Abstract
Inflammation drives the development of depression and may affect neurotransmitters and thus neurocircuits increase the risk of depression. To investigate the influence of inhibition of inflammatory pathways on the biogenic amine neurotransmitters metabolism in depressive rats, sertraline, and meloxicam, the inhibitors of arachidonic acid - cyclooxygenase-2/lipoxygenase (AA-COX-2/5-LO) pathways, were given to depressive rats. After the development of depression model by chronic unpredictable mild stress (CUMS) for 6 weeks, Successful modeling rats were selected and randomly divided into CUMS group and medication administration group. After given medicine, The biogenic amine neurotransmitters in rat cortex and hippocampus were measured by high-performance liquid chromatography equipped with an electrochemical detector (HPLC-ECD). Compared with the normal group, the concentration of norepinephrine (NE) significantly decreased and the concentrations of Tyrosine (Tyr), Tryptophan (Trp), 3,4-dihydroxyphenyl acetic acid (DOPAC), 3-methoxy-4-hydroxyphenylglycol (MHPG), homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA) significantly increased in the CUMS group. Sertraline significantly inhibited the elevation of 5-HIAA. Meloxicam inhibited the decrease of NE level in CUMS-induced rat and the increase of Trp, MHPG, and 5-HIAA level in a dose-dependent manner. Caffeic acid inhibited the decrease of NE and the increase of Trp and MHPG in a dose-dependent manner. The inhibition of AA-COX-2/5-LO pathways can improve the behaviors of depression rats and suppress CUMS-induced changes in biogenic amines. Compared with the single-dose lipoxygenase (5-LO) or Cyclooxygenase-2 (COX-2) inhibitor, the combination treatment with meloxicam 1 mg/kg and caffeic acid 10 mg/kg have no significant improvement in CUMS-induced depression behavior and the level of cortical monoamine neurotransmitters and their metabolites.
Keywords: AA-COX-2/5-LO inflammatory pathways, Bioamine neurotransmitters, Caffeic acid, Depression rat, Meloxicam, Sertraline
Introduction
Depression is a kind of emotional disorder with the major characteristics of sustained depressed mood.1 Although the mechanism of depression has not yet been completely understood, it involved the decrease of monoamine neurotransmitters such as serotonin (5-HT)/norepinephrine (NE) or the alteration of their receptors in number and sensitivity, hypothalamic–pituitary–adrenal axis dysfunction, and neuroplasticity dysfunction.2 In 1995, Maes proposed that depression was a disease caused by the dysfunction of neuropsychiatric immune and the activation of the inflammatory response system.3 Now, the anti-inflammatory is a potential therapy, because pathogenesis of depression is increasingly recognized as immune activation and secretion of proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), leukotrienes (LTs), and prostaglandins (PGs).4, 5, 6
Arachidonic acid (AA) is a precursor of many biologically active substances and leukotrienes and prostaglandins are inflammatory metabolites of it. At present, there are mainly three types of enzymes involved in the metabolism of arachidonic acid, namely cyclooxygenase (COX), lipoxygenase (LO), and cytochrome P450(CYP450). The key enzymes and metabolites of these three metabolic pathways are closely related to the occurrence and development of inflammation.7 Arachidonic acid is converted into PGH2 with the participation of COX-1/2 and then into PGE2 with microsomal PGE2 synthetase. In inflammatory reactions, PGE2 involves all the classical processes of inflammation. PGE2 can be considered as proinflammatory cytokines to regulate the differentiation of immune cells or up-regulate the expression of cytokines during inflammation, thus promoting inflammation.8, 9 Similarly, Arachidonic acid can be converted into leukotrienes with 5-LO. In inflammatory cells, leukotrienes are converted into leukotriene B4 (LTB4) and cysteinyl leukotrienes (CysLTs) which participate in central inflammatory responses.10 LTB4 is a chemokine and agonist of inflammatory cells and plays a role in allergic and inflammatory reactions. CysLTs are considered as an important inflammatory mediator. In asthma, CysLTS can significantly modulate inflammatory responses and regulate the production and secretion of cytokines.11 CysLTs can also stimulate most cells to release inflammatory mediators such as histamine.12 The key link between the inflammation and depression may be 5-LO. The pro-inflammatory cytokines are closely related to the pathogenesis of depression. The possible mechanism is that excessive expression of pro-inflammatory cytokines is related to the depletion of central 5-HT and structural changes of the brain, which can promote the death of nerve cells.13
When inflammation occurs, the expression levels of COX-2 not only strongly increased in cells which were closely related to inflammation such as monocytes, macrophages, and central neurons but also are related to the severity of inflammation.14 5-LO can promote the production of inflammatory factor LTs. The inhibition of COX-2 pathway can lead to the compensatory of 5-LO, resulting in more LTs. It is reported that 5-LO may be a common biologic mechanism involved in both atherosclerosis and depression and that 5-LO not only affects the deposition of amyloid-beta, but is involved in the regulation of neurotransmitters receptor in the brain.15, 16 The increased expression levels of COX-2 and 5-LO can accelerate the production of inflammatory factors, thus affecting the metabolism of monoamine neurotransmitters.17, 18
Therefore, the purpose of our study was to explore the influence of inhibiting of AA-COX-2/5-LO inflammatory pathways on the monoamine neurotransmitters metabolism including Tyrosine (Tyr), Tryptophan (Trp), Dopamine (DA), NE, epinephrine(E), 5-HT, 3-methoxytyramine (3-MT), 3,4-dihydroxyphenyl acetic acid (DOPAC), 3-methoxy-4-hydroxyphenylglycol (MHPG), homovanillicacid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA). HPLC-ECD was adopted to determine the neurotransmitters. In our study, meloxicam was applied to improve the depression symptom by inhibiting COX-2 pathway, however, caffeic acid was used to inhibit 5-LO pathway to improve the depression symptom. It was expected that AA-COX-2/5-LO inflammatory pathways and the changes of monoamine neurotransmitters metabolism were effectively linked to depression.
