Abstract
To investigate the association of specific ultrasonography features identified during the diagnosis of early pregnancy loss (EPL) and abnormal karyotype. This was a systematic review and meta-analysis conducted in accordance with PRISMA criteria. We searched PubMed, Cochrane and Ovid MEDLINE from 1977 to Jan 2017 to identify the articles that described EPL with karyotype and ultrasonography features. Risk differences were pooled to estimate the chromosomal abnormality rates in ultrasonography features, including pre-embryonic, enlarged yolk sac (YS), short crown rump length (CRL), small gestational sac (GS), symmetrical arrested growth embryo, or gestational sac with only a YS. Quality assessment of included studies was performed using Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklists for Observational Studies (2007 version). Thirteen studies were included in the meta-analysis. Chromosomal abnormality was more likely to occur in embryonic EPL and enlarged YS. On the other hand, short CRL, small GS, symmetrical arrested growth embryo, or gestational sac with only a YS, were not associated with an increased risk of fetal chromosomal abnormality. Ultrasonography features at the time of diagnosis of EPL have limited predictive value of fetal chromosomal abnormality.
Keywords: Ultrasonography, Early pregnancy loss, Chromosomal abnormality, Predictive value
Introduction
In natural conception, about 15% of clinical pregnancies results in miscarriage1; whereas in in vitro fertilization (IVF), the miscarriage rate fluctuates at a higher level of 20%–30%.2, 3 Most pregnancy loss occurs in the first trimester, especially during 6–8 weeks of gestation.4, 5, 6 Chromosomal abnormalities account for about half of all the early pregnancy losses (EPLs) in both natural and assisted reproductive conceived pregnancies.1, 7
Transvaginal ultrasound (TVS) has been proven to be a reliable technique to monitor the morphological development of human embryo as early as 5 weeks of gestation.8 After the implantation of the embryo, the first embryonic structure that can be seen by TVS is a sac structure formed by extraembryonic mesoblast, clinically known as the gestational sac (GS) at around 5 weeks of gestation. At around 6 weeks of gestation, the secondary yolk sac (YS) and the primary germ layers, referred to as “fetal pole” in ultrasound terminology, could be detected within the gestational sac. During the 7th week, the primitive cardiac tube could be detected by TVS, referred to as “fetal heart”. These developmental changes could be identified by transabdominal ultrasound (TAS) about 5 days later compared with TVS,9 so TVS were usually preferred in early pregnancy scanning. Several ultrasonography features were observed in early pregnancy. Based on the presence or absence of an embryonic pole (fetal pole) during ultrasound scanning, sonographic features of EPL were commonly categorized into embryonic or anembryonic/pre-embryonic, also referred to as ‘with’ or ‘without embryo’; whereas, ‘empty sac’ was described by continued absence of embryonic structures inside the GS in serial scans.10 A slightly difference may exist between features of an ‘empty sac’ versus an ‘anembryonic’ pregnancy, in terms of whether or not a YS can be seen or not.6, 7 Secondary yolk sac is considered as enlarged when the diameter exceeds 95th centile for expected size or more than 6 mm; short crown rump length (CRL) and small GS are usually described when the measurement were less than 5th centile for expected size; whilst, early symmetrical arrested growth refers to simultaneous arrest of growth of both gestational sac and CRL.10 However, during this rapidly changing stage after implantation, the ability of early ultrasound to predict pregnancy outcomes appears to be limited. Therefore, in this study we are trying to identify the association between ultrasonography features and abnormal karyotypes in early pregnancy loss.
Methods
Literature search
A literature review was performed according to PRISMA guidelines.11 Relevant citations on chromosomal abnormalities and ultrasound morphologies in early pregnancy loss were extracted from PubMed, Cochrane and Ovid MEDLINE from 1977 to Jan 2017 to identify the relevant articles. The following keywords and their synonyms were used: (“chromosomal abnormalities” OR “chromosomal imbalance” OR “chromosomal anomalies” OR “cytogenetic analysis” OR “genetic abnormalities” OR “genomic imbalance” OR “genetic anomalies” OR “aneuploidy” OR “karyotype” OR “array” OR “microarray” OR “sequencing”) AND (“early pregnancy loss” OR “pregnancy loss” OR “miscarriage” OR “abortion” OR “spontaneous abortion” OR “missed abortion” OR “failed pregnancy” OR “products of conception”) AND (“ultrasound” OR “scanning” OR “sonography” OR “transvaginal ultrasound” OR “crown rump length” OR “fetal pole” OR “gestational sac” OR “yolk sac” OR “embryonic pregnancy” OR “anembryonic pregnancy” OR “embryonic gestation” OR “anembryonic gestation”). No restrictions on language or type of publication were applied to the primary search.
Inclusion and exclusion criteria
During the screening and selection of literature, all the studies investigating the association between ultrasonographic morphology and chromosomal abnormalities in early pregnancy loss were considered eligible for inclusion. The articles had to be written in the English language. All pregnancy loss between 5 and 12 weeks of gestation with ultrasound assessment and karyotyping by either conventional G-banding or microarray were included. Studies that included only ultrasound fetal structural abnormalities or genetic screening without ultrasound parameters in early pregnancy were excluded. The pregnancies with chromosomal abnormalities but miscarried in gestational weeks after 12 weeks were also excluded. The main study outcomes were chromosomal abnormality rates in early pregnancy loss between 5 and 12 weeks of gestation, with or without a transvaginal ultrasound identifiable embryo (“fetal pole”). Other outcomes were chromosomal abnormal rates in early pregnancy loss with or without a small GS, short CRL, enlarged YS, or YS only.10 All outcomes were reported as per ultrasonography features.
