a thin film, which can be seen in the interlaced network of small vessels (Fig. 4A). The weight of KD tissue in the AMF siRNA group was 101.6 ± 5.2 mg, and it was 128.1 ± 7.9 mg in the PBS control group; the difference was statistically significant (P < 0.05) (Fig. 4B). In the PBS control group, HE staining showed tissue with wide, eosinophilic, refractory homogeneous lamellar collagen fiber deposition, closely arranged disordered arrangement, nodular or spiral in shape, and rich in capillaries; however, in the AMF siRNA group, the flaky collagen fibers became thinner, less dense, nodular, and swirled, and decreased microvessels (Fig. 4C). Masson staining indicated that a large amount of collagen in tissue sections in the two groups was dyed blue, and the blue collagen clusters in the PBS control group was in a coarse, disordered arrangement, with multiple visible blood vessels; in the AMF siRNA group, the collagen was relatively fine, with loose arrangement and few blood vessels (Fig. 4D).
Figure 4.
The therapeutic effect of AMF siRNA on KD in nude mice. KD-bearing mice and KD specimens (A), the weight of keloid (B), HE staining, Scale bars: 25 μm (C) and Masson staining, Scale bars: 50 μm (D) at the end of different treatments. *P < 0.05.
Discussion
KD is a common disease in plastic surgery that has a long course and difficulty healing. Patients often experienced pain and itching as the main symptoms [5]. KD often affected facial or limbs appearance or caused dysfunction in the joints and other parts of the body, which seriously affected quality of life.25 The growth of KD without self-limitation is a process of the migration of fibroblasts from the original growth site to normal skin.26 The main features of KFs are excessive proliferation, antiapoptosis ability, and excessive synthesis and deposition of collagen.27, 28, 29 KDs have a rich blood supply and are associated with tumor-related genes and cytokines such as TGF-β, VEGF, and CTGF, and its pathogenesis has gene regulation similar to that of tumor; therefore, KD has biological characteristics of malignant tumor.25 The pathogenesis of KD is not clear30; however, the clinical treatment of KD cannot be cured completely, and the recurrence rate remains high.30, 31
AMF was originally isolated from human melanoma cell lines, elevated in the body fluids of patients with multiple malignancies. AMF is closely related to high transfer characteristics of various cells,32 and can stimulate cell movement effectively. In this study, the results indicated that the expression of AMF in KFs was significantly higher than that of NFs and HFs, and the stimulating proliferation effect of AMF on KFs was concentration-dependent and time-dependent, with the increase of concentration of AMF and prolonging of stimulation time, the proliferation ability of KFs became stronger and stronger; in addition, AMF can also effectively promote the migration of KFs. Hence, AMF is expected to be a potential target for treatment of KD.
RhoA is one of the members of the family of guanosine triphosphate and plays an important role in cell proliferation and skeleton rearrangement.33 The previous study indicated that AMF stimulated the melanoma cell A375, which could significantly increase the activity of RhoA, with the downstream molecule ROCK1 increasing9; after RhoA inhibitors, the activity of RhoA was significantly inhibited. This study demonstrated that the proliferation and migration of KFs were significantly inhibited through Y-27632 treating KFs, which indicated that RhoA/ROCK1 signaling pathway played an important role in proliferation and migration of KFs. In addition, compared with the Y-27632 + AMF group, the Y-27632 group showed stronger inhibition migration ability, which further declared that AMF was a promotion factor in the progression of KFs.
Gene therapy is a novel and tremendous potential method of researching and treating disease. RNAi, one of the most effective gene silencing technology methods,23, 34 has attractive characteristics including high efficiency, specificity, position effect, and transmissibility. Lentiviral vector, an emerging viral vector, has strong carrying capacity, high transfection efficiency, and no immunogenicity.35, 36 In this study, AMF mRNA was as a target sequence, AMF siRNA was designed, and lentiviral vector was selected as the carrier to import AMF siRNA into KFs. We found that AMF siRNA could effectively inhibit the growth of subcutaneous KD in nude mice.