Materials and methods
Standards and reagents
Sertraline, Meloxicam and Caffeic acid were provided by Kunshan Rotam Reddy Pharmaceutical Co., Ltd (Kunshan, China). Sodium carboxymethyl cellulose (CMC-Na) was purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Tyr, Trp, DA, DOPAC, E, NE and vanillic acid as the internal standard (IS) were purchased from Aladdin (Shanghai, China). 5-HT, 5-HIAA, 3-MT, HVA, MHPG were purchased from Sigma–Aldrich. 1-octanesulfonic acid sodium salt (OSA) was supplied by J&K Scientific Ltd (Beijing, China). Acetonitrile of HPLC grade was purchased from TEDIA (America). Sodium acetate, citric acid and ethylene-diamine-tetra-acetic acid disodium salt (EDTA-2Na) were of analytical grade. Ultrapure water was produced by a Milli-Q system.
Animals
A total of 90 male Sprague–Dawley (SD) rats weighing 180–220 g, 8 weeks old, were provided by the Experimental Animal Center of Chongqing Medical University and housed under standard laboratory conditions (24 ± 1 °C, 60–70% humidity, 12: 12 h dark: light cycle) with free access to both water and food. Rats were acclimatized for one week prior to experiments. All procedures were carried out by the National Institutes of Health guide for the care and use of Laboratory Animals. The animals in this research was approved by Chongqing Medical University Animal Experimental Center Ethics Committee.
Animal handling
Rats were randomly divided into control group (n = 10) and experimental group (n = 80). Control group were group housed five per cage, while the experimental group was individually housed in plastic cages with wooden shavings. The CUMS model was established according to the protocol described by Brotto with some modifications. Rats in the experimental group were subjected to different stressors for six weeks. Stressors included the following: tail nip for 1 min (1 cm from the end of the tail), cold water swimming (5 min at 4 °C), hot water swimming (5 min at 45 °C), wet bedding (24 h), noise (92 dB, 1500 Hz, 2 h), food and water privation (24 h), water privation plus cage tilting 45°(24 h), and inversion of light/dark cycle (24 h). The eight stressors were applied randomly and each stressor was not applied consecutively during 72 h. The control group was housed in a separated room without contacting with stressed rats. After six-week CUMS exposure, 70 rats of experimental group with significant differences in behavior indexes were selected, randomly divided into CUMS group (n = 10), Sertraline 5 mg/kg group (n = 10), Meloxicam 3 mg/kg group (n = 10), Meloxicam 1 mg/kg group (n = 10), Caffeic acid 30 mg/kg group (n = 10), Caffeic acid 10 mg/kg group (n = 10), Meloxicam 1 mg/kg + Caffeic acid 10 mg/kg group (n = 10). The rats of the control group and CUMS group were oral gavage with 0.5% CMC-Na, others were orally treated with drugs depending on their weight. Drug administration started after six-week CUMS exposure and lasted for 3 weeks.
Behavior testing
Open Field Test (OFT)
OFT proceeded in dark with visibility of 5 m. The open field apparatus was a wooden box with 25 black squares in the bottom (100 × 100 × 50 cm). SD rats were individually placed in the center of black squares and their activity of vertical movement and horizontal movement was recorded during 5 min. The apparatus was cleaned with 75% alcohol and dried after each trial to avoid residual odors. The experiment room was quiet, with dim lighting.
Forced swimming test (FST)
The test was performed according to the method described by Makino. Et al with minor modification. Animals were individually placed in an opened transparent cylindrical container (30 × 50 cm) with water depth of 30 cm and water temperature of 24 ± 2 °C. After 2 min of adaptable swimming, the total duration of immobility was recorded in the following 5 min. When the rat floated without struggling and kept its head above the water, the spent time was defined as the duration of immobility.
Neurotransmitters determination by HPLC-ECD
Sample preparation and HPLC-ECD analysis were based on the method of our previous research.19
Preparation of standard solution
The individual stock solution of analytes was prepared by dissolving the dry standards or its salt with 0.1 mol/L HClO4 solution, obtaining a concentration of 1.0 mg/mL for all analytes, except dl-tyrosine stock solution which was 100 μg/ml. The stock solution was stored at −20 °C and further diluted to mixed working standard solutions with 0.1 mol/L HClO4 solution until use.
Sample preparation
The cortex of rat was separated immediately after decapitation on an ice plate and was stored at −80 °C until use. The isolated tissues were weighed and then homogenized with 6 μL chilled 0.1 mol/L HClO4 containing 1 μg/mL vanillic acid (IS) per mg tissue sample. The homogenates were centrifuged at 4 °C for 15 min at 20,000 × g. Then the supernatant was filtered using a 0.2 μm Millipore® filter (Millex, Millipore, Ireland) attached to a syringe. Finally, 20 μL of liquor from the resulting solution was injected into the HPLC-ECD system for analysis.