Titles and abstracts of all identified studies were screened and the full paper of the preselected articles was read by two researchers (J.H. and W.Z.). If 2 × 2 tables of any ultrasonographic features could be constructed the study was selected for final inclusion.
Quality assessment
The relevance and methodological quality of each included study was scored by using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklists for Observational Studies (2007 version). The following characteristics of the studies were also taken into consideration: data collection method (prospective or retrospective), selection bias (i.e. products of conception were collected by patient at home and then submitted to laboratory), information bias (i.e. pregnancy in IVF setting would be more precisely dated than that of the LMP in natural pregnancy), and attrition bias (i.e. pregnancy loss caused by unidentified uterine factors).
Data extraction
Both researchers (J.H. and W.Z.) extracted the data from the article independently by using standardized data extraction forms. In the 2 × 2 tables, the numbers of chromosomally normal and chromosomally abnormal pregnancies for one ultrasound parameter were recorded. If multiple parameters were reported in the same study, each parameter would be documented in a 2 × 2 table.
Statistical analysis
The odds ratios (ORs) and 95% confidence intervals (CIs) were used to demonstrate the association of chromosomal abnormalities with different ultrasound features, with p value less than 0.05 considered as statistically significant. Between-study heterogeneity was measured by I2 A random-effects model was applied to pool the data. All statistical analyses in this study were performed using Mantel-Haenszel (M-H) method, random effects Review Manager 5.3 (http://community.cochrane.org/tools/review-production-tools) and listed separately.
Results
Systematic search, selection and data extraction
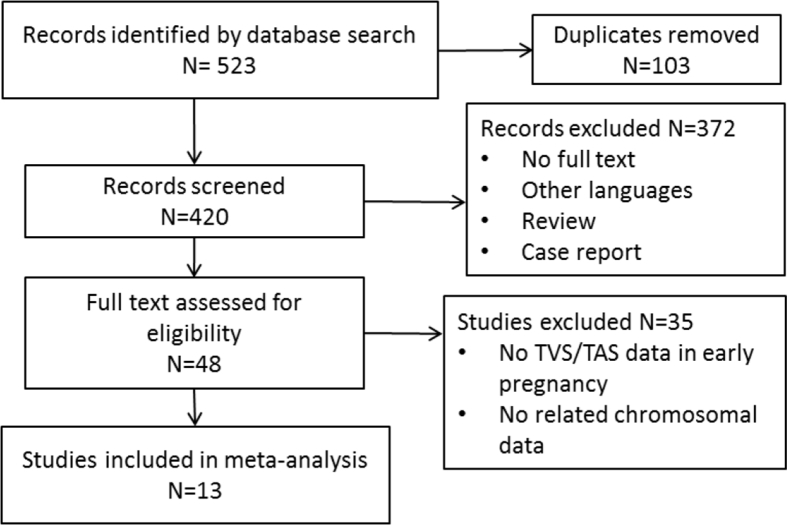
The search revealed 523 records (Fig. 1). There were 103 duplicated records that were removed. Following the screening of the titles and abstracts, 372 articles were excluded as full text was not available, non-English languages, or unsuitable article types (review, case report or book chapter). There were 48 studies identified to be potentially eligible for inclusion. After reviewing the manuscripts and assessing the inclusion criteria and methodological quality, 13 studies were eligible for final inclusion.
Figure 1.
Flow chart of systematic search, selection and data extraction according to PRISMA.
From the 13 included studies, 2 × 2 tables for the chromosomal abnormality rate in EPL with or without ultrasound identifiable embryo could be constructed6, 7, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21; four of these studies were included for the chromosomal abnormality rate in EPL with short CRL7, 10, 14, 20; two studies for the chromosomal abnormality rate in EPL with small GS7, 10; two studies for the chromosomal abnormality rate in EPL with YS only7, 17; two studies for the chromosomal abnormality rate in EPL with symmetrical arrested growth7, 10; one study for the chromosomal abnormality rate in EPL with enlarged YS.10 Table 1 summarized the general information of the 13 included studies in the analysis.
Table 1.
Summary of general information of the 13 included studies.