In this study, we first revealed that AMF is an activator of RhoA, and speculate that the RhoA/ROCK1 signaling pathway plays an important role in the occurrence and progression of KD, and AMF siRNA, through silencing AMF gene, can effectively inhibit expression of AMF in KD, and finally inhibit the progression of KD. In conclusion, AMF siRNA is expected to be a novel strategy for treatment of KD through inhibiting the RhoA/ROCK1 signaling pathway.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81071596).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.gendis.2018.05.002.
Contributor Information
Yuan Cheng, Email: 892697696@qq.com.
Hongyun Zhao, Email: zhaohongyunchina@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Tuan T.L., Zhu J.Y., Sun B. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol. 1996;106(5):1007–1011. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- 2.Slemp A.E., Kirschner R.E. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18(4):396–402. doi: 10.1097/01.mop.0000236389.41462.ef. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R. Keloids as a serious disease such as malignancy. Plast Reconstr Surg. 2008;122(3):993–994. doi: 10.1097/PRS.0b013e31818120a0. [DOI] [PubMed] [Google Scholar]
- 4.Yao X., Cui X., Wu X. Tumor suppressive role of miR-1224-5p in keloid proliferation, apoptosis and invasion via the TGF-beta 1/Smad 3 signaling pathway. Biochem Biophys Res Commun. 2018;495(1):713–720. doi: 10.1016/j.bbrc.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 5.Taheri A., Molaei H., Aghili M. Outcomes of surgical excision and brachytherapy in intractable keloids. World J Plast Surg. 2017;6(3):280–284. [PMC free article] [PubMed] [Google Scholar]
- 6.Bijlard E., Timman R., Verduijn G.M. Intralesional cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid treatment: randomised controlled trials. J Plast Reconstr Aesthetic Surg. 2018;71(6):847–856. doi: 10.1016/j.bjps.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Ud-Din S., Bayat A. Strategic management of keloid disease in ethnic skin: a structured approach supported by the emerging literature. Br J Dermatol. 2013;3(169 suppl):71–81. doi: 10.1111/bjd.12588. [DOI] [PubMed] [Google Scholar]
- 8.Al-Attar A., Mess S., Thomassen J.M. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi S., Gupta S.K., Hogan V. Activation of small GTPase Rho is required for autocrine motility factor signaling. Cancer Res. 2002;62(15):4484–4490. [PubMed] [Google Scholar]
- 10.Liotta L.A., Mandler R., Murano G. Tumor cell autocrine motility factor. Proc Natl Acad Sci USA. 1986;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endo K., Shirai A., Furukawa M. Prognostic value of cell motility activation factors in patients with tongue squamous cell carcinoma. Hum Pathol. 2006;37(8):1111–1116. doi: 10.1016/j.humpath.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W.G., Raz A., Douglas-Jones A. Expression of autocrine motility factor (AMF) and its receptor, AMFR, in human breast cancer. J Histochem Cytochem. 2006;54(2):231–241. doi: 10.1369/jhc.5A6785.2005. [DOI] [PubMed] [Google Scholar]
- 13.Wang W., Yang L.Y., Yang Z.L. Elevated expression of autocrine motility factor receptor correlates with overexpression of RhoC and indicates poor prognosis in hepatocellular carcinoma. Dig Dis Sci. 2007;52(3):770–775. doi: 10.1007/s10620-006-9479-4. [DOI] [PubMed] [Google Scholar]
- 14.Funasaka T., Yanagawa T., Hogan V. Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. FASEB J. 2005;19(11):1422–1430. doi: 10.1096/fj.05-3699com. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T., Nishiura D., Wu R.C. Nuclear Rho kinase, Rock2, targets p300 acetyltransferase. J Biol Chem. 2006;281(22):15320–15329. doi: 10.1074/jbc.M510954200. [DOI] [PubMed] [Google Scholar]