Apparatus and analytical conditions
The SHIMADZU HPLC system consisted of a CBM-20A communication bus module, a DGU-20A3R degassing unit, and two LC-20AD pumps. Electrochemical detection was performed using an amperometric detector ED723 coupled with three electrodes including a diamond working electrode with a surface area of 1.44 cm2, an Ag/AgCl reference electrode and a stainless steel counter electrode. The flow cell is a thin layer type with the volume of 1.5 μL. Separation of analytes was performed on a Hypersil ODS2 column (250 mm × 4.6 mm, 5.0 μm particle size, Elite analysis instrument co., LTD, Dalian, China) fitted with a C18 security guard cartridge (phenomenex, American) at a flow rate of 1.0 mL/min. The mobile phase was composed of an aqueous solution and acetonitrile in the ratio of 90/10. The aqueous portion contained 25 mmol/L sodium acetate, 25 mmol/L citric acid, 0.01 mmol/L EDTA-2Na and 1.0 mmol/L OSA, adjusting pH to 3.5 with acetic acid. The mobile phase was vacuum-filtered through a 0.22 μm cellulose acetate membrane and degassed for 10 min. The column oven temperature was set at 30 °C and the injection volume was 20 μL. The analytical potential of the detector was set at 700 mV within the output range of 10 μA. The chromatograms were integrated with Shimadzu Software.
Statistical analysis
All results were expressed as mean ± SD (standard deviation). Statistical significance was assessed through one-way analysis of variance (ANOVA) followed by least significant difference using SPSS17.0 software. A value of P < 0.05 was considered to be statistically significant.
Results
Behavior testing
Open Field Test (OFT)
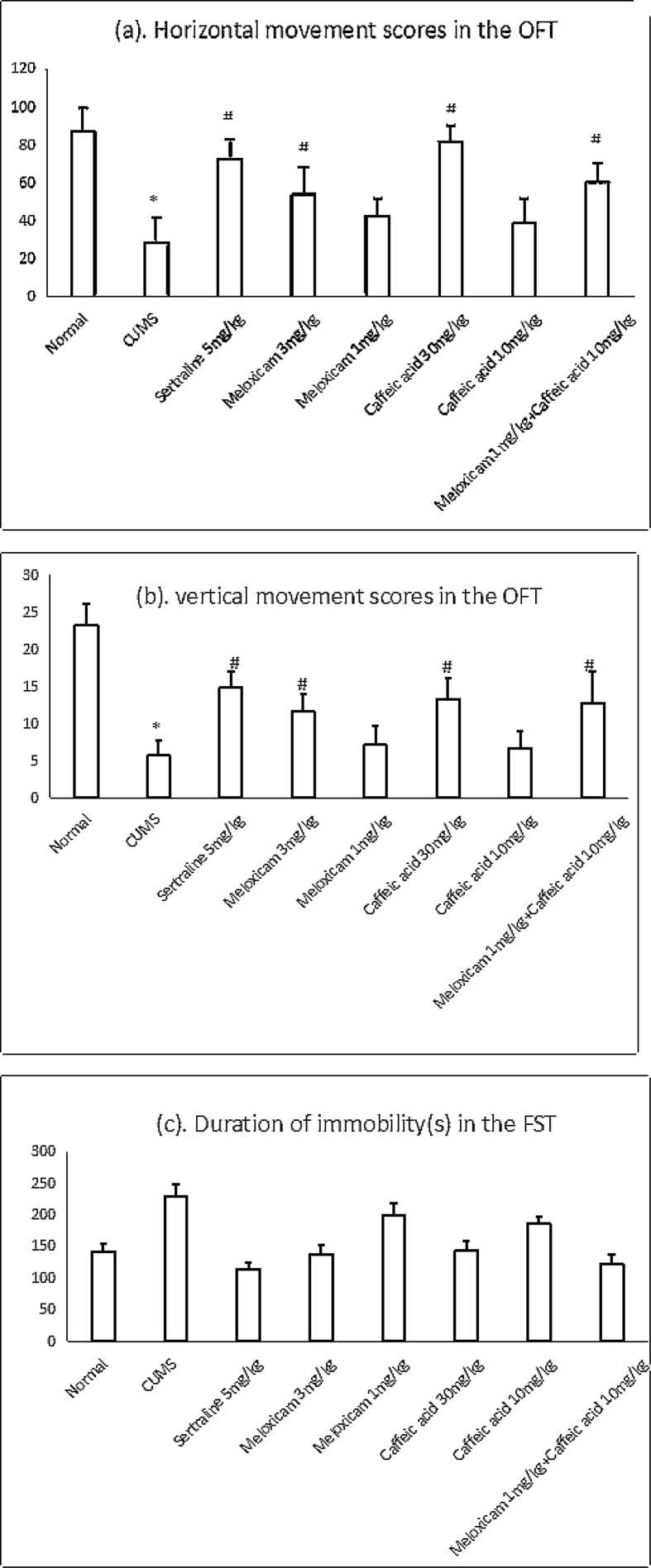
Fig. 1a and Fig. 1b indicated that CUMS rats showed a significant decrease in vertical and Horizontal movements in Open Field Test compared with the control group (*P < 0.01), which indicated that CUMS-induced rats displayed depression-like obviously. The administration of Sertraline 5 mg/kg, Meloxicam 3 mg/kg, Caffeic acid 30 mg/kg or Meloxicam 1 mg/kg + Caffeic acid 10 mg/kg prevented a decrease in locomotive activity (#P < 0.05) in those rats compared with CUMS rats. Whereas administration of Meloxicam 1 mg/kg or Caffeic acid 10 mg/kg showed no significant significant decrease in vertical and Horizontal movements in those rats compared with CUMS rats.
