Abstract
The emergence of antibiotic resistant bacteria in the healthcare is a serious concern. In the Healthcare premises precisely intensive care unit are major sources of microbial diversity. Recent findings have demonstrated not only microbial diversity but also drug resistant microbes largely habitat in ICU. Pseudomonas aeruginosa found as a part of normal intestinal flora and a significant pathogen responsible for wide range of ICU acquired infection in critically ill patients. Nosocomial infection associated with this organism including gastrointestinal infection, urinary tract infections and blood stream infection. Infection caused by this organism are difficult to treat because of the presence of its innate resistance to many antibiotics (β-lactam and penem group of antibiotics), and its ability to acquire further resistance mechanism to multiple class of antibiotics, including Beta-lactams, aminoglycosides and fluoroquinolones. In the molecular evolution microbes adopted several mechanism to maintain genomic plasticity. The tool microbe use for its survival is mainly biofilm formation, quorum sensing, and horizontal gene transfer and enzyme promiscuity. Such genomic plasticity provide an ideal habitat to grow and survive in hearse environment mainly antibiotics pressure. This review focus on infection caused by Pseudomonas aeruginosa, its mechanisms of resistance and available treatment options. The present study provides a systemic review on major source of Pseudomonas aeruginosa in ICU. Further, study also emphasizes virulence gene/s associated with Pseudomonas aeruginosa genome for extended drug resistance. Study gives detailed overview of antibiotic drug resistance mechanism.
Keywords: Antibiotics, Blood stream infections, Infection, Pathogen, Resistance, Urinary tract infections
Introduction
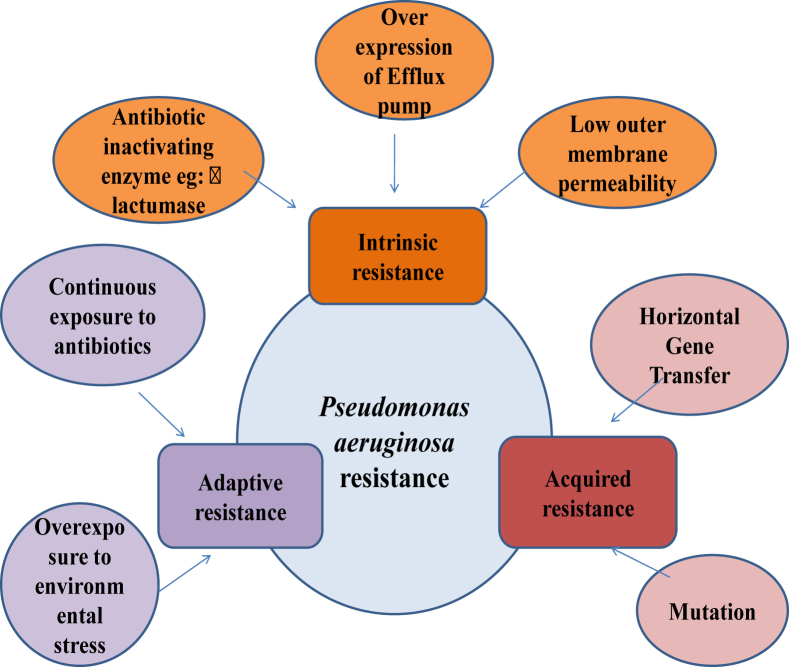
Incidence of infection caused by drug resistance organism is increasing in hospital and other clinical care settings. Though antibiotic drug resistant microbes are distributed ubiquitous, however recent finding have demonstrated an exponential increase in ICU. Infections caused by these drug resistance organism are difficult to treat include challenges related to diagnosis and treatment and causes increased morbidity and mortality.1 ICU are the major source of creating, disseminating and amplifying these drug resistant organism2 where the selection pressures is highest for the emergence of resistance of drug-resistant pathogens3 due to increased use of antibiotics to treat infections in patients. In addition to this, ICU patients have an increased risk of infection due to their reduced host defense, delayed immune response, and use of multiple procedure and invasive devices such as mechanical ventilation, central venous catheterizations (CVC), and urinary tract catheterizations.4 In this era of antibiotic resistance, Pseudomonas aeurogenosa represents one of the most concerning pathogens involved in antibiotic resistance and as such, together with other highly important MDR pathogens including E. coli, Klebsiella, Acinetobacter baumannii, Pseudomonas aeurogenosa has been classified as an ESKAPE organism1 and responsible for wide range of ICU acquired infection in critically ill patients5 (see Fig. 1).
Figure 1.
Proposed mechanism of antibiotic resistant in P. aeruginosa.
Due to the presence of its innate resistance to many antibiotics and antiseptics, ability to acquire further resistance mechanism to multiple classes of antibiotics, ability to survive in moist environments, P. aeruginosa is a common pathogen in hospitals and particularly in intensive care units. It involves in various life threatening infection in ICU such as endocarditis and septicemia, urinary tract infections, cystitis, pneumonia, surgical wound infections. Various mechanism involve in drug resistance of Pseudomonas, in that innate resistance involve the presence of over expressed efflux pump, and its low permeability of outer membrane,6 whereas acquired resistance involve the acquisition of resistance gene or mutation in genes encoding porins, efflux pumps, penicillin-binding proteins, and chromosomal β-lactamase, all contributing to resistance to β-lactams, carbapenems, aminoglycosides, and fluoroquinolones.7 Pseudomonas aeruginosa remain known to acquired capacity to pose infection by manipulating host pathogen interactions. The antibiotics resistance Pseudomonas aeruginosa posed threat to next level by limiting efficacy of antibiotics approved for clinical uses. Often these mechanisms exist simultaneously, thus conferring combined resistance to many antibiotics,8 and thus the treatment option for these drug resistance Pseudomonas, are very limited. This review will focus on infection caused by Pseudomonas aeruginosa, its mechanisms of resistance, available treatment options (see Table 1, Table 2, Table 3).
Table 1.
The list of antibiotics, classes and their mechanism.103
| Antibiotic class | Mechanism of action | Drug |
|---|---|---|
| Penicillins | Bacterial cell wall synthesis inhibition | Ticarcillin |
| Penicillin/Beta-lactamase inhibitor | Bacterial cell wall synthesis inhibition | Ticarcillin/Clavulanic acid |
| Piperacillin/Tazobactam | ||
| Cephalosporins | Bacterial cell wall synthesis inhibition | Ceftazidime |
| Cefepime | ||
| Monobactams | Bacterial cell wall synthesis inhibition | Aztreonam |
| Carbapenems | Bacterial cell wall synthesis inhibition | Imipenem |
| Meropenem | ||
| Doripenem | ||
| Fluoroquinolones | Block of DNA synthesis | Ciprofloxacin |
| Levofloxacin | ||
| Ofloxacin | ||
| Aminoglycosides | Protein synthesis inhibition | Gentamycin |
| Tobramycin | ||
| Amikacin |
Table 2.
Different combinations of antibiotics with enhanced activity against MDR P. aeruginosa.
| Antibiotic combination | Reference |
|---|---|
| Cephalosporins, Quinolones | 109 |
| Ceftazidime, Colistin | 109 |
| Macrolides, Tobramycin, Trimethoprim, Rifampin | 109 |
| Fosfomycin, colistin | 110 |
| Meropenem, levofloxacin | 110 |
| Colistin, ceftazidime Colistin, ciprofloxacin |
110 |
| Ceftolozane + tazobactam, tobramycin Ceftolozane + tazobactam, amikacin |
111 |
| Imipenem, tigecycline, amikacin Tigecycline, amikacin, cefepime Imipenem, amikacin, cefepime |
112 |
Table 3.