| Study | Inclusion criteria for original study | Maternal age | No. of cases | Monitoring strategy for miscarriage | Way of conception | Gestational period | Previous miscarriage | Tissue collect method | Culture/direct | Detection methods | Maternal contamination | US |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li (2016) | Singleton EPL | 32.2 | 2172 | serum β-hCG levels were checked 2wks after ET; patients with increased β-hCG were referred for TVS to confirm the pregnancy and assess embryo viability 4–5wks after ET, at least two TVS between 6th and 12th week of gestation. | IVF | ≤12 wks | NA | NA | Direct | CMA + FISH | NA | TVS |
| Liu (2015) | Miscarriage for D&C | 32.8 | 183 | First TVS 6–7wks. If no fetal cardiac activity, repeat 1 week later; if with fetal cardiac activity, repeat every 2wks until 10–12wks. | NA | ≤12 wks | Yes | SE | Culture | G-banding | NA | TVS |
| Romero (2015) | Pregnancy loss <20 wks | 31.1 | 64 | NA | NA | ≤20 wks | Yes | SP, SE | Direct | CMA | Excluded | NA |
| Cheng (2014) | POC for cytogenetic tests | 32.4 | 223 | NA | NA | NA | Yes | SE | Culture | G-banding | NA | TVS/TAS |
| Angiolucci (2011) | Singleton EPL | 35.6 | 156 | Spontaneous conception, at least one TVS scan performed prior to documentation of EPL, and successful karyotyping from POC. The second scan was performed 3–30 days after the first scan to document EPL. | Natural conception | ≤12 wks | Yes | SE | Both | G-banding | Excluded | TVS |
| Ljunger (2010) | Miscarriage for D&C | 31.8 | 259 | NA | NA | ≤12 wks | NA | SE | Direct | G-banding | NA | TVS |
| Munoz (2010) | Singleton EPL | 35.2 | 185 | All missed miscarriage will be offered a CVS before evacuation of POC | NA | ≤12 wks | Yes | CVS before SE | Direct | G-banding | NA | TVS |
| Lathi (2007) | Singleton EPL | 36.8 | 272 | NA | Mixed | 6–10 wks | NA | SE | Culture | G-banding | NA | TVS |
| Ginsberg (2001) | Singleton EPL | >35 | 129 | Missed abortions for early prenatal diagnosis by CVS | NA | 10–12wks | NA | CVS before SE | Both | G-banding | NA | TVS/TAS |
| Brajenovic-Milic (1998) | Miscarriage for D&C | 28.6 | 106 | Two consecutive ultrasonographic examinations at 7- to 10-day intervals. | NA | 7–16wks | NA | SE | Culture | G-banding | NA | NA |
| Coulam (1997) | Miscarriage for D&C | 36.3 | 137 | NA | Mixed | ≤12 wks | NA | SE | Both | G-banding | NA | TVS |
| Goldstein (1996) | Singleton EPL | NA | 102 | NA | NA | ≤72 days | NA | SE | NA | G-banding | NA | TVS |
| Dickey (1994) | Singleton EPL | 31.2 | 99 | Initial vaginal ultrasound after hCG value expected to be 2000 mlU. | Mixed | ≤12 wks | NA | SE | NA | G-banding | NA | TVS |
Only first author of each study is given. CMA, chromosomal microarray; CVS, chorionic villus sampling; D&C, dilatation and curettage; EPL, early pregnancy loss; ET, embryo transfer; FISH, fluorescent in situ hybridization; hCG, human chorionic gonadotropin; NA, not available; POC, product of conception; SE, Surgical evacuation; SP, Spontaneous passage; TAS, transabdominal scan; TVS, transvaginal scan; US, ultrasound; wks, weeks.
Study characteristics
Characteristics of included studies were summarized in Table 2. Apart from 3 studies that did not describe their study design, there were five prospective studies and five retrospective studies. All the studies reported on ultrasound findings in details and the corresponding karyotype. In this particular meta-analysis the study design of the incorporated studies might not be so important to the outcome of the results, since all the information needed for analysis was well-recorded regardless of the study type.
Table 2.
Characteristics of the 13 studies included.
| Studies | Consecutive | Design | Pro-/retro-spective | Outcome | Selection bias | Information bias | Attrition bias | Verification bias |
