Abstract
Aims: The EXPLORE-J study aimed to assess lipid management in patients hospitalized for acute coronary syndrome (ACS) and their cardiovascular risk despite undergoing standard therapy. Here, we focused on background characteristics of patients in the EXPLORE-J study to elucidate the current lipid-lowering therapy and its issues in Japan.
Methods: In this multicenter, prospective, observational study (UMIN000018946), consecutive Japanese ACS patients who required hospitalization were registered between April 2015 and August 2016. Background and lipid profile data collected within 14 days of hospitalization were analyzed according to risk factors such as diabetes mellitus status.
Results: In total, 1944 patients were analyzed (80.3% male). The mean and standard deviation (SD) age and body mass index of all patients were 66.0 years (SD: 12.2) and 24.24 kg/m2 (SD: 3.59), respectively. The most common lipid-modifying medication used at the time of ACS was statins (27.3%). The low-density lipoprotein cholesterol (LDL-C) level (first measurement after hospitalization) of patients overall was 121.2 mg/dL (SD: 39.7); 30.3% had an LDL-C level < 100 mg/dL (current target level for secondary prevention of cardiovascular events in Japan), compared with 52.1% of patients with a previous history of coronary artery disease (CAD), and 57.2% of patients with a history of CAD and diabetes.
Conclusions: Many patients were not meeting Japanese LDL-C target levels at the time of ACS, and a large proportion of patients meeting target levels developed ACS; therefore, more stringent management and further evaluation of the target LDL-C levels is warranted in high-risk patients and those with previous history of CAD.
Keywords: Acute coronary syndrome, Cardiovascular risk, Japan, Low-density lipoprotein cholesterol
Introduction
A positive correlation between low-density lipoprotein cholesterol (LDL-C) levels and the incidence of cardiovascular events has been reported. Specifically, LDL-C-lowering therapy was shown to decrease the incidence of cardiovascular events1, 2). Therefore, target LDL-C levels have been established worldwide to reduce the risk of cardiovascular diseases, including coronary artery disease (CAD), which represents a burden for patients and society.
Target LDL-C levels vary by country. Guidelines outside of Japan recommend an LDL-C level < 70 mg/dL for the secondary prevention of atherosclerotic cardiovascular disease (ASCVD); however, in Japan, this level is recommended only to patients with a very high-risk profile for secondary prevention. High-risk secondary prevention patients include those with acute coronary syndrome (ACS) and familial hypercholesterolemia and those with diabetes mellitus (DM) complicated with non-cardiogenic cerebral infarction, peripheral artery disease (PAD), chronic kidney disease (CKD), metabolic syndrome, positive smoking status, and more than one major risk factor3, 4). The target LDL-C level in Japanese guidelines is less strict than those in European5) and American guidelines6) because there is a lack of evidence for the efficacy of more aggressive lipid-lowering therapy in Japanese patients. Although the target levels in Japan are less strict, many patients cannot achieve these target levels.
Despite a sharp decrease in the incidence of atherosclerotic diseases in general in Japan from the 1960s to the early 2000s, there is evidence of a recent increase in ischemic heart disease owing to westernization of the lifestyle and an increase in the incidence of dyslipidemia7–9). A recent study showed an overall prevalence of ASCVD of 1869 per 100,000 individuals among the employed Japanese population10) with an event rate (any cardiovascular event) of 4.0% within the first year after diagnosis of ASCVD, representing substantial clinical and economic burdens. Furthermore, DM is not only a notable risk factor for secondary prevention of ASCVD, but also it is on the rise in Japan and throughout Asia11). Thus, there is a need to determine whether more aggressive lipid-lowering therapy is warranted in Japanese patients with ACS. This article describes the baseline characteristics of patients in the EXPLORE-J study, including comorbidities (DM and hypertension), lipid profile, and cardiovascular risk status, to elucidate the current lipid management status of patients after ACS with a focus on high-risk patients with history of CAD (secondary prevention) and DM.