Figure 1.
Effect of drugs treatment on depressive-like behaviors in rats. *p<0.01 vs corresponding value of Normal, #p<0.05 vs corresponding value of CUMS.
Forced swimming test (FST)
Fig. 1c indicated that CUMS rats showed that the duration of immobility in the CUMS rats prolonged significantly (*p < 0.01) in FST compared with the control group. Whereas administration of Sertraline 5 mg/kg, Meloxicam 3 mg/kg, Caffeic acid 30 mg/kg or Meloxicam 1 mg/kg + Caffeic acid 10 mg/kg inhibited the prolongation of immobility time (#p < 0.05) in those rats compared with CUMS rats. Administration of Meloxicam 1 mg/kg or Caffeic acid 10 mg/kg showed no significant inhibition the prolongation of immobility time in those rats compared with CUMS rats.
Determination of monoamine neurotransmitters
The monoamine neurotransmitters and related metabolites in the rat cortex were determined with our method. Representative chromatograms were shown in Fig. 2. The results of the control group, CUMS group, Sertraline 5 mg/kg group, Meloxicam 3 mg/kg group, Meloxicam 1 mg/kg group, Caffeic acid 30 mg/kg group, Caffeic acid 10 mg/kg group, and Meloxicam 1 mg/kg + Caffeic acid 10 mg/kg group were shown in Table 1.
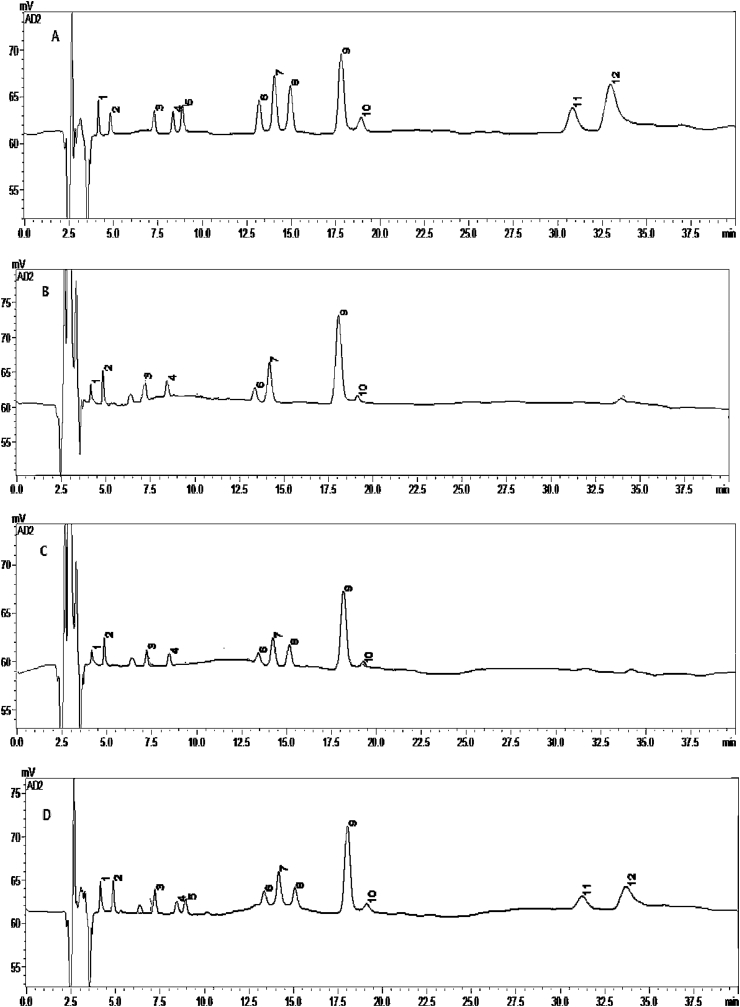
Figure 2.
Representative chromatographs of mixed standard solution (A), matrices of cortex (B), matrices spiked with IS (C), and matrices spiked with standards and IS (D). Figure note: Tyr (1), MHPG (2), NE (3), DOPAC(4), E (5), 5-HIAA (6), DA (7), vanillic acid (8), Trp (9), HVA (10), 3-MT (11), 5-HT (12).
Table 1.