|---|---|---|---|---|---|---|---|---|
| Li 2016 |
Yes | Cohort | Retrospective | EPL | Yes | No | No | NA |
| Liu 2015 |
Yes | Cohort | Retrospective | EPL | Yes | NA | No | NA |
| Romero 2015 | Yes | Cohort | Prospective | EPL | Yes | No | No | NA |
| Cheng 2014 | Yes | Cohort | Retrospective | EPL | Yes | Yes | No | NA |
| Angiolucci 2011 |
Yes | Cohort | Prospective | EPL | Yes | No | NA | NA |
| Ljunger 2010 | Yes | Cohort | Prospective | EPL | Yes | NA | No | NA |
| Munoz 2010 |
Yes | Cohort | Prospective | EPL | NA | NA | NA | NA |
| Lathi 2007 |
Yes | Cohort | Retrospective | EPL | Yes | No | No | NA |
| Ginsberg 2001 | Yes | Cohort | NA | EPL | Yes | Yes | NA | NA |
| Brajenovic 1998 | Yes | Cohort | NA | EPL | NA | No | NA | NA |
| Coulam 1997 |
Yes | Cohort | NA | EPL | NA | No | NA | NA |
| Goldstein 1996 Dickey 1994 |
Yes | Cohort | Retrospective | EPL | Yes | NA | No | NA |
| Yes | Cohort | Prospectively | EPL | Yes | No | No | NA |
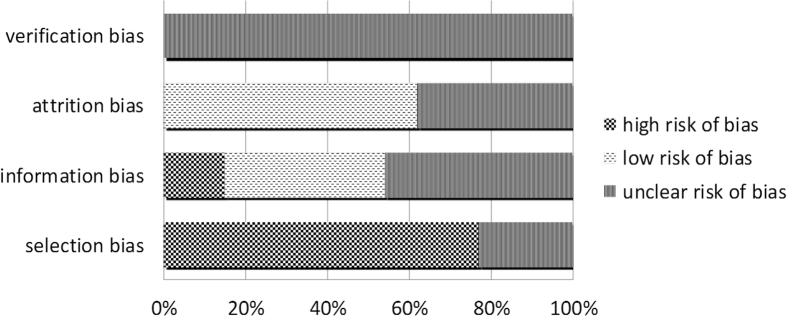
Fig. 2 exhibits the overall risk of bias in this meta-analysis. The overall selection bias was high as all the included studies need to provide information for both ultrasonography features and chromosomal tests in EPL. Apart from 3 studies that did not list their recruitment criteria,12, 14, 21 the other 10 studies described similar requirements when selecting the cases, such as the specific gestational age and at least one ultrasound measurement before identification of miscarriage. The information bias could be affected by a series of factors, including whether the patients conceived naturally or by IVF, and the methods for karyotype determination. There were two studies using a combination of trans-vaginal and trans-abdominal ultrasound scan method, which could contribute to the information bias.13, 16 Seven studies described that the researchers had designed the study carefully to minimize measurement error.6, 7, 10, 12, 14, 15, 18 The information bias might be under-estimated as the majority of the studies did not mention whether the ultrasound scans were performed by the same doctor, or whether the managing doctors were blinded to the patients’ history. For the attrition bias, eight studies indicated that their cases were well followed-up. Nevertheless, the other five studies did not report the attrition problems. The attrition bias is unlikely to affect the result of this meta-analysis, as the study focuses on the association between the ultrasound morphology and karyotype records of early pregnancy loss, which does not require long-term follow-up.
Figure 2.
Quality assessment of 13 included studies using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklists for Observational Studies (2007 version).
Odds ratios
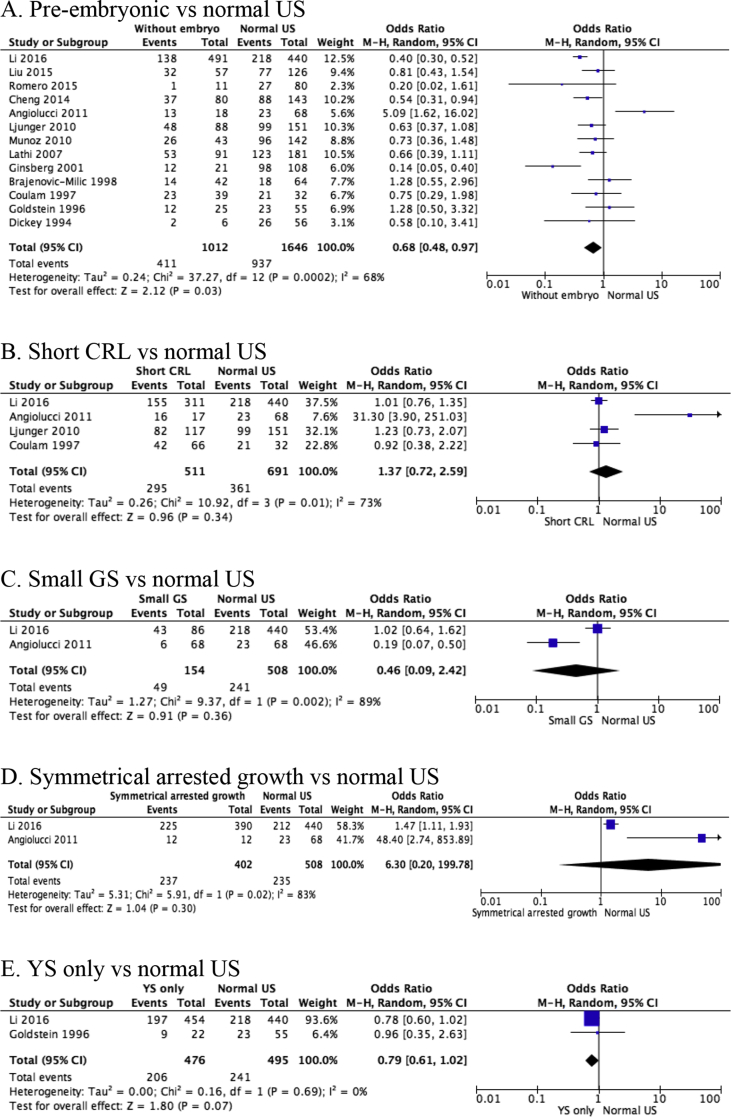
The forest plots of 13 included studies and pooled results from the meta-analysis are shown in Fig. 3. Individual studies were considered heterogeneous with I2 mainly over 60%. A negative association have been found between abnormal karyotype and pre-embryonic ultrasound features in early pregnancy loss (p = 0.03, OR = 0.68, 95%CI = 0.48–0.97). It seemed that enlarged YS was highly related with aneuploidy (p < 0.0001, OR = 27.39, 95%CI = 5.99–125.24), but this feature only involved one study.10 The other ultrasound features, including short CRL (p = 0.34, OR = 1.37, 95%CI = 0.72–2.59), small GS (p = 0.36, OR = 0.46, 95%CI = 0.09–2.42), symmetrical arrested growth embryo (p = 0.30, OR = 6.30, 95%CI = 0.20–199.78), or gestational sac with only a yolk sac (p = 0.07, OR = 0.79, 95%CI = 0.61–1.02), did not affect the likelihood of chromosomal abnormalities in early pregnancy loss.