- 16.Wang H., Eto M., Steers W.D. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277(34):30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 17.van Nieuw Amerongen G.P., Koolwijk P., Versteilen A. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol. 2003;23(2):211–217. doi: 10.1161/01.atv.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- 18.Qvigstad E., Sjaastad L., Brattelid T. Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res. 2005;97(3):268–276. doi: 10.1161/01.RES.0000176970.22603.8d. [DOI] [PubMed] [Google Scholar]
- 19.Han H. RNA interference to knock down gene expression. Meth Mol Biol. 2018;1706:293–302. doi: 10.1007/978-1-4939-7471-9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nygren P., Sørbye H., Osterlund P. Targeted drugs in metastatic colorectal cancer with special emphasis on guidelines for the use of bevacizumab and cetuximab: an Acta Oncologica expert report. Acta Oncol. 2005;44(3):203–217. doi: 10.1080/02841860510029798. [DOI] [PubMed] [Google Scholar]
- 21.ter Brake O., Legrand N., von Eije K.J. Evaluation of safety and efficacy of RNAi against HIV-1 in the human immune system (Rag-2(-/-)gammac(-/-)) mouse model. Gene Ther. 2009;16(1):148–153. doi: 10.1038/gt.2008.124. [DOI] [PubMed] [Google Scholar]
- 22.Das A.T., Brummelkamp T.R., Westerhout E.M. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J Virol. 2004;78(5):2601–2605. doi: 10.1128/JVI.78.5.2601-2605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judge A.D., Robbins M., Tavakoli L. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119(3):661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z., Nie F., Kang C. Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med. 2014;34(1):253–261. doi: 10.3892/ijmm.2014.1760. [DOI] [PubMed] [Google Scholar]
- 25.Varmeh S., Egia A., McGrouther D. Cellular senescence as a possible mechanism for halting progression of keloid lesions. Genes Cancer. 2011;2(11):1061–1066. doi: 10.1177/1947601912440877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler P.D., Longaker M.T., Yang G.P. Current progress in keloid research and treatment. J Am Coll Surg. 2008;206(4):731–741. doi: 10.1016/j.jamcollsurg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Jumper N., Hodgkinson T., Paus R. A role for Neuregulin-1 in promoting keloid fibroblast migration via ErbB2-mediated signaling. Acta Derm Venereol. 2017;97(6):675–684. doi: 10.2340/00015555-2587. [DOI] [PubMed] [Google Scholar]
- 28.Jurzak M., Adamczyk K., Antonczak P. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFbeta isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol Pharm. 2014;71(6):972–986. [PubMed] [Google Scholar]
- 29.Noishiki C., Takagi G., Kubota T. Endothelial dysfunction may promote keloid growth. Wound Repair Regen. 2017;25(6):976–983. doi: 10.1111/wrr.12601. [DOI] [PubMed] [Google Scholar]
- 30.Feng J., Xue S., Pang Q. miR-141-3p inhibits fibroblast proliferation and migration by targeting GAB1 in keloids. Biochem Biophys Res Commun. 2017;490(2):302–308. doi: 10.1016/j.bbrc.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Yun I.S., Lee M.H., Rah D.K. Heat shock protein 90 inhibitor (17-AAG) induces apoptosis and decreases cell migration/motility of keloid fibroblasts. Plast Reconstr Surg. 2015;136(1):44e–53e. doi: 10.1097/PRS.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 32.Euer N., Schwirzke M., Evtmova V. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 2002;22(2a):733–740. [PubMed] [Google Scholar]
- 33.Ridley A.J., Paterson H.F., Johnston C.L. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Wei X., Sun X. A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system. Int J Nanomed. 2018:13617–13631. doi: 10.2147/IJN.S142797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart H.J., Fong-Wong L., Strickland L. A stable producer cell line for the manufacture of a lentiviral vector for gene therapy of Parkinson's disease. Hum Gene Ther. 2011;22(3):357–369. doi: 10.1089/hum.2010.142. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y.P., Liu H., Zeng H. Downregulation of lentivirus-mediated ILK RNAi on tractional force generation in human retinal Muller cells. Acta Pharmacol Sin. 2009;30(12):1625–1633. doi: 10.1038/aps.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.