Effect of Sertraline, Meloxicam, Caffeic acid on monoamine neurotransmitter together with their precursor amino acids and metabolites (mean ± s, n = 5, μg/g).
| Tyr | MHPG | NE | 5-HIAA | Trp | DA | DOPAC | HVA | |
|---|---|---|---|---|---|---|---|---|
| Control | 41.80 ± 9.19 | 1.50 ± 0.36 | 0.94 ± 0.05 | 0.19 ± 0.02 | 3.28 ± 0.57 | 0.15 ± 0.05 | 0.36 ± 0.04 | 0.18 ± 0.02 |
| CUMS | 123.71 ± 12.53∗ | 2.41 ± 0.48∗ | 0.74 ± 0.06∗ | 0.24 ± 0.03∗ | 5.08 ± 1.07∗ | – | 0.58 ± 0.19∗ | 0.33 ± 0.07∗ |
| Sertraline 5 mg/kg | 128.09 ± 9.77∗ | 2.48 ± 0.31∗ | 0.65 ± 0.13∗ | 0.14 ± 0.01∗# | 10.38 ± 0.72∗# | – | – | – |
| Meloxicam 3 mg/kg | 103.76 ± 7.87∗# | 2.42 ± 0.28∗ | 0.81 ± 0.15∗ | 0.17 ± 0.02∗# | 6.09 ± 1.68∗ | – | 0.22 ± 0.06∗# | 0.13 ± 0.01∗# |
| Meloxicam 1 mg/kg | 93.71 ± 4.38∗# | 1.82 ± 0.32∗# | 0.67 ± 0.05∗# | 0.16 ± 0.02∗# | 5.84 ± 0.47∗ | – | 0.18 ± 0.06∗# | 0.13 ± 001∗# |
| Caffeic acid 30 mg/kg | 52.96 ± 7.55∗# | 2.27 ± 0.39∗ | 0.80 ± 0.05∗# | 0.15 ± 0.03∗# | 3.62 ± 0.23# | – | 0.08 ± 0.01∗# | – |
| Caffeic acid 10 mg/kg | 42.13 ± 8.50# | 1.77 ± 0.42# | 0.62 ± 0.01∗# | 0.18 ± 0.04# | 4.90 ± 0.93∗ | – | 0.18 ± 0.04∗# | – |
| Meloxica + Caffeic acid | 109.09 ± 6.93∗# | 1.95 ± 0.23∗# | 0.61 ± 0.08∗# | 0.17 ± 0.03# | 8.43 ± 0.80∗# | – | 0.45 ± 0.16# | 0.23 ± 0.01∗# |
Table note: *, P < 0.05, VS control group; #, P < 0.05, VS CUMS group.
The results showed that DA could be quantitatively detected only in the control group, revealing that the level of DA in the cerebral cortex of depressive rats was decreased and that the increment of DA could not reach at the detection level while varieties of anti-inflammation treatments were applied to improve depressive symptoms. Compared with the control group, the NE concentration in the CUMS group was dramatically declined while the levels of Tyr, MHPG, 5-HIAA, Trp, DOPAC, and HVA were significantly increased, which suggested that the decreased level of NE and increased levels of its related metabolites in the cerebral cortex were probably connected to the presence of depression. What's more, the increased Tyr and Trp may be associated with the decline of NE and the compensatory increase of monoamine synthesis precursors. Treatment with sertraline (5 mg/kg/d) could not elevate the level of NE but significantly increase Trp level and reduce 5-HIAA level, uncovering that sertraline may increase 5-HT synthesis and reduce its metabolism mainly through the 5-HT system, thus getting clinical depression symptoms improved. Meloxicam inhibited the decline of NE and increase of Trp, MHPG, and Tyr with a dose-dependent manner and significantly reduced the level of DOPAC, 5-HIAA, and HVA in the cerebral cortex of CUMS-reduced rats, showing that the improved depression symptom by meloxicam may be associated with inhibited COX-2. Caffeic acid could significantly inhibit the increase of Tyr, MHPG, Trp, and 5-HIAA in a dose-dependent manner, especially when treated with caffeic acid 10 mg/kg, the increasing tendency of Tyr, MHPG, and 5-HIAA could be dramatically inhibited, which were no significant difference with the control group. Caffeic acid inhibited reduction of NE and increases of Trp and MHPG in a dose-dependent manner and inhibited the increases of 5-HIAA, Tyr, and DOPAC at the same time, revealing that 5-LO system had a relationship with NE, 5-HT, DA, and it's precursor metabolism and that inhibition of 5-LO activity may slow down the metabolic rate of NE, DA, and it's precursor metabolism, thus improving the symptom of depression.
Compared with CUMS group, the elevation of Tyr, MHPG, and 5-HIAA caused by depression could be restored to normal levels by the inhibition of 5-LO pathway, the elevation of 5-HIAA and DOPAC could be restored to normal levels by the inhibition of AA-COX-2/5-LO inflammation pathway, revealing that the inhibition of AA-COX-2/5-LO pathway indeed influenced the level of monoamine neurotransmitters and their metabolites and that the combination therapy had no obvious advantages than the single therapy.