Figure 3.
Forest plots of chromosomal abnormality rate of specific abnormal ultrasound features compared with normal ultrasound findings (without the specified ultrasound features) in early pregnancy loss. Only first author of each study is given. CRL, crown rump length; M-H, Mantel-Haenszel; US, ultrasound; YS, yolk sac.
Distribution of chromosomal abnormalities
As summarized in Table 3, there was no report of trisomy 1 in any of the studies. Monosomies were not common in EPL, except 45,X. Compared with sex chromosomal abnormalities, autosomal trisomies were the most common type of chromosomal abnormalities in EPL. Furthermore, the three most common trisomies in EPL with embryo structures identified by ultrasound scanning involved chromosomes 16, 22 and 21; while without embryo involved chromosomes 16, 22 and 2. Viable aneuploidy include trisomy 13, trisomy 18, trisomy 21, and 45,X. The rate of viable aneuploidy was 29.6% in EPL with “fetal pole” identified by ultrasound, while 21.8% in those without.
Table 3.
Prevalence of different type of chromosomal abnormalities in pre-embryonic or embryonic EPL.
| Abnormality | Pre-embryonic n = 456 (%) | Embryonic n = 1031 (%) |
|---|---|---|
| Autosomal trisomy | 344 (75.4) | 780 (75.7) |
| Trisomy 2 | 27 (5.9) | 9 (0.9) |
| Trisomy 3 | 10 (2.2) | 11 (1.1) |
| Trisomy 4 | 9 (2.0) | 22 (2.1) |
| Trisomy 5 | 1 (0.2) | 7 (0.7) |
| Trisomy 6 | 6 (1.3) | 14 (1.4) |
| Trisomy 7 | 11 (2.4) | 20 (1.9) |
| Trisomy 8 | 5 (1.1) | 23 (2.2) |
| Trisomy 9 | 3 (0.7) | 32 (3.1) |
| Trisomy 10 | 3 (0.7) | 12 (1.2) |
| Trisomy 11 | 7 (1.5) | 11 (1.1) |
| Trisomy 12 | 4 (0.9) | 14 (1.4) |
| Trisomy 13 | 19 (4.2) | 54 (5.2) |
| Trisomy 14 | 3 (0.7) | 31 (3.0) |
| Trisomy 15 | 12 (2.6) | 75 (7.3) |
| Trisomy 16 | 114 (25.1) | 142 (13.8) |
| Trisomy 17 | 7 (1.5) | 15 (1.5) |
| Trisomy 18 | 15 (3.3) | 30 (2.9) |
| Trisomy 19 | 2 (0.4) | 1 (0.1) |
| Trisomy 20 | 20 (4.4) | 20 (1.9) |
| Trisomy 21 | 15 (3.3) | 98 (9.5) |
| Trisomy 22 | 51 (11.2) | 139 (13.5) |
| Monosomy | 56 (12.3) | 126 (12.2) |
| 45,X | 50 (11.0) | 124 (12.0) |
| Monosomy 21 | 5 (1.1) | 2 (0.2) |
| Monosomy 18 | 1 (0.2) | 0 (0) |
| Triploidy | 6 (1.3) | 36 (3.5) |
| Tetraploidy | 9 (2.0) | 5 (0.5) |
| Other | 37 (8.1) | 61 (5.9) |
Discussion
This study was the first comprehensive systematic review and meta-analysis of the association between the ultrasound morphological features of EPL and specific chromosomal abnormalities. A total of 4231 EPLs with sonographic morphological data, including 2163 abnormal karyotypes, were analyzed. In accordance with previous studies, chromosomal abnormalities account for about 50% of EPLs. Though the results of this meta-analysis showed that EPL without embryo has a lower chromosomal abnormality rate than those with embryo, the proportion of viable chromosomal abnormalities in embryonic EPLs was higher than that of pre-embryonic ones. This provides evidence that the earlier the pregnancy loss happens, the higher the possibility of the detrimental embryonic chromosomal abnormalities. It could also be possible that besides non-viable embryonic chromosomal abnormalities, other factors, such as maternal causes including immune disorders and uterine anomalies, would assume an increasingly important role in affecting the developing embryo and thus cause its demise. Whilst viable pregnancies with chromosomal abnormalities but miscarried after 12 weeks in gestational weeks were excluded in this particular study, we acknowledge that the inclusion of these cases in future study should help to understand if pregnancies with chromosomal abnormality are associated with specific structural features in the early stage of pregnancy.
In formation of chromosomal abnormalities during meiosis of gametes formation, inaccurate chromosome recombination and segregation will result in aneuploidy, containing either extra or insufficient copies of one or more chromosomes. Subsequent fertilization with such gametes can develop the offspring with incorrect copy number of chromosomes. It is reported that over 90% of autosomal aneuploidy originates from the oocyte.22 However, mosaicism of chromosomal abnormalities occurs in mitotic division of post-fertilization stage,23 which was not common in EPL, but recently raises concerns in embryo selection for embryo transfer in assisted reproductive medicine.