Methods
Study Design
This is a multicenter, prospective, observational study of Japanese patients presenting with ACS. This article describes the baseline characteristics of those patients, focusing on the lipid profile collected 1) at the first measurement after hospitalization, and 2) at Visit 1 (i.e., at registration [after informed consent], which was within 14 days of hospitalization) (Supplemental Fig. 1). All other laboratory measurements (including other lipid parameters, glycosylated hemoglobin [HbA1c], and fasting blood glucose [FBG]) were collected at Visit 1. ACS patients who required hospitalization were consecutively recruited and registered in 59 sites between April 2015 and August 2016 (enrollment period). Participating patients provided written informed consent within 7 days after hospitalization. Next, those who met the inclusion criteria were registered successively to avoid selection bias by the investigator. The study protocol conforms to the ethical guidelines of the 2013 revision of the Declaration of Helsinki and was approved by each institution's ethics committee for research involving humans. Additional details of the study methods have been described previously12).
Supplemental Fig. 1.
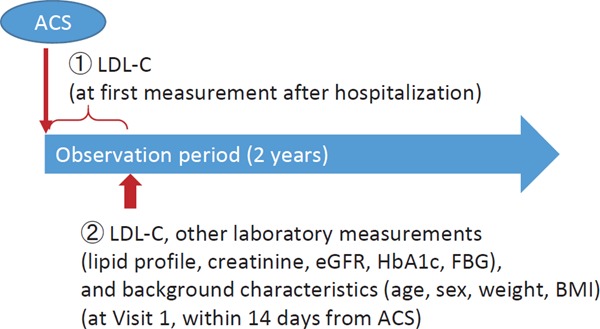
Study design
ACS, acute coronary syndrome; BMI, body mass index; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein Cholesterol
Patients
The inclusion criteria were as follows: patients aged ≥ 20 years at the time of informed consent, who developed ACS, including ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), or unstable angina (UA). The main exclusion criteria were as follows: patients with chest pain caused by pre-specified significant comorbidities, patients with in-stent thrombosis, patients enrolled in interventional studies that could affect the lipid profile, and those judged as inappropriate for inclusion by the investigators or subinvestigators.
Background Characteristics
Background data (demographic characteristics, physical findings, laboratory data, and medical history) were collected within 14 days of hospitalization (at Visit 1) using electronic case report forms, and have been described previously12). The presence of a comorbidity was determined at the attending/reporting physician's discretion.
Lipid Profile
The first LDL-C measurement (regardless of the method) after hospitalization was reported. LDL-C levels, both direct and calculated, and other lipid parameters were obtained at Visit 1 (within 14 days after hospitalization for ACS). LDL-C levels were evaluated according to the type of treatment (statin therapy, intensive statin therapy, any lipid-lowering therapy, or no lipid-lowering therapy). Intensive statin therapy was defined as atorvastatin ≥ 20 mg, rosuvastatin ≥ 10 mg, or pitavastatin ≥ 4 mg. The definition of intensive statin therapy in the current study differs from that in the Adult Treatment Panel III of the National Cholesterol Education Program guidelines13), as lower doses of statins are approved and used in Japan owing to concerns about adverse events.
An additional analysis was conducted in which patients were stratified according to previous history of CAD using the Suita score14). The Suita score categorizes patients into high-, medium-, and low-risk groups according to patient characteristics (e.g., age, sex, smoking history, blood pressure, high-density lipoprotein cholesterol [HDL-C], and LDL-C, among others). The achievement rate of LDL-C target levels for each of the resulting risk groups (< 100 mg/dL [secondary prevention], < 120 mg/dL [high risk], < 140 mg/dL [medium risk], and < 160 mg/dL [low-risk]) was calculated. Because blood pressure was not reported in EXPLORE-J, patients with hypertension were all given a score of 4, which is equivalent to SBP 90–99/DBP 140–159.
Laboratory Parameters in Patients with and without DM
Lipid parameters, HbA1c, and FBG were described in patients with and without DM. Serum cholesterol levels were evaluated at each participating study center.