Discussion
In our previous study, we compared the 5-HT concentrations between cortex and hippocampus and found that the level of 5-HT in the cortex was too low to be quantified.19 The level of DA was also extremely low. It may due to that DA was mainly synthesized, stored and released by dopaminergic neurons which were primarily located in the nigrostriatal system, mesolimbic system, mesocortical system and tuberoinfundibular system. Previous studies reported that anhedonia in major depression and abnormal hippocampus activity in schizophrenia were associated with the dopaminergic system.20, 21, 22 Previous animal experiments have confirmed that inflammatory cytokines or cytokines inducers affected the metabolism of 5-HT, DA, and NE.23, 24 Trp was involved in two pathways for 5-HT pathway and kynurenine metabolic pathway (KP), in which the first rate-limiting enzyme, indoleamine 2,3-dioxygenase (IDO), was inflammatory-inducible enzyme. When IDO was activated, Trp was more likely to decomposed into kynurenine (Kyn), thus reducing the synthesis of 5-HT and leading to depression. What's more, the metabolites of KP presented an influence on the metabolism of neurotransmitters. 3-hydroxy-kynurenine (3-HK) could generate a large amount of oxygen free radicals to enhance the activity of monoamine oxidase and accelerate the metabolism of 5-HT in synaptic cleft.25, 26, 27 Studies confirmed that cytokines could promote the synthesis of nitric oxide and lessen tetrahydropterpine which was an important cofactor of rate-limiting tyrosine hydroxylase in the process of tyrosine converting into DA. The reduction of tetrahydropterpine could result in tyrosine hydroxylase activity declining, therefore decreasing the synthesis of DA.28, 29
There was a potential antidepressant mechanism that neurogenesis and synaptic connectivity could get enhanced by synaptogenesis, reorganization, or reintegration of new neurons into depression neurocircuitry.30, 31 Sertraline, as one kind of selective serotonin reuptake inhibitors (SSRI), could inhibit the reuptake of 5-HT to increase the synaptic cleft content of 5-HT. 5-HT was primarily released by serotonergic neurons of which the activity was negatively regulated by 5-HT1A receptors.32 Depression animal models have shown that depression could decrease the 5-HT1A autoreceptor susceptibility33 and that chronic unpredictable stress (CUS) significantly reduced 5-HT neural activity and 5-HT1A autoreceptor sensitivity.34 There was a hypothesis that the antidepressant effect of sertraline was based on the presence of altered brain substrates such as neural modifications, expression changes of serotonergic receptors, or alteration in the firing of 5-HT neurons.35 Sertraline may resist the reduction number of 5-HT neurons. Consequently, the decreased metabolism of 5-HT caused by sertraline may compensate for the alteration of 5-HT neurons. Compared with CUMS group, the increased Trp and declined 5-HIAA in sertraline group may reveal that sertraline may affect both on the central serotonergic system and IDO activity to affect the metabolism of monoamine neurotransmitters in the brain.
A literature reported that Caffeic acid (3,4-dihydroxycinnamic acid), could enhance the activity of superoxide dismutase, catalase, glutathione peroxidase to maintain the balance of intracellular redox36 and that caffeic acid could resist oxidative damages in a variety ways to protect the pathophysiological process. This capacity of antioxidant made up an important foundation for many pharmacological effects of caffeic acid.37, 38 Caffeic acid may present it's anti-inflammation on the connection with the antioxidant activity, inhibition of 5-LO and protein kinase C39, 40. Caffeic acid could regulate inflammatory mediator release and immune function, thus effectively control the neuronal oxidative damages caused by inflammation, which was responsed by inhibiting 5-LO.40, 41 Caffeic acid could significantly inhibit the increases of Tyr, MHPG, Trp, and 5-HIAA in a dose-dependent manner, especially when treated with caffeic acid 10 mg/kg, the increasing tendency of Tyr, MHPG, and 5-HIAA could be dramatically inhibited, which were no significant difference with the control group. It was a hypothesis that Caffeic acid, an inhibitor of the 5-LO pathway, mainly affected the synthesis processes of NE and 5-HT, thereby affecting the metabolism of other neurotransmitters.
Meloxicam inhibited both COX-1 and COX-2 activities, primarily COX-2 to exert it's anti-inflammatory and anti-tumor activity. When inflammation occurs, over-expression of COX-2 may cause accumulation of PGE2, which could activate diverse biological effects by its binding to a family of receptors.42, 43 We found that despite the different doses of meloxicam, they all had a significant inhibitory effect on the elevation of 5-HIAA, DOPAC, and HVA caused by depression. It was a hypothesis that meloxicam may affect the metabolic processes of 5-HT and DA and that the inhibition of COX2 activity may result in the oxidation of Tyr and Trp.
AA can produce leukotrienes and Lipoxin via the LO metabolic pathway. Lipoxin is thought to have a neuroprotective effect: lipoxin induces a large release of the immunosuppressive factor IL-10 and also reverses microvascular blood permeation during inflammation. What's more, it can inhibit lipopolysaccharide-induced inflammatory responses in endothelial cells and macrophages by inhibiting activation of nuclear factor κB (NF-κB).44, 45 A study shows that COX-2 plays a neuroprotective role in the central nervous system and that the inhibition of its activity aggravates the lipopolysaccharide-induced neuroinflammatory response.46 Intrinsic COX-1 can cause damage to the central nervous system with pro-inflammatory factors (lipopolysaccharide, amyloid β-protein (Aβ), interleukin).47 Studies have shown that up-regulation of COX-2 results in the accumulation of Aβ when inflammation occurs.48 COX and LO, as the main metabolic pathway of AA, are highly expressed in inflammation. The compensation mechanism between COX and LO follows a compensatory mechanism, namely, there is a balance between the two sides. When one path is inhibited, metabolism will rush to the other path. Actually, in Ying Luo's previous study, she had measured the expression of the mRNA and protein of Cox-2/5-LO, superoxide dismutase (SOD) activity, malondialdehyde (MDA) content, and inflammatory factors such as IL-6, TNF-A, PGE2, and LTB4 levels in depression rats brain tissue, which were the same batches as ours, when caffeic acid, meloxicam, and combination therapy administered together. She founded that the combined effect of AA-COX2/5-LO pathway intervention on learning and memory dysfunction in depressed rats was significantly better than single-channel intervention. However, our experimental results show that the combined effect of COX-2 and 5-LO inhibitors did not have a clear advantage over neurotransmitters when compared to the effect of a single administration. This may be due to this compensatory mechanism resulting in a reduction of lipoxin and weakness of its inhibitory effect on the lipopolysaccharide-induced inflammatory response when simultaneous administration of caffeic acid and meloxicam was adopted. When inflammation occurs, Aβ may accumulate and meloxicam mainly inhibits COX-2, which may aggravate lipopolysaccharide-induced neuritis. COX-1 may cause nerve damage with the effect of Aβ. The simultaneous administration of caffeic acid and meloxicam may weaken the body's inhibitory effect on lipopolysaccharide-induced inflammatory responses, thereby aggravating central nervous system injury. Therefore, the simultaneous administration of caffeic acid and meloxicam is not superior to a single dosing regimen in improving depression-related monoamine substances. Of course, the reason remains to be further studied.