The traditional histoembryology system to describe the development of human embryo is the Carnegie stages, which classified the development from stage 1 (zygote) to 23 (56–60 days embryo) based on morphologic characteristics. The collection of all the 23 stages has been available since 1900s at University of New South Wales Embryology (http://embryology.med.unsw.edu.au). It is the reference of human embryo morphology in relation to both size and age. At around 5 weeks of gestation, equal to human embryo Carnegie stage 7, about one week later, the secondary yolk sac and the primary germ layers formed, equal to Carnegie stage 10. However, the evaluation by Carnegie staging system is not commonly used in early pregnancy, and according to the indirect evidence from ultrasound monitoring of intrauterine embryonic size, the embryonic size variation in very early stage of pregnancy is not negligible; whilst after 46 days of embryo development, the fetal sizes in different studies becomes consistent.24, 25 The early embryonic size variation reduced in pregnancies from assisted reproduction with subsequent normal live birth.26, 27 While in general early pregnancy group with unknown outcome, the variation remained obvious.28 It could be suggestive of the morphological difference between miscarriage and live birth in early stage of pregnancy. Therefore, establishment and standardization of precisely timed morphological assessment of embryo structural development is essential, especially for the non-invasive monitoring of early embryo development. The precisely timed morphology change could be more accurately documented in IVF pregnancy.15, 29, 30
The categorical discrepancy between features of an ‘empty sac’ versus an ‘anembryonic’ pregnancy increased the heterogeneity of the studies related with YS presented or not. Among the 13 included studies, two analyzed YS only as an independent feature7, 10; two combined YS only and empty sacs in analysis of anembryonic EPL6, 13; Other studies did not clearly define the categorization of the ultrasound findings of with or without the presence of embryo. This less stringent in categorization of ultrasound parameters is likely to lead to information bias, as well as insufficient data for further stratification for the analysis of the association of detailed morphological features with specific chromosomal abnormalities.
An enlarged YS has been shown to be associated with an increased risk of EPL,31 but only one eligible study,10 investigated the association between this ultrasound feature and chromosomal abnormalities. According to this single study, the majority of the EPL with enlarged YS are at high risk of chromosomal abnormalities of which trisomy 22 were especially high. During the submission of this study, a newly published cohort study including 151 patients with pregnancy <12 weeks’ gestation echoed our finding that an enlarged yolk sac suggested an abnormal fetal karyotype, whereas an pre-embryonic feature was with less association.32
EPL with symmetrical arrested growth of both the CRL and the gestational sac showed a trend towards an increased risk of chromosomal abnormalities which was however not statistically significant, and no association with specific abnormality was identified.
There appears to be limited correlation between sonographic features and specific chromosomal abnormalities, but certain types of chromosomal abnormalities are more likely to be identified in some ultrasonography features in EPL. First, monosomy is less common than trisomy in EPL. It is known that deletion of certain region of the genome usually causes more severe outcomes that duplication.33 Therefore, monosomy is often more detrimental than trisomy, which could cause the embryo demise at the first few days after fertilization.34 Second, chromosome 1 aneuploidies were rarely identified in EPL. But in day 5 embryos the occurrence of chromosome 1 aneuploidies was comparable to that of other chromosomes.34 Furthermore, only a few cases of mosaic trisomy 1q live birth with significant defects and a very short life span have been reported.35 Third, no live birth has been reported with trisomy 16 or 22, but live births of mosaic trisomy 1536, 1637, 2238 have been reported. In light of these findings, it might suggest that during the long and consecutive development of human embryos, chromosomes would demonstrate stage specific roles and some of the chromosomes, like chromosome 1, 16, and 22 are critical during the early stage of the embryo development.
A limitation of the study is that heterogeneity amongst the included studies in this analysis is generally high, especially in relation to ultrasound features datasets of without embryo, short CRL, small GS, and symmetrical arrested growth; while in YS only dataset the heterogeneity is ignorable. It indicates that the major origin of the heterogeneity came from the documentation of ultrasound morphology. During the acquisition of morphological features, both the approach of measurement (TVS or TAS), the inter-observer inconsistency and different reference normal ranges could contribute to the discrepancy.28 Thus, we use the random effects model for forest plot analysis. Furthermore, the presence of YS is rarely affected by subjective assessment, so the dataset heterogeneity is quite low. Therefore, for better investigation of association between the ultrasound morphology and specific chromosomal abnormalities, it would be necessary to identify more objectively acquired sonographic markers for assessment. For example, Virtual organ computer-aided analysis (VOCAL) was proposed as a more precise approach with less inter-observer variation to measure GS.39 Studies have attempted to explore a range of ultrasonography features for predicting pregnancy outcomes and the association with chromosomal abnormalities. For example, Dickey's et al found that EPL with larger gestational sac diameter-CRL difference30 and shorter CRL29 were more likely to have chromosomal abnormalities. However, there seems no consensus so far on the early ultrasonography features and chromosomal abnormalities. With the introduction of high resolution transvaginal ultrasonography, it is possible that structural anomalies in variable pregnancy up to 10 weeks of gestation (but miscarried later on or diagnosed as having chromosomal abnormality) may be detected, although at present there is very little literature information available regarding this area.