Table demonstrates pattern of drug resistance and virulence factors associated in P. aeruginosa.120
| S No | Pattern of Drug Resistance | Virulence factors |
|---|---|---|
| 1 | Biofilm formation | Proteases |
| 2 | Quorum Sensing | Hemolysins |
| 3 | Horizontal Gene Transfer | Endotoxin A |
| 4 | Enzyme Promiscuity | Exoenzyme S |
| 5 | Increased virulence Capacity | Pyocyanin |
| 6 | Enhanced motility | Adhesins |
Microbiology
Pseudomonas aeruginosa is a member of the genus Pseudomonas, colloquially called the pseudomonads. P. aeruginosa is a gram-negative aerobic bacterium, motile, non-spore forming rods, oxidase positive and lactose non fermenters. Due to the production of water soluble pigments such as pyoverdine which is a yellow-green, fluorescent pigment, and pyocyanin that is a blue-green pigment it is easily detectable on agar.9, 10 P. aeruginosa found in soil and water ecosystem and also associated with infection of plants, animal and humans.11. Human colonization begins within the gastrointestinal tract, with subsequent spread to moist cutaneous sites such as the perineum and axilla. As a group, pseudomonads have minimal nutritional requirements for its growth and can utilize a wide variety of environmental sources for nutrition; P. aeruginosa often only needs acetate and ammonia as the source of carbon and nitrogen, respectively. In addition Pseudomonas aeruginosa can grow anaerobically, and does not carry out fermentation, rather obtaining energy from the oxidation of sugars. This minimal nutritional requirement allow it to grow in marginal environments such as dry surfaces of hospital operating rooms, hospital rooms, clinics, and medical equipment as well as sinks, showers, even contaminating distilled water12 and thus proven as important source of nosocomial infection. In a report conducting in Portugal hospital environment were evaluated for the presence Pseudomonas and found high level contamination which could increase the risk of transmission of infection.13
Epidemiology
Pseudomonas aeruginosa, first isolated in 1882 by Gessard from green pus. The ubiquitous life-style of P. aeruginosa allows this bacterium to contribute to frequent infections in humans. It may be found as part of normal intestinal flora; however it is not well adhere to normal intact epithelium. Therefore, it does not cause infection in healthy individuals14 but an opportunistic pathogen in immune compromised patients. Due to its adaptable nature and high surviving ability it can survive on dry inanimate surfaces of hospital environment from 6 h to 6 month15 and frequently contaminated the healthcare equipment and surfaces such as., monitors, ventilator buttons, bedrails, respiratory equipment, dialysis tubing. P. aeruginosa shows inherent and acquired resistance too many common antimicrobial agents,16 it can also be cultured from hand creams,17 and certain cleaning solutions and can also survive in some antiseptic solutions used to disinfect endoscopes and surgical instruments.18, 19, 20
Pseudomonas aeruginosa is involved in a variety of hospital acquired infections ranging from ventilator associated pneumonia, to blood stream infection. It is the fourth most commonly-isolated nosocomial pathogen accounting for 10% of all hospital-acquired infections and second most common cause of pneumonia and the third most common gram-negative cause of blood stream infection (BSI).21 In a recent analysis of 8247 inpatient with nosocomial infections in different tertiary care hospital of Greece, 746 patients with an active HAI were identified and P. aeruginosa was the second most frequently isolated (16%) bacteria and accounted for 49.4% of resistance towards penem group of antibiotics.22 Pseudomonas aeruginosa have been associated with increased mortality in blood stream infection compare to infection with other gram positive organism.23
Nosocomial infection caused by Pseudomonas aeruginosa
Hospital acquired pneumonia
Hospital acquired pneumonia is the most common life-threatening hospital acquired infection, and the majority of the cases are associated with mechanical ventilation. It is the second most common nosocomial infection in the intensive care unit (ICU) and the most common in mechanically ventilated patients.24, 25 Ventilator associated pneumonia occurs in approximately 10–20% of patients who are on ventilators for longer than 48 h and is associated with significant increases in length of hospital stay, mortality, and costs.26, 27 The microbiologic flora responsible for VAP usually depends on the duration of mechanical ventilation. In general, early onset of VAP is associated with pathogens that are sensitive to antibiotics, VAP include Streptococcus pneumoniae (as well as other streptococcus species), Hemophilus influenzae, methicillin-sensitive Staphylococcus aureus (MSSA), antibiotic-sensitive enteric gram-negative bacilli, Escherichia coli, Klebsiella pneumonia, Enterobacter species, Proteus species and Serratia marcescens, are associated with early onset of VAP whereas multi-drug resistant bacteria, such as methicillin-resistant S. aureus (MRSA), Acinetobacter, Pseudomonas aeruginosa, and extended-spectrum beta-lactamase producing bacteria (ESBL)28 are associated with late onset of VAP and are more difficult to treat. VAP caused by Pseudomonas aeruginosa has been associated with higher case fatality rates than that by other bacteria.29 Tracheobronchial colonization is one of the most important factors for VAP and the predominant organisms responsible for infection are Staphylococcus aureus, P. aeruginosa, and Enterobacteriaceae. Large quantities of P. aeruginosa in the trachea of ventilated patients are associated with an increased risk of death.30 The effectiveness of Pseudomonas aeruginosa in posing infections incapable to combat with conventional antibiotics/single drug is mainly due to complex gene/s involve in drug resistance. These gene/s are either present as separate extrachromosomal DNA i.e. plasmid and or integrated into bacterial genome.
Several studies have demonstrated that about 3–5% of nosocomial pneumonia is caused by Pseudomonas spp. Ranjan et al,31 showed that Pseudomonas account for 25.7%–38% of total gram negative isolates of VAP. Similar trends were observed in other study with percentage of occurrence of 38% of total bacterial isolates of VAP in tertiary care hospital.32 P. aeruginosa along with Staphylococcus aureus is one of the most common bacteria causing VAP, with a prevalence of approximately 4%,33 and its attributable mortality is as high as 13.5%, even with adequate antibiotic treatment.34 P. aeruginosa is among the three top microorganisms causing health care respiratory infection and is now resistant to multiple class of drug35 and, even associated with early-onset of VAP. Infection by MDR P. aeruginosa compared with susceptible strain, increase risk of mortality up to tow fold with the excess of 12%.36 Various Risk factors are associated with VAP for P. aeruginosa are mainly prior antibiotic exposure and MV longer than 5 days.37, 38, 39 Patients with chronic diseases such as obstructive pulmonary disease and other chronic respiratory diseases may carry endogenous colonization and are at higher risk of developing a severe respiratory infection following intubation and MV. Various studies report an increased incidence of Pseudomonas infections in patients with immunosuppression (e.g. hematologic malignancies) or chronic diseases like cystic fibrosis.40, 41
Urinary tract infections
Urinary tract infections is one of the leading causes of nosocomial infections accounting for between 20 and 49% of all nosocomial infection42, 43 and about–10% of urinary tract infections are caused by Pseudomonas aeruginosa. These infections caused by P. aeruginosa are usually occur secondary to catheterization, instrumentation of urinary system or surgery,44 Outcomes of UTI ranges from patient discomfort, pyelonephritis, morbidity and, in rare cases, death. Pathogens use catheters as a source of host entry,45, 46, 47 because the insertion of the catheter may causes disruption of mucosal epithelial layers, promoting bacterial colonization.44, 48 In UTI Pseudomonas aeruginosa is key uropathogen with high prevalence in reported cases worldwide.