Conclusion
The inhibition of AA-COX-2/5-LO pathway indeed influenced monoamine neurotransmitters and their metabolites. The anti-inflammatory therapy with inhibition of AA-COX-2/5-LO activity can improve the behaviors of depression rats, which the mechanism may be associated with the decrease of inflammation-related monoamine neurotransmitters and oxidative metabolism of their precursors. Compared with the single-dose COX-2 or 5-LO inhibitor, the combination treatment with meloxicam 1 mg/kg and caffeic acid 10 mg/kg have no significant improvement in CUMS-induced depression behavior and the level of cortical monoamine neurotransmitters and their metabolites.
Conflict of interest
There are no conflicts of interest.
Acknowledgement
This research was carried out by all authors in collaboration. This work was supported by pharmacy school of Chongqing Medical University. This research work was financially supported by Research Fund of Chongqing Science & Technology Commission (No: cstc2013jcyjA10040) and Research Start-up Fund of Pharmacy School of Chongqing Medical University.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Yoo H.J., Hong J.P., Cho M.J. Lifetime suicidal ideation and attempt in adults with full major depressive disorder versus sustained depressed mood. J Affect Disord. 2016;203:275–280. doi: 10.1016/j.jad.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Leonard B.E. Impact of inflammation on neurotransmitter changes in major depression: an insight into the action of antidepressants. Prog Neuro-psychoph Biol Psychiatr. 2014;48:261–267. doi: 10.1016/j.pnpbp.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Maes M., Vandoolaeghe E., Ranjan R., Bosmans E., Bergmans R., Desnyder R. Increased serum interleukin-1-receptor antagonist concentration in major depression. Affect Disord M. 1995;36:29–36. doi: 10.1016/0165-0327(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 4.Nunes S.O., Reiche E.M., Morimoto H.K. Immune and hormonal activity in adults suffering from depression. Braz J Med Biol Res. 2002;35:581–587. doi: 10.1590/s0100-879x2002000500011. [DOI] [PubMed] [Google Scholar]
- 5.Miller A.H. Raison. CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikova O., Yakimova R., Bosmans E., Kenis G., Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur J Pharmacol. 2001;11:203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 7.Calder P.C. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009:91791–91795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Yao C., Sakata D., Esaki Y. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med. 2009;6:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 9.Legler D.F., Bruckner M., Uetzvon Allmen E., Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010;2:198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y., Calhoun W.J. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–798. doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Bigby T.D. The yin and the yang of5-lipoxygenase pathway activation. Mol Pharmacol. 2002;62:200–202. doi: 10.1124/mol.62.2.200. [DOI] [PubMed] [Google Scholar]
- 12.Kaori S., Kaori M., Hiroyuki K. Cysteinyl leukotrienes enhance the degranulation of bone marrow-derived mast cells through the autocrine mechanism. Tohoku J Exp Med. 2009;3:185–191. doi: 10.1620/tjem.217.185. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblat J.D., Cha D.S., Mansur R.B., Mclntyre R.S. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;53:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Minghetti L. Cyclooxy genase-2 (COX-2)in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 15.Manev H., Manev R. 5-lipoxygenase as a possible biological link between depressive symptoms and atherosclerosis. Arch Gen Psychiatr. 2007;64:1333. doi: 10.1001/archpsyc.64.11.1333. [DOI] [PubMed] [Google Scholar]
- 16.Rådmar O., Samuelsson B. 5-Lipoxygenase: regulation and possible involvement in atherosclerosis. Prostag Other Lipid Mediat. 2007;83:162–174. doi: 10.1016/j.prostaglandins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Leo L.M., Almeida S., Canetti C.A., Canetti C.A., Amaral O.B. Age-dependent relevance of endogenous 5-lipoxygenase derivatives in anxiety-like. PLoS One. 2014;9:500–509. doi: 10.1371/journal.pone.0085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manev H., Manev R., Vidovich M.I. Depression, inflammation, and cardiovascular disease: is 5-lipoxygenase the missing link. Am Coll Cardiol. 2008;51:1990–1991. doi: 10.1016/j.jacc.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Yang J.Q., Luo Y., Shang J.C., Jiang X.H. Simultaneous determination of eleven compounds related to metabolism of bioamines in rat cortex and hippocampus by HPLC-ECD with boron-doped diamond working electrode. J Pharmaceut Biomed Anal. 2016;118:41–51. doi: 10.1016/j.jpba.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Treadway M.T., Zald D.H. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill K.M., Lodge D.J., Cook J.M., Aras S., Grace A.A. A novel alpha5GABA (A) R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton M.H., Bizup B.T., Grace A.A. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci. 2013;33:16865–16873. doi: 10.1523/JNEUROSCI.2449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anisman H., Merali Z., Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:71–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Felger J.C., Alagbe O., Hu F. Effects of interferon-alpha on rhesus monkeys: a non-human primate model of cytokine-induced depression. Biol Psychiatr. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura H., Ozaki N., Sawada M., Isobe K., Ohta T., Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 26.Kim S., Miller B.J., Stefanek M.E., Miller A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer. 2015;121:2129–2136. doi: 10.1002/cncr.29302. [DOI] [PubMed] [Google Scholar]