Though ultrasonography features at the time of diagnosis of EPL have limited predictive value of fetal chromosomal abnormality, this study investigated ultrasound soft markers as a screening tool to predict the possibility of chromosomal abnormalities in early pregnancy loss, which is before the common non-invasive prenatal testing (NIPT) approaches looking at gestation of usually 11 weeks of gestation in clinical practice. In future, certain combination of ultrasonography features may increase the predictive value for fetal chromosomal abnormality. The ultrasonography features should be more objective and systematically documented, and in the repeat features may be of great interest in EPL.
Conflict of interest
None of the authors have conflict of interest to declare.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Li T.C., Makris M., Tomsu M., Tuckerman E., Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8(5):463–481. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 2.Saravelos S.H., Kong G.W., Chung J.P. A prospective randomized controlled trial of 3D versus 2D ultrasound-guided embryo transfer in women undergoing ART treatment. Hum Reprod. 2016;31(10):2255–2260. doi: 10.1093/humrep/dew206. [DOI] [PubMed] [Google Scholar]
- 3.Li Z., Wang Y.A., Ledger W., Edgar D.H., Sullivan E.A. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29(12):2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang Y., Tan Y., Yi Y. Correlation between chromosomal distribution and embryonic findings on ultrasound in early pregnancy loss after IVF-embryo transfer. Hum Reprod. 2016;31(10):2212–2218. doi: 10.1093/humrep/dew201. [DOI] [PubMed] [Google Scholar]
- 5.Pereza N., Ostojic S., Kapovic M., Peterlin B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril. 2016;107(1):150–159. doi: 10.1016/j.fertnstert.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Romero S.T., Geiersbach K.B., Paxton C.N. Differentiation of genetic abnormalities in early pregnancy loss. Ultrasound Obstet Gynecol. 2015;45(1):89–94. doi: 10.1002/uog.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Ouyang Y., Yi Y., Tan Y., Lu G. Correlation analysis between ultrasound findings and abnormal karyotypes in the embryos from early pregnancy loss after in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2016;34(1):43–50. doi: 10.1007/s10815-016-0821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jauniaux E., Johns J., Burton G.J. The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet Gynecol. 2005;25(6):613–624. doi: 10.1002/uog.1892. [DOI] [PubMed] [Google Scholar]
- 9.Fossum G.T., Davajan V., Kletzky O.A. Early detection of pregnancy with transvaginal ultrasound. Fertil Steril. 1988;49(5):788–791. doi: 10.1016/s0015-0282(16)59884-7. [DOI] [PubMed] [Google Scholar]
- 10.Angiolucci M., Murru R., Melis G., Carcassi C., Mais V. Association between different morphological types and abnormal karyotypes in early pregnancy loss. Ultrasound Obstet Gynecol. 2011;37(2):219–225. doi: 10.1002/uog.7681. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brajenović-Milić B., Petrović O., Krašević M., Ristić S., Kapović M. Chromosomal anomalies in abnormal human pregnancies. Fetal Diagn Ther. 1998;13(3):187–191. doi: 10.1159/000020836. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H.H., Ou C.Y., Tsai C.C. Chromosome distribution of early miscarriages with present or absent embryos: female predominance. J Assist Reprod Genet. 2014;31(8):1059–1064. doi: 10.1007/s10815-014-0261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulam C.B., Goodman C., Dorfmann A. Comparison of ultrasonographic findings in spontaneous abortions with normal and abnormal karyotypes. Hum Reprod. 1997;12(4):823–826. doi: 10.1093/humrep/12.4.823. [DOI] [PubMed] [Google Scholar]
- 15.Dickey R.P., Gasser R., Olar T.T. Relationship of initial chorionic sac diameter to abortion and abortus karyotype based on new growth curves for the 16th to 49th post-ovulation day. Hum Reprod. 1994;9(3):559–565. doi: 10.1093/oxfordjournals.humrep.a138544. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg N.A., Strom C., Verlinsky Y. Crown-rump lengths in missed miscarriages and trisomy 21. Ultrasound Obstet Gynecol. 2001;18(5):488–490. doi: 10.1046/j.0960-7692.2001.00571.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein S., Kerenyi T., Scher J., Papp C. Correlation between karyotype and ultrasound findings in patients with failed early pregnancy. Ultrasound Obstet Gynecol. 1996;8(5):314–317. doi: 10.1046/j.1469-0705.1996.08050314.x. [DOI] [PubMed] [Google Scholar]
- 18.Lathi R.B., Mark S.D., Westphal L.M., Milki A.A. Cytogenetic testing of anembryonic pregnancies compared to embryonic missed abortions. J Assist Reprod Genet. 2007;24(11):521–524. doi: 10.1007/s10815-007-9166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Liu Y., Chen H. Relationship of karyotype to embryo crown-rump length and maternal serum human chorionic gonadotropin level in early miscarriage. Am J Perinatol. 2015;32(1):15–22. doi: 10.1055/s-0034-1371708. [DOI] [PubMed] [Google Scholar]