Blood stream infections
Blood stream infection is a serious life threatening condition and an important cause of increased morbidity and mortality. Incidence of BSI caused by pseudomonas is increasing. Studies associated with risk factor for P. aeruginosa with BSI are very few. Various study documented different source of infection for BSI with Pseudomonas. Marra et al,49 found that the most frequent sources of BSI were the respiratory tract and central venous catheters. Immuno compromised patients in ICU are at the high risk of BSI associated with Pseudomonas. In a case control study performed by50 found that risk factor BSI with Pseudomonas is the presence of lung cancer and previous antimicrobial therapy. Other risk factor including septic shock, pneumonia, having severe underlying disease, inappropriate empirical therapy, delayed antimicrobial therapy and multiple drug resistance.51
Antibiotic resistance in P. aeruginosa
A general definition of antimicrobial resistance is the ability of an organism to resist the action of an antimicrobial agent to which it was previously susceptible.52 Nosocomial infection caused by antibiotic resistant P. aeruginosa, have emerged as major concern in clinical care settings as the increasing development of MDR strains (i.e. resistance to at least three antibiotics).53 P. aeruginosa exhibits intrinsic resistance to various antimicrobial agents (β-lactam and penem group of antibiotics) because of its outer membrane with low permeability (1/100 of the permeability of E. coli outer membrane.54, 55 Although some other mechanisms are also responsible for their intrinsic resistance including, efflux system which expel antibiotic out of the bacterial the cell,56, 57, 58 and production of antibiotic inactivating enzyme. However, this bacterium is a highly diverse pathogen that is capable of adaptation to the surrounding environment. When subjected to antibiotic selective pressure, the induced response facilitates bacterial survival and develops antibiotic resistance.59 The emergence of antibiotic resistance has been reported during host colonization of CF patients, whereby P. aeruginosa strains develop and acquire resistance during antimicrobial therapy.60 Studies have reported a strong correlation between increased uses of ciprofloxacin with increased prevalence of ciprofloxacin resistant strains.36 Therefore, another factor associated with the increase in MDR-P. Aeruginosa is due to the frequent use of antimicrobial agents. This acquired resistance is may be due to the consequences of mutational event or the acquisition of resistance gene through horizontal gene transfer and can occur during the antibiotic therapy61 mutational event may lead to over expression of endogenous beta lactamases or efflux pump, expression of specific porins.
Resistance to beta lactam
Beta lactam antibiotics involve in the inhibition of synthesis of bacterial peptidoglycan cell wall.53 This class includes penicillin, cephalosporin, carbapenem and monobactam. Among these group, piperacillin and ticarcillin (penicillins), ceftazidime (3rd generation cephalosporin), cefepime (4th generation cephalosporin), aztreonam (monobactam), imipenem, meropenem and doripenem (carbapenems) are most effective beta lactam that are commonly used in the treatment of Pseudomonas aeruginosa.62 Resistance to the β-lactam mediated by the action of β-lactamases, these enzymes destroy the amide bond of the β-lactam ring, causes the antimicrobial to be ineffective. This inactivation of drug is may be due to expression of endogenous β-lactamases or through the expression of acquired beta lactamases. Hundreds of β-lactamases have been identified till date and they are characterizing as their substrate specificity. On the basis of Amber's molecular classification system there are four major classes of beta-lactamases have been identified in P. aeruginosa: A-D.63 Classes A, C and D inactivate the β-lactams through the catalytic activity of serine-residue, whereas class B or metallo- β-lactamases (MBLs) need zinc for their action.64
AmpC beta lactamase (Cephelosporinase)
Furthermore, production of endogenous beta lactamase such as AmpC beta lactamase (chromosomal cephalosporinase). In P. aeruginosa can be induced by a number of beta lactams such as benzyl penicillin, narrow spectrum cephalosporin and imipenem. P. aeruginosa is naturally susceptible to carboxypenicillins, ceftazidime and aztreonam but it can acquire resistance through a gene mutation which leads to cause hyper production of AmpC beta-lactamase65, 66 P. aeruginosa produces an inducible chromosome-encoded AmpC beta-lactamase (cephalosporinase) that belongs to molecular class C, based on Ambler and the first functional group according to Bush et al,.67 Usually the enzyme is produced in low quantities (‘low-level’ expression) and determines resistance to aminopenicillins and most of the early cephalosporins.68
However, chromosomal cephalosporin's production in P. aeruginosa may increase from 100 to 1000 times in the presence of inducing beta-lactams (especially imipenem).69 AmpC cephalosporinase activity is not inhibited by beta-lactamase inhibitors used in clinical practice, for example clavulanic acid, sulbactam and tazobactam.70 AmpC beta-lactamase is encoded by the ampC gene.71 Several genes are involved in induction of ampC gene including ampR, ampG and ampD (75). ampR, encodes a positive transcriptional regulator, this regulator is necessary for the beta-lactamase induction.71 The second gene involve is ampG, encodes a transmembrane protein that acts as a permease for 1,6-anhydromurapeptides, which are considered to be the signal molecules involved in ampC induction.72 The third gene, ampD, encodes a cytosolic N-acetyl-anhydromuramyl-l-alanine amidase, which hydrolyses 1,6-anhydromurapeptides, acting as a repressor of ampC expression.73 The fourth gene, ampE, encodes a cytoplasmic membrane protein that act as a sensory transducer molecule necessary for induction.74 Activity of these AmpC beta lactamase is not inhibited by commercially available beta lactum except avibactam.
Class A carbenicillin hydrolysing b-lactamases
Four carbenicillin hydrolyzing beta-lactamases enzyme (PSE-of Pseudomonas specific enzyme) were identified in P. aeruginosa: PSE-1 (CARB-2), PSE-4 (CARB-1), CARB-3 and CARB-4.75 These enzymes belong to the group of molecular class A of β-lactamases and their substrate profile includes carboxypenicillins, ureidopenicillins and cefsulodine. These enzymes belong to molecular class A and functional group 2c.76 Activity of these beta lactamase can be inhibited by commercially available beta lactam inhibitor such as clavulanic acid, tazobactam and sulbactam.77
Resistance to aminoglycoside
Aminoglycosides are the inhibitor of microbial protein synthesis and act by binding to the bacterial 30S ribosomal subunit and interferes with initiation of protein synthesis. In Pseudomonas resistance to aminoglycosides is mediated by transferable aminoglycoside modifying enzymes (AMEs), low outer membrane permeability, active efflux and, rarely target modification.78, 79, 80
Aminoglycoside-modifying enzymes
AMEs inactivate the aminoglycoside by attaching a phosphate, adenyl or acetyl radical of the antibiotic molecule, and thus modified antibiotics have the decrease the binding affinity to its target in the bacterial cell (30S ribosomal subunit).81, 82 There are three class of AMEs involve in aminoglycoside modification: aminoglycoside phosphoryl transferases (APHs), aminoglycoside adenylyl transferases (also known as nucleotidyltransferases) (AADs or ANTs) and aminoglycoside acetyltransferases (AACs) (101). Most frequently P. aeruginosa expresses the following AMEs: AAC (69)—II (determines resistance to gentamicin, tobramycin and netilmicin), AAC (3)—I (resistance to gentamicin), AAC (3)—II (resistance to gentamicin, tobramycin and netilmicin), 69)—I (resistance to tobramycin, netilmicin and amikacin) and ANT (29)-I (resistance to gentamicin and tobramycin).83
Low outer membrane permeability
Resistance to aminoglycosides independent from AMEs has been reported in clinical isolates of cystic fibrosis patients. This type of resistance is attributable due to reduced uptake through the decrease permeability of outer membrane and is typically referred to as impermeability resistance.84
Efflux systems
P. aeruginosa expresses several efflux pumps that expel drugs together with other substances out of the bacterial cell. Efflux mediated aminoglycoside resistance is relatively rare and mostly due to the over expression of the MexXY-OprM efflux pump.