- 27.Karu N., Mckercher C., Nichols D.S. Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: Tasmanian Chronic Kidney Disease pilot study. BMC Nephrol. 2016:17. doi: 10.1186/s12882-016-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagami T., Yamada K., Miura H., Hashimoto R., Nabeshima T., Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- 29.Krzyaniak M.D., Eser B.E., Ellis H.R., Fitzpatrick P.F., McCracken J. A pulsed EPR study of amino acid and Tetrahydropterin binding in a tyrosine hydroxylase nitric oxide complex: evidence for substrate rearrangements in formation of the oxygen-reactive complex. Biochemistry. 2013;52:8430–8441. doi: 10.1021/bi4010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duman R.S., Li N.A. Neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B Biol Sci. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahar I., Bambico F.R., Mechawar N., Nobrega J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Hall H., Lundkvist C., Halldin C. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H] WAY-100635 and [11C] WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 33.Froger N., Palazzo E., Boni C. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bambico F.R., Nguyen N.T., Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur J Pharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Ulloa J.L., Castaneda P., Fiedler J.L. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticoaterone administration. Pharmacol Biochem Behav. 2010;97:213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Facino R.M., Carini M., Aldini G., Saibene L., Pietta P., Mauri P. Echinacoside and caffeoyl conjugates protect collagen from free radical-induced degradation - a potential use of echinacea extracts in the prevention of skin photodamage. Planta Med. 1995;61:510–514. doi: 10.1055/s-2006-959359. [DOI] [PubMed] [Google Scholar]
- 37.Bakir S., Ozbay M., Gun R. The protective role of caffeic acid phenethyl ester against streptomycin ototoxicity. Am J Otolaryngol. 2013;34:16–21. doi: 10.1016/j.amjoto.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Khanduja K.L., Avti P.K., Kumar S., Mittal N., Sohi K.K., Pathak C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: a Bcl-2 independent mechanism. Biochim Biophys Acta. 2006;1760:283–289. doi: 10.1016/j.bbagen.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Gamaro G.D., Suyenaga E., Borsoi M., Lermen J., Pereira P., Ardenghi P. Effect of rosmarinic and caffeic acid on inflammatory and Nociception process in rats. ISRN Pharmacol. 2011 doi: 10.5402/2011/451682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S.R., Jung Y.R., Kim D.H. Caffeic acid regulates LPS-induced NF-jB activation through NIK/IKK and c-Src/ERK signaling pathways in endothelial cells. Arch Pharm Res. 2014;37:539–547. doi: 10.1007/s12272-013-0211-6. [DOI] [PubMed] [Google Scholar]
- 41.Mehrotra A., Shanbhag R., Chamallamudi M.R., Singh V.P., Mudgal J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur J Pharmacol. 2011;666:80–86. doi: 10.1016/j.ejphar.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 42.Engelhardt G., Schnitzer C., Utzmann R., Utzmann R. Meloxican: influence on arachidonic acid metabolism: Part 1. In vitro findings. Biochem Pharmacol. 1996;51:21–28. doi: 10.1016/0006-2952(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 43.Dong X.F., Li R., Li J. Meloxicam executes its antitumor effects against hepatocellular carcinoma in COX-2- dependent and -independent pathways. PLoS One. 2014;9:e92864. doi: 10.1371/journal.pone.0092864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ereso A.Q., Cureton E.L., Cripps M.W. Lipoxin A(4) attenuates microvascular fluid leak during inflammation. J Surg Res. 2009;2:183–188. doi: 10.1016/j.jss.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kure I., Nishiumi S., Nishitani Y. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Therapeut. 2010;2:541–548. doi: 10.1124/jpet.109.159046. [DOI] [PubMed] [Google Scholar]
- 46.Aid S., Langenbach R., Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:1–14. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aid S., Bosetti F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: therapeutic implications. Biochimie. 2011;93:46–51. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferretti M.T., Bruno M.A., Ducatenzeiler A., Klein W.L., Cuello A.C. Intracellular A beta-oligomers and early inflammation in a model of Alzheimer's disease. Neurobiol Aging. 2012;33:1329–1342. doi: 10.1016/j.neurobiolaging.2011.01.007. [DOI] [PubMed] [Google Scholar]