- 20.Ljunger E., Stavreus-Evers A., Cnattingius S. Ultrasonographic findings in spontaneous miscarriage: relation to euploidy and aneuploidy. Fertil Steril. 2011;95(1):221–224. doi: 10.1016/j.fertnstert.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Munoz M., Arigita M., Bennasar M., Soler A., Sanchez A., Borrell A. Chromosomal anomaly spectrum in early pregnancy loss in relation to presence or absence of an embryonic pole. Fertil Steril. 2010;94(7):2564–2568. doi: 10.1016/j.fertnstert.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Hassold T., Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 23.Kort D.H., Chia G., Treff N.R. Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum Reprod. 2016;31(2):312–323. doi: 10.1093/humrep/dev281. [DOI] [PubMed] [Google Scholar]
- 24.Grisolia G., Milano K., Pilu G. Biometry of early pregnancy with transvaginal sonography. Ultrasound Obstet Gynecol. 1993;3(6):403–411. doi: 10.1046/j.1469-0705.1993.03060403.x. [DOI] [PubMed] [Google Scholar]
- 25.Verburg B.O., Steegers E.A., De Ridder M. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31(4):388–396. doi: 10.1002/uog.5225. [DOI] [PubMed] [Google Scholar]
- 26.Coulam C.B., Britten S., Soenksen D.M. Early (34-56 days from last menstrual period) ultrasonographic measurements in normal pregnancies. Hum Reprod. 1996;11(8):1771–1774. doi: 10.1093/oxfordjournals.humrep.a019483. [DOI] [PubMed] [Google Scholar]
- 27.Guirgis R.R., Alshawaf T., Dave R., Craft I.L. Transvaginal crown-rump length measurements of 224 successful pregnancies which resulted from gamete intra-Fallopian transfer or in-vitro fertilization. Hum Reprod. 1993;8(11):1933–1937. doi: 10.1093/oxfordjournals.humrep.a137963. [DOI] [PubMed] [Google Scholar]
- 28.Papaioannou G.I., Syngelaki A., Poon L.C., Ross J.A., Nicolaides K.H. Normal ranges of embryonic length, embryonic heart rate, gestational sac diameter and yolk sac diameter at 6-10 weeks. Fetal Diagn Ther. 2010;28(4):207–219. doi: 10.1159/000319589. [DOI] [PubMed] [Google Scholar]
- 29.Dickey R.P., Gasser R.F., Olar T.T. The relationship of initial embryo crown--rump length to pregnancy outcome and abortus karyotype based on new growth curves for the 2-31 mm embryo. Hum Reprod. 1994;9(2):366–373. doi: 10.1093/oxfordjournals.humrep.a138510. [DOI] [PubMed] [Google Scholar]
- 30.Dickey R.P., Olar T.T., Taylor S.N., Curole D.N., Matulich E.M. Relationship of small gestational sac-crown-rump length differences to abortion and abortus karyotypes. Obstet Gynecol. 1992;79(4):554–557. [PubMed] [Google Scholar]
- 31.Berdahl D.M., Blaine J., Van Voorhis B., Dokras A. Detection of enlarged yolk sac on early ultrasound is associated with adverse pregnancy outcomes. Fertil Steril. 2010;94(4):1535–1537. doi: 10.1016/j.fertnstert.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 32.Yoneda S., Shiozaki A., Yoneda N. A yolk sac larger than 5 mm suggests an abnormal fetal karyotype, whereas an absent embryo indicates a normal fetal karyotype. J Ultrasound Med. 2017;37(5):1233–1241. doi: 10.1002/jum.14467. [DOI] [PubMed] [Google Scholar]
- 33.Kong G.W., Cao Y., Huang J. Prenatal detection of 10q22q23 duplications: dilemmas in phenotype prediction. Prenat Diagn. 2016;36(13):1211–1216. doi: 10.1002/pd.4959. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Purata J., Lee J., Whitehouse M. Embryo selection versus natural selection: how do outcomes of comprehensive chromosome screening of blastocysts compare with the analysis of products of conception from early pregnancy loss (dilation and curettage) among an assisted reproductive technology population? Fertil Steril. 2015;104(6):1460–1466. doi: 10.1016/j.fertnstert.2015.08.007. e1461–1412. [DOI] [PubMed] [Google Scholar]
- 35.Patel C., Hardy G., Cox P., Bowdin S., McKeown C., Russell A.B. Mosaic trisomy 1q: the longest surviving case. Am J Med Genet A. 2009;149A(8):1795–1800. doi: 10.1002/ajmg.a.32959. [DOI] [PubMed] [Google Scholar]
- 36.McPadden J., Helm B.M., Spangler B.B., Ross L.P., Boles D.B., Schrier Vergano S.A. Mosaic trisomy 15 in a liveborn infant. Am J Med Genet A. 2015;167A(4):821–825. doi: 10.1002/ajmg.a.36958. [DOI] [PubMed] [Google Scholar]
- 37.Sparks T.N., Thao K., Norton M.E. Mosaic trisomy 16: what are the obstetric and long-term childhood outcomes? Genet Med. 2017;79(10):1164–1170. doi: 10.1038/gim.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrich T., Nanda I., Rehn M. Live-born trisomy 22: patient report and review. Mol Syndromol. 2013;3(6):262–269. doi: 10.1159/000346189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee W., Deter R.L., McNie B. Quantitative and morphological assessment of early gestational sacs using three-dimensional ultrasonography. Ultrasound Obstet Gynecol. 2006;28(3):255–260. doi: 10.1002/uog.2840. [DOI] [PubMed] [Google Scholar]