Target modification
Bacteria may be resistant to aminoglycoside because of low affinity of the drug for the bacterial ribosome. This may be accomplished by target modification through Methylation of 16S rRNA. Different 16S rRNA methylases have been described for P. aeruginosa: RmtA, firstly reported in clinical isolates of aminoglycoside resistant Pseudomonas aeruginosa and confer resistance to all parenterally administered aminoglycosides, including amikacin, tobramycin, isepamicin, kanamycin, arbekacin and gentamicin,85 other 16S rRNA methylases including RmtB, ArmA (29)and RmtD.
Resistance to fluoroquinolones
Resistance to fluoroquinolones develops via mutation in bacterial chromosomal gene encoding DNA gyrase or topoisomerase 1 V or by active transport of drug out of cell.86 Mutation for the topoisomerase 1 V may occur in gyrA/gyrB genes within the QRDR (quinolone-resistant-determinative region) motif, which is considered as the enzyme's active site. This leads to the altered amino acid sequences of A and B subunit, and hence a modified topoisomerase II with low binding affinity to quinolone molecules. Modifications of a secondary target (topoisomerase IV) occur as a result of point mutations in parC and parE genes encoding ParC and ParE enzyme subunits, respectively. Other mode for fluoroquinolone resistance in Pseudomonas involve the over expression of efflux. Mutations in nalB, nfxB and nfxC gene leading, to overexpression of fallowing efflux MexA–MexB–OprM, MexC–MexD–OprJ and MexE–MexF–OprN.87
Treatment options for MDR Pseudomonas aeruginosa
The treatment options entirely depend on nature of antibiotic drug resistance and gene/s involved in drug resistance. P. aeruginosa has a broad range of intrinsic and adaptive antimicrobial resistance mechanisms. P. aeruginosa encodes an chromosomal AmpC beta-lactamase (which confer resistance to many penicillins and cephalosporins), has an outer membrane porin, OprD which can be variably expressed (loss of which confers resistance to carbapenems), and have some drug efflux pumps such as MexAB-OprM (which expel some class of antibiotics out of the cell); all these mechanism are inducible, and regulation is mediated by the environment encountered by the organism.88 The conventional antibiotics including beta lactum and similar narrow spectrum antibiotics seem ineffective as Pseudomonas aeruginosa is capable to degrade ring responsible to deliver activity. Despite the intrinsic resistance of P. aeruginosa to many antimicrobials, some antibiotics are active against this microorganism and are belong to the class of Beta-lactams, Quinolones, and Aminoglycosides.89
In addition P. aeruginosa can import further resistance mechanisms, which acquired through horizontal gene transfer and make it resistance to different anti-pseudomonal compounds and thus very few options are available for the testament of severe nosocomial pseudomonas infection, particularly in patients confined to ICUs. Colistin and polymyxin B may be effective alternative agents for treatment of multi-drug-resistant Pseudomonas aeruginosa P. aeruginosa binds to lipopolysaccharides and phospholipids in the outer cell membrane of gram-negative bacteria. It competitively displaces divalent cations from the phosphate groups of membrane lipids, which leads to disruption of the outer cell membrane, leakage of intracellular contents, and bacterial death.90, 91, 92 Memer et al conducted a study to find out the role of colistin for treating the Multidrug resistant Pseudomonas and found effectiveness of the colistin is higher than another beta lactam drugs.93 Combination of colistin with an anti-pseudomonas agent such as imipenem, piperacillin, aztreonam, ceftazidime, azlocillin, rifampin or ciprofloxacin was more efficient than only colistin against MDR P. aeruginosa.94, 95, 96 However, increasing administration of colistin for antibiotic therapy of infections by MDR organisms may lead to the emergence of colistin-resistant strains in some countries.97 But they remain to date relatively rare for Pseudomonas aeruginosa.98
Fosfomycin, is an another option for the treatment of MDR Pseudomonas aeruginosa. It inactivates pyruvil-transferase enzyme, which is required for the synthesis of the cell wall peptidoglycan. In systematic review of different study, fosfomycin were found to effective against 90% of MDR P. aeruginosa including MDR strains.99 In a prospective observational study 375 of 385 (97.4%) Pseudomonas spp were found susceptible to fosfomycin.100 Fosfomycin together with aminoglycosides, cephalosporin's and penicillin's, ceftazidime, gentamicin, imipenem, and levofloxacin, ciprofloxacin, have been used for the better result for the treatment of drug resistance Pseudomonas aeruginosa.99, 101 As far as newer carbapenem compounds are concerned, data suggest that doripenem and Tigecycline is not option for treatment of MDR P. aeruginosa. Furthermore, time-kill studies on 12 MBL-producing P. aeruginosa isolates performed with combinations of piperacillin-tazobactam with levofloxacin and cefoperazone-sulbactam with levofloxacin, aztreonam alone and in combination with ceftazidime and amikacin, showed bactericidal activity against one and eight isolates respectively.102
Combination therapy
The application of combination therapy instead of monotherapy in cases of non-MDR P. aeruginosa remains to date a controversial issue.104 Combination treatment against MDR strains instead seems to be some times necessary (for example in cases of pan-resistance or resistance to all except a single agent). Frequently used combinations include a β-lactam plus an aminoglycoside group of antibiotics.105 Both of these drugs have different bacterial targets and modes of entry into the bacterial cell, thus having enhanced antimicrobial activity. There are different advantages of using combination therapy over monotherapy including: an increased likelihood that the infecting pathogen will be susceptible to at least one of the antimicrobials, combination therapy allows for different mechanisms of bacterial killing, prevention of emergence of resistance, and additive or even synergistic effect of the combination. Several old and newer studies have showed that different combinations of antibiotics have enhanced in vitro activity against MDR Pseudomonas aeruginosa even though; the mechanisms of positive interaction between the various agents are rarely known.106 In a retrospective cohort study of consecutive patients with Pseudomonas aeruginosa bacteremia, combination therapy was found to be significantly better than monotherapy in improving outcome in terms of decreasing mortality.107, 108
Challenges
The growing challenge in context with Antimicrobial Drug Resistance is microbial capacity to acquire genomic plasticity.113 The constant changing of microbial genome is bigger threat than infection itself. There are several mechanism microbes utilize to alter their genome such as horizontal gene transfer, transposon mediated genomic alterations and promiscuous nature of gene and genomic products. The promiscuity is another concept recently studied gives an idea how a single gene product such as protein/enzyme create a favorable environment for microbes.27, 114 As a result microbes need not to carry multiple gene/s and by rearranging within limited genome can survive in hearse conditions. Now, if we consider antibiotic drug resistance, bacteria genome is capable to encode enzyme which can destroy multiple substrate by developing affinity against them. Here, promiscuity in enzyme encoded by bacterial genome allow microbe to nullify bactericidal effect of several antibiotics more precisely several generation of a class of antibiotic. Beta lactamase and extended beta lactamase are classical example to understand enzyme promiscuity. The concept of promiscuity is well established in the context of biophysical aspect for multisubstrate affinity of single enzyme and capacity to deliver multiple functions.115, 116, 117, 118 Both the enzyme encoded by most of resistant microbial species including P. aeruginosa selectively target beta-lactam antibiotics including penicillin and cephalosporins. The growing evidence of promiscuous nature of beta lactamase will allow enzyme to develop affinity for new generation beta lactum antibiotics and bacteria need not to have additional genes for new generation antibiotics.
ICU and infections
ICUs are believed major source of microbial infections and become major threat. The rate of nosocomial infections in the intensive care unit (ICU) is about 2–5 times higher than in the general in-patient hospital population. It has been observed that ICUs are potential source of both gram positive and gram negative pathogens associated with variety of human disease.119 These contagious pathogens are not only limited to acute infections but also leads to chronic lethal disease. The studies have demonstrated P. aeruginosa is major pathogen in ICU followed by E. coli, Enterobacter, Staphylococcus, Enterococci and K. pneumonia. Another finding demonstrated that these contagious infections present in pediatric intensive care unit as well.120, 121 The infectious agents present in ICU are more than 50% are largely gram negative microbes. It has been reported that ICU-acquired infections and extended- (XDR) or multi-drug resistant (MDR) strains are increasingly the ones isolated, including carbapenemase-producing Klebsiella pneumoniae (KPC), Acinetobacter spp. and Pseudomonas aeruginosa. These infections are responsible for high ICU mortality which further increased due to XDR/MDR bacteria.122 In the ICU based infection Pseudomonas aeruginosa alone represent more than 30% contagious infection. In recent time, ICU based infection and associated diseases are major threat for modern healthcare and associated with high mortality rate. Among ICU infection the major diseases are respiratory, catheter-related bacteremia, non-catheter related bacteremia, secondary peritonitis, surgical wound infections and a few urinary tract infections.123, 124
Conclusion
Hospitals and healthcare settings are proven as reservoirs for large numbers of pathogenic strains of Pseudomonas aeruginosa and it has been proven as an important nosocomial pathogen because of presence of intrinsic mechanism of resistance to many class of antibiotics as well as its ability to acquire resistance (via mutations) against all relevant treatments. Multidrug resistance (MDR) is common and increasing. Occurrence of these MDR strain in clinical care settings makes them difficult and expensive to treat because these drug resistant strain are exhibit resistance to essentially all reliable antipseudomonal antibiotics. Improved methods for antimicrobial susceptibility testing should be implemented for early detection of drug resistance Pseudomonas aeruginosa. It is also essential to understand pattern of drug resistance and mechanism behind. There is growing scientific evidence that microbe utilize several mechanism to acquire antibiotic drug resistance mainly horizontal gene transfer and biofilm formation. Further, promiscuous nature of enzyme encoded by microbe genome and quorum sensing are additional tool microbes' use to survive in hearse conditions. The available clinical solution for antibiotic resistance Pseudomonas aeruginosa infections require a precise diagnostic and combination antibiotic therapy based on diagnostics. Several infections which are recurrent need additional care to stop proliferation of antibiotic resistance Pseudomonas aeruginosa in order to contaminate surrounding environment. These microbes precisely antibiotic drug resistance Pseudomonas aeruginosa and many more are potential source for gene transfer to non pathogenic microbes. Clinical studies are required to identify risk factors for the development MDR, and also determine the most effective antimicrobial regimens and duration of therapy for successful treatment of severe infections due to drug resistant P. aeruginosa.
Conflict of interest
Author declares no conflict of interest.
Acknowledgment
Author would like to acknowledge and thanks Department of Biotechnology, Barkatullah University, Bhopal 462026, Madhya Pradesh, India for providing facility and resources for present work.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Preeti Pachori, Email: preeti_pachori@yahoo.com.
Ragini Gothalwal, Email: ragini_gothalwal@yahoo.com.
Puneet Gandhi, Email: puneetgandhi67@yahoo.com.
References
- 1.Boucher H.W., George H., Talbot J. Bad bugs, No drugs: No ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Carlet J., Ben A.A., Tabah A. Multidrug resistant infections in the ICU: mechanisms, prevention and treatment. In: Kuhlen R., Moreno R., Ranieri V.M., Rhodes A., editors. 25 Years of Progress and Innovation in Intensive Care Medicine. Medizinisch Wissenschaftliche Verlagsgesellschaft; Berlin, Germany: 2007. pp. 199–211. [Google Scholar]
- 3.Esposito S., Leone S. Antimicrobial treatment for intensive care unit (ICU) infections including the role of the infectious disease specialist. Int J Antimicrob Agents. 2007;29:494–500. doi: 10.1016/j.ijantimicag.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Ranjan N., Chaudhary U., Chaudhry D., Ranjan K.P. Ventilator associated pneumonia in a tertiary care intensive care unit: analysis of incidence, risk factors and mortality. Indian J Crit Care Med. 2007;18:200–204. doi: 10.4103/0972-5229.130570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore L.S., Freeman R., Gilchrist M. Homogeneity of antimicrobial policy, yet heterogeneity of antimicrobial resistance: antimicrobial non-susceptibility among 108,717 clinical isolates from primary, secondary and tertiary care patients in London. J Antimicrob Chemother. 2014;69(12):3409–3422. doi: 10.1093/jac/dku307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santajit S., Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016:1–8. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oie S., Fukui Y., Yamamoto M., Masuda Y., Kamiya A. In vitro antimicrobial effects of aztreonam, colistin, and the 3-drug combination of aztreonam, ceftazidime and amikacin on metallo b-lactamase-producing Pseudomonas aeruginosa. BMC Infect Dis. 2009;9:123. doi: 10.1186/1471-2334-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memar M.Y., Pormehrali R., Alizadeh N., Ghotaslou R., Baghi H.B. Colistin, an option for treatment of multiple drug resistant Pseudomonas aeruginosa. Physiol Pharmacol. 2016;20:130–136. [Google Scholar]
- 9.Lamont I.L., Martin L.W. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiol. 2003;149(4):833–842. doi: 10.1099/mic.0.26085-0. [DOI] [PubMed] [Google Scholar]
- 10.Peix A., Ramirez-Bahena M.H. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infect Genet Evol. 2009;9(6):1132–1147. doi: 10.1016/j.meegid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Trautmann M., Lepper P.M., Haller M. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am J Infect Contr. 2009;33:S41–S49. doi: 10.1016/j.ajic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Abreu P.M.D., Farias P.D., Paiva G.S., Almeida A.M., Vasconcelos P. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol. 2017;10:317–328. doi: 10.1186/1471-2180-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engleberg N.C., Dermondy T.S. 4th ed. 2007. Lippincott Williams & Wilkins. [Google Scholar]
- 14.Roy F.C., Simmons S., Dale C. The role of the healthcare environment in the spread of multidrug-resistant organisms: update on current best practices for containment. Therapeut Adv Inf Dis. 2014;2:79–90. doi: 10.1177/2049936114543287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favero M.S., Carson L.A., Bond W.W., Petersen N.J. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science. 1997;173(999):836–838. doi: 10.1126/science.173.3999.836. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski R.P., Pandya A.N., Karenchak L.M. An in vitro resistance study of levofloxacin, ciprofloxacin, and ofloxacin using keratitis isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Ophthalmology. 2001;108(10):1826–1829. doi: 10.1016/s0161-6420(01)00724-2. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy A.M., Elward A.M., Fraser V.J. Survey of knowledge, beliefs, and practices of neonatal intensive care unit healthcare workers regarding nosocomial infections, central venous catheter care, and hand hygiene. Infect Control Hosp Epidemiol. 2004;25(9):747–752. doi: 10.1086/502471. [DOI] [PubMed] [Google Scholar]
- 18.Magill S.S., Edwards J.R., Bamberg W. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kritsotakis E.I., Kontopidou F., Astrinaki E., Roumbelaki M., Ioannidou E., Gikas A. Prevalence, incidence burden, and clinical impact of healthcare-associated infections and antimicrobial resistance: a national prevalent cohort study in acute care hospitals in Greece. Infect Drug Resist. 2017 Oct 10;10:317–328. doi: 10.2147/IDR.S147459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaden J.T., Park L.P., Maskarinec S.A., Ruffin F., Fowler V.G., Van Duin D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother. 2017;24(61):2671–2716. doi: 10.1128/AAC.02671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afshari A., Pagani L., Harbarth S. Year in review 2011: critical care–infection. Crit Care. 2012;16:242. doi: 10.1186/cc10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter J.D. Ventilator associated pneumonia. BMJ. 2012;344:40–44. doi: 10.1136/bmj.e3325. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis W.R., Schlosser J., Chinn R.Y., Tweeten S., Marguerite J. National prevalence of methicillin resistant Staphylococcus aureus in inpatients at US health care facilities. Am J infect cont. 2007;35(10):631–637. doi: 10.1016/j.ajic.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Tumbarello M., Pascale G.D., Trecarichi E.M. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39(4):682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 25.Kollef M.H., Chastre J., Fagon J.Y. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med. 2014;42(10):2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 26.Crouch B.S., Wunderink R.G., Jones C.B., Leeper K.V., Jr. Ventilator associated pneumonia due to Pseudomonas aeruginosa. Chest. 1986;109(4):1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y., Yu H., Guo Q. Distribution of 16S rRNA methylases among different species of Gram- negative bacilli with high-level resist-ance to aminoglycosides. Eur J Clin Microbiol Infect Dis. 2010;29:1349–1353. doi: 10.1007/s10096-010-1004-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q.T., BSMed B.H., Zhu H. Potential for cost-savings in the care of hospitalized low-risk community-acquired pneumonia patients in China. Value Health. 2009;12(1):14–46. doi: 10.1111/j.1524-4733.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Raineri R., Porcella L., Acquarolo A., Crema L., Albertario F., Candiani A. Ventilator-associated pneumonia caused by Pseudomonas aeruginosa in intensive care unit: epidemiology and risk factors. J Med Microbiol Diagn. 2014;3:149. [Google Scholar]
- 30.Rello J., Ausina V., Puzo C., Quintana E., Net A., Prats G. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994;20(3):193–198. doi: 10.1007/BF01704699. [DOI] [PubMed] [Google Scholar]
- 31.Leroy O., d'Escrivan T., Devos P., Dubreuil L., Kipnis E., Georges H. Hospital-acquired pneumonia in critically ill patients: factors associated with episodes due to imipenem-resistant organisms. Infection. 2005;33(3):129–135. doi: 10.1007/s15010-005-4021-8. [DOI] [PubMed] [Google Scholar]
- 32.Nathwani D., Raman G., Sulham K., Gavaghan M., Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Contr. 2014;3(1):32. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rello J., Borgatta B., Lagunes L. Management of Pseudomonas aeruginosa pneumonia: one size does not fit all. Crit Care. 2014;18(2):136. doi: 10.1186/cc13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montero M., Sala M., Riu M. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:335–339. doi: 10.1007/s10096-009-0850-1. [DOI] [PubMed] [Google Scholar]
- 35.Meier P. Nosocomial urinary tract infections: many unresolved questions. Clin Microbiol Infect. 2001;7:521–522. [PubMed] [Google Scholar]
- 36.Horcajada J.P., Shaw E., Padilla B. Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect. 2013;9:962–968. doi: 10.1111/1469-0691.12089. [DOI] [PubMed] [Google Scholar]
- 37.Bouza E., San Juan R., Muñoz P., Voss A., Kluytmans J. A European perspective on nosocomial urinary tract infections I. Report on the microbiology workload, etiology and antimicrobial susceptibility. Clin Microbiol Infect. 2001;7:523–531. doi: 10.1046/j.1198-743x.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- 38.Djordjevic Z., Folic M.M., Zivic Z., Markovic V., Jankovic S.M. Nosocomial urinary tract infections caused by Pseudomonas aeruginosa and Acinetobacter species: sensitivity to antibiotics and risk factors. Am J Infect Control. 2013;41(12):1182–1187. doi: 10.1016/j.ajic.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Mittal R., Aggarwal S., Sharma S., Chhibber S., Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a mini review. J Infect Public Health. 2009;2(3):101–111. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Inglis T.J.J. Evidence for dynamic phenomena in residual tracheal tube biofilm. Br J Anaesth. 1993;70(1):22–24. doi: 10.1093/bja/70.1.22. [DOI] [PubMed] [Google Scholar]
- 41.Morris N.S., Stickler D.J., McLean R.J. The development of bacterial biofilms on indwelling urethral catheters. World J Urol. 1999;17(6):345–350. doi: 10.1007/s003450050159. [DOI] [PubMed] [Google Scholar]
- 42.Donlan R.M. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maki D.G., Tambyah P.A. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7(2):342–347. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss W.J., Beers M.C., Johnson E. Pilot study of antibiotic cycling in a pediatric intensive care unit. Crit Care Med. 2002;30(8):1877–1882. doi: 10.1097/00003246-200208000-00034. [DOI] [PubMed] [Google Scholar]
- 45.Nseir S., Di Pompeo C., Pronnier P. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J. 2002;20(6):1483–1489. doi: 10.1183/09031936.02.00012902. [DOI] [PubMed] [Google Scholar]
- 46.Obritsch M.D., Fish D.N., MacLaren R., Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–1364. doi: 10.1592/phco.2005.25.10.1353. [DOI] [PubMed] [Google Scholar]
- 47.Tanya S., Daniel Y. Pseudomonas aeruginosa : a phenomenon of bacterial resistance. J Med Microbiol. 2000;58:1133–1148. doi: 10.1099/jmm.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 48.Angus B.L., Carey A.M., Caron D.A., Kropinski A.M.B., Hancock R.E.W. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-super susceptible mutant. Antimicrob Agents Chemother. 1982;21:299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Therrien C., Levesque R.C. Molecular basis of antibiotic resistance and β-lactamase inhibition by mechanism-based inactivators: perspectives and future direction. FEMS Microbiol Rev. 2000;24(3):251–262. doi: 10.1111/j.1574-6976.2000.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 50.Vidaur L., Sirgo G., Rodriguez A.H., Rello J. Clinical approach to the patient with suspected ventilator-associated pneumonia. Respir Care. 2005;50(7):965–974. [PubMed] [Google Scholar]
- 51.Bert F., Branger C., Lambert-Zechovsky N. Identification of PSE and OXA β-lactamase genes in Pseudomonas aeruginosa using PCR–restriction fragment length polymorphism. J Antimicrob Chemother. 2000;50(1):11–18. doi: 10.1093/jac/dkf069. [DOI] [PubMed] [Google Scholar]
- 52.Bagge N., Hentzer M., Andersen J.B., Ciofu O., Givskov M., Hoiby N. Dynamics and spatial distribution of-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2004;48(4):1168–1174. doi: 10.1128/AAC.48.4.1168-1174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juan C., Macia M.D., Gutierrez O., Vidal C., Perez J.L., Oliver A. Molecular mechanisms of beta-lactam resistance mediated by AmpC hyper production in Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother. 2005;49(11):4733–4738. doi: 10.1128/AAC.49.11.4733-4738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bush K., Jacoby G.A., Medeiros A.A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39(6):1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-Loeches I., Deja M., Koulenti D. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39(4):672–681. doi: 10.1007/s00134-012-2808-5. [DOI] [PubMed] [Google Scholar]
- 56.Okomoto K., Gotoh N., Nishino T. Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: penem resistance mechanisms and their interplay. Antimicrob Agents Chemother. 2001;45(7):1964–1971. doi: 10.1128/AAC.45.7.1964-1971.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paul M., Benuri-Silbiger I., Soares-Weiser K., Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:479–487. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rello J., Allegri C., Rodriguez A. Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology. 2006;105(4):709–714. doi: 10.1097/00000542-200610000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Dietz H., Pfeifle D., Wiedemann B. The signal molecule for beta-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob Agents Chemother. 1997;4(10):2113–2120. doi: 10.1128/aac.41.10.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honoré N., Nicolas M.H., Cole S.T. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol Microbiol. 1989;3(8):1121–1130. doi: 10.1111/j.1365-2958.1989.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 62.Köck R., Becker K., Cookson B. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15(41):19688. doi: 10.2807/ese.15.41.19688-en. [DOI] [PubMed] [Google Scholar]
- 63.Vakulenko S.B., Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramirez M.S., Tolmasky M.E. Aminoglycoside modifying enzymes. Drug Res. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Llano-Sotelo B., Azucena E.F., Kotra L.P., Mobashery S., Chow C.S. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem Biol. 2002;9:455–463. doi: 10.1016/s1074-5521(02)00125-4. [DOI] [PubMed] [Google Scholar]
- 67.Miller G.H., Sabatelli F.J., Hare R.S., the Aminoglycoside Resistance Study Group The most frequent aminoglycoside resistance mechanisms – changes with time and geographic area: a reflection of aminoglycoside usage patterns? Clin Infect Dis. 1997;24:S46–S62. doi: 10.1093/clinids/24.supplement_1.s46. [DOI] [PubMed] [Google Scholar]
- 68.MacLeod D.L., Nelson L.E., Shawar R.M. Aminoglycoside-resistance mechanisms for cystic fibrosis Pseudomonas aeruginosa isolates are unchanged by long-term, intermittent, inhaled tobramycin treatment. J Infect Dis. 2002;181(3):1180–1184. doi: 10.1086/315312. [DOI] [PubMed] [Google Scholar]
- 69.Yamane K., Doi Y., Yokoyama K. Genetic environments of the rmtA gene in Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2002;48:2069–2074. doi: 10.1128/AAC.48.6.2069-2074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gast Gurung M., Moon D.C., Tamang M.D. Emergence of 16S rRNA methylase gene armA and cocarriage of blaIMP-1 in Pseudomonas aeruginosa isolates from South Korea. Diagn Microbiol Infect Dis. 2010;68:468–470. doi: 10.1016/j.diagmicrobio.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 71.Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2011;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 72.Lu Q., Eggimann P., Luyt C.E. Pseudomonas aeruginosa serotypes in nosocomial pneumonia: prevalence and clinical outcomes. Crit Care. 2014;18(1):R17. doi: 10.1186/cc13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magiorakos A.P., Srinivasan A., Carey R.B. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol. 2000;174(3):135–142. doi: 10.1007/s002030000188. [DOI] [PubMed] [Google Scholar]
- 74.Liljequist B., Paterson D.L., Rice L.B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 75.Furtado G.H., d'Azevedo P.A., Santos A.F., Gales A.C., Pignatari A.C., Medeiros E.A. Intravenous polymyxin B for the treatment of nosocomial pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:315. doi: 10.1016/j.ijantimicag.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 76.Kallel H., Hergafi L., Bahloul M. Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case-control study. Intensive Care Med. 2007;33:1162. doi: 10.1007/s00134-007-0675-2. [DOI] [PubMed] [Google Scholar]
- 77.Gunderson B.W., Ibrahim K.H., Hovde L.B., Fromm T.L., Reed M.D., Rotschafer J.C. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zavascki A.P., Goldani L.Z., Li J., Natio R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 79.D'Souza B.B., Padmaraj S.R., Rekha P.D., Tellis R.C., Prabhu S., Pothen P. In Vitro synergistic activity of colistin and ceftazidime or ciprofloxacin against multidrug-resistant clinical strains of Pseudomonas aeruginosa. Microb Drug Resist. 2010;20(6):550–554. doi: 10.1089/mdr.2014.0006. [DOI] [PubMed] [Google Scholar]
- 80.Bialvaei A.Z., Samadi Kafil H. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin. 2015;31:707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 81.Falagas M.E., Kastoris A.C., Karageorgopoulos D.E., Rafailidis P.I. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents. 2009;34:111–120. doi: 10.1016/j.ijantimicag.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Patwardhan V., Singh S. Fosfomycin for the treatment of drug-resistant urinary tract infections: potential of an old drug not explored fully. Int Urol Nephrol. 2017;49(9):1637–1643. doi: 10.1007/s11255-017-1627-6. [DOI] [PubMed] [Google Scholar]
- 83.Okazaki M., Suzuki K., Asano N. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J Infect Chemother. 2002;8:37–42. doi: 10.1007/s101560200004. [DOI] [PubMed] [Google Scholar]
- 84.Yamada S., Hyo Y., Ohmori S., Ohuchi M. Role of ciprofloxacin in its synergistic effect with fosfomycin on drugresistant strains of Pseudomonas aeruginosa. Chemother. 2007;53:202–220. doi: 10.1159/000100811. [DOI] [PubMed] [Google Scholar]
- 85.Mizuta M., Linkin D.R., Nachamkin I. Identification of optimal combinations for empirical dual antimicrobial therapy of Pseudomonas aeruginosa infection: potential role of a Combination Antibiogram. Infect Control Hosp Epidemiol. 2006;27(4):413–415. doi: 10.1086/503175. [DOI] [PubMed] [Google Scholar]
- 86.Rahal J.J. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43:S95–S99. doi: 10.1086/504486. [DOI] [PubMed] [Google Scholar]
- 87.Par S.Y., Park H.J., Moon S.M. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis. 2012;12(308):1471–2334. doi: 10.1186/1471-2334-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fish D.N., Choi M.K., Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother. 2002;50:1045–1049. doi: 10.1093/jac/dkf211. [DOI] [PubMed] [Google Scholar]
- 89.Saiman L., Chen Y., San Gabriel P., Knirsch C. Synergistic activities of macrolides antibiotics against Pseudomonas aeruginosa, Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. Antimicrob Agents Chemother. 2002;46:1105–1107. doi: 10.1128/AAC.46.4.1105-1107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di X., Wang R., Liu B. In vitro activity of fosfomycin in combination with colistin against clinical isolates of carbapenem-resistant Pseudomas aeruginosa. J Antibiot. 2015;68(9):551–555. doi: 10.1038/ja.2015.27. [DOI] [PubMed] [Google Scholar]
- 91.Louie A., Michael W.L., Guilder M.V. Combination treatment with meropenem plus levofloxacin is synergistic against Pseudomonas aeruginosa infection in a murine model of pneumonia. JID (J Infect Dis) 2015;21(8):1326–1333. doi: 10.1093/infdis/jiu603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aboulmagd E., Alsultan A.A. Synergic bactericidal activity of novel antibiotic combinations against extreme drug resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Afr J Microbiol Res. 2014;8(9):856–861. [Google Scholar]
- 93.Afessa B., Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the pulmonary complications, ICU support, and prognostic factors of hospitalized patients with HIV (PIP) study. Chest. 2000;117(4):1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- 94.Gentry L.O., Rodriguez G.G. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34(1):40–43. doi: 10.1128/aac.34.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmad S.I. Treatment of post-burns bacterial infections by bacteriophages, specifically ubiquitous Pseudomonas spp. notoriously resistant to antibiotics. Med Hypotheses. 2002;58(4):327–331. doi: 10.1054/mehy.2001.1522. [DOI] [PubMed] [Google Scholar]
- 96.Allegranzi B., Luzzati R., Luzzani A. Impact of antibiotic changes in empirical therapy on antimicrobial resistance in intensive care unit-acquired infections. J Hosp Infect. 2002;52(2):136–140. doi: 10.1053/jhin.2002.1277. [DOI] [PubMed] [Google Scholar]
- 97.Arancibia F., Bauer T.T., Ewig S. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162(16):1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 98.Baltch A., Smith R. Marcel Dekker; New York, NY: 1994. Pseudomonas aeruginosa: Infections and Treatment. [Google Scholar]
- 99.Berra L., Sampson J., Wiener-Kronish J. Pseudomonas aeruginosa: acute lung injury or ventilator associated pneumonia? Minerva Anestesiol. 2010;76(10):824–832. [PubMed] [Google Scholar]
- 100.Centers for Disease Control and Prevention Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep. 2009;58:256–260. [PubMed] [Google Scholar]
- 101.Centers for Disease Control and Prevention Pseudomonas dermatitis/folliculitis associated with pools and hot tubs--Colorado and Maine, 1999-2000. MMWR Morb Mortal Wkly Rep. 2000;49(48):1087–1091. [PubMed] [Google Scholar]
- 102.Chatzinikolaou I., Abi-Said D., Bodey G.P., Rolston K.V., Tarrand J.J., Samonis G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med. 2002;160:501. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- 103.Chaudhary R., Thapa S.K., Rana J.C., Shah P.K. Surgical site infections and antimicrobial resistance pattern. J Nepal Health Res Counc. 2017;15(36):120–123. doi: 10.3126/jnhrc.v15i2.18185. [DOI] [PubMed] [Google Scholar]
- 104.Cosgrove S.E. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42:S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 105.Dassner A.M., Sutherland C., Girotto J., Nicolau D.P. In vitro activity of ceftolozane/tazobactam alone or with an aminoglycoside against multi-drug- resistant Pseudomonas aeruginosa from pediatric cystic fibrosis patients. Infect Dis Ther. 2017;6(1):129–136. doi: 10.1007/s40121-016-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dean C.R., Visalli M.A., Projan S.J., Sum P.E., Bradford P.A. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother. 2013;47:972–978. doi: 10.1128/AAC.47.3.972-978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dietz H., Pfeifle D., Wiedemann B. The signal molecule for beta-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob Agents Chemother. 2017;4(10):2113–2120. doi: 10.1128/aac.41.10.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doi Y., Ghilardi A.C., Adams J., Garcia D.O., Paterson D.L. High prevalence of metallo-β-lactamase and 16S rRNA methylase coproduction among imipenem- resistant Pseudomonas aeruginosa isolates in Brazil. Antimicrob Agents Chemother. 2007;51:3388–3390. doi: 10.1128/AAC.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verma Y.K., Verma M.K. CT gene modulates differential expression of chitinase gene under variant habitats in Vibrio's. Asian Pac J Trop Dis. 2013;3(1):20–25. [Google Scholar]
- 110.Firych J.K., Kozłowska A., Sukhadia S., Al-Mosawi L.K. Hospital-acquired infections caused by antibiotic resistant bacteria. Postępy Nauk MedycznycHh. 2011;11 [Google Scholar]
- 111.Lambert P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;95:22–26. [PMC free article] [PubMed] [Google Scholar]
- 112.Liew Y.X., Tan T.T., Lee W. Risk factors for extreme-drug .resistant Pseudomonas aeruginosa infections in patients with hematologic malignancies. Am J Infect Contr. 2013;41:140–144. doi: 10.1016/j.ajic.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 113.Richards M.J., Edwards J.R., Culver D.H., Gaynes R.P. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27(5):887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 114.Verma M.K., Pulicherla K.K. Broad substrate affinity and catalytic diversity of fibrinolytic enzyme from Pheretima posthumous—purification and molecular characterization study. Int J Biol Macromol. 2017;95:1011–1021. doi: 10.1016/j.ijbiomac.2016.10.090. [DOI] [PubMed] [Google Scholar]
- 115.Verma M.K., Pulicherla K.K. Enzyme promiscuity in Earthworm serine protease- Substrate versatility and therapeutic potential. Amino Acids. 2016;48(4):941–948. doi: 10.1007/s00726-015-2162-3. [DOI] [PubMed] [Google Scholar]
- 116.Verma M.K., Pulicherla K.K. Targeting therapeutics across the Blood-Brain Barrier (BBB), Prerequisite towards thrombolytic therapy for a cerebrovascular disorders-an overview and advancements AAPS. Pharm Sci Tech. 2015;16(2):223–233. doi: 10.1208/s12249-015-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verma M.K., Xavier F., Verma Y.K., Sobha K. Evaluation of the cytotoxic and antitumor activity of serine proteases isolated and purified from the Indian earthworm Pheretmia posthuma. Asian Pac J Trop Biomed. 2013;3(11):896–901. [Google Scholar]
- 118.Urrea M., Pons M., Serra M., Latorre C., Palomeque A. Prospective incidence study of nosocomial infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2003;22:490–494. doi: 10.1097/01.inf.0000069758.00079.d3. PubMed PMID: 12799503. [DOI] [PubMed] [Google Scholar]
- 119.Vincent J.L., Rello J., Marshall J. International study of the prevalence and outcomes of infection in intensive care units. J Am Med Assoc. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 120.Brusselaers N., Vogelaers D., Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47. doi: 10.1186/2110-5820-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boucher H.W., Talbot G.H., Bradley J.S. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 122.Fagon J.Y., Novara A., Stephan F., Girou E., Safar M. Mortality attributable to nosocomial infections in the ICU. Infect Control Hosp Epidemiol. 1994;15(7):428–434. doi: 10.1086/646946. [DOI] [PubMed] [Google Scholar]
- 123.Vosylius S., Sipylaite J., Ivaskevicius J. Intensive care unit acquired infection: a prevalence and impact on morbidity and mortality. Acta Anaesthesiol Scand. 2003;47:1132–1137. doi: 10.1034/j.1399-6576.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 124.Papia G., McLellan B.A., El-Helou P. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma. 1999;47:923–927. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]