Statistical Analysis
Details of the sample size calculations were provided previously12). Briefly, based on a previous study on Japanese ACS patients (PACIFIC registry)15) in which the incidence of major adverse cardiovascular events (MACE) was 6.4% at 2 years, the planned sample size was 2000 patients with an estimated precision of approximately 1%, and 95% confidence interval of 5.3%–7.4%.
The background characteristics of the patients were summarized using mean, median, standard deviation (SD), range for continuous data, and number and proportion of patients in each category for categorical data.
Fisher's exact test was used for comparison of categorical variables, and t-tests, including Welch's t-test, or the Wilcoxon rank sum test were used for continuous variables as appropriate. A two-sided p value of < 0.05 was considered significant.
Results
The disposition of patients is shown in Supplemental Fig. 2. A total of 2016 consecutive ACS patients were registered, 72 of whom were excluded from the analysis for the following reasons: failure to obtain informed consent within 7 days of hospitalization (n = 62), unapproved informed consent (n = 4), no informed consent obtained (n = 2), duplicate entry (n = 2), erroneous entry (n = 1), and withdrawal because the target number of cases for enrollment was achieved at registration (n = 1). Finally, 1944 patients were included in our analysis.
Supplemental Fig. 2.
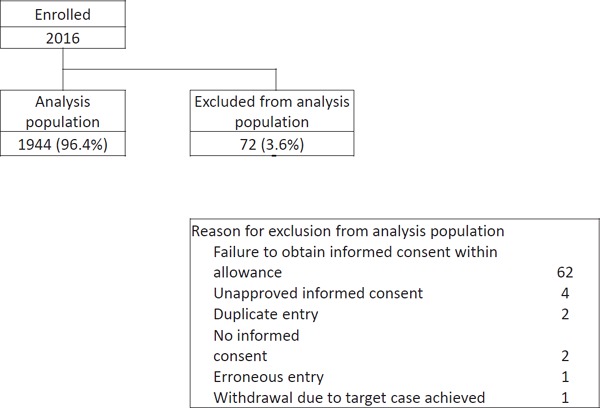
Patient disposition
Background Characteristics
The mean and SD age of patients overall (N = 1944) and those with (N = 355) and without history of CAD (N = 1589) were 66.0 (12.2) years, 69.4 (11.0) years, and 65.2 (12.3) years, respectively. The mean and SD body mass index of patients overall and those with and without history of CAD were 24.24 (3.59) kg/m2, 24.39 (3.64) kg/m2, and 24.21 (3.58) kg/m2, respectively. Among all patients, 1561/1944 (80.3%) patients were male; among those with and without history of CAD, 301 (84.8%) and 1260 (79.3%) were male (Table 1). The most common ACS type was STEMI (61.5%), followed by UA (22.6%), and NSTEMI (15.9%). Patients with a history of CAD were more likely to have NSTEMI and UA. The most common lipid-modifying medication used at the time of ACS was statins (overall, 27.3%). Statins were used by 85.0% (531/626) of patients who were taking any lipid-lowering therapies at the time of ACS in this study. Other medications that were commonly used included antihypertensives (51.2%), antiplatelet agents (23.1%), and antiglycemic medications other than insulin (19.7%) (Table 2).
Table 1. Background characteristics of patients overall and of those with and without a history of CAD.
| N | All patients Mean ± SD | History of CAD Mean ± SD | No history of CAD Mean ± SD | p value (history of CAD vs no history of CAD) | Statistical test | |
|---|---|---|---|---|---|---|
| No. of patients | 1944 | 355 | 1589 | |||
| Patient characteristic | ||||||
| Age (years) | 1944 | 66.0 ± 12.2 | 69.4 ± 11.0 | 65.2 ± 12.3 | < 0.001 | Welch's t-test |
| Male, n (%) | 1944 | 1561 (80.3) | 301 (84.8) | 1260 (79.3) | 0.018 | Fisher's exact test |
| BMI (kg/m2) | 1937 | 24.24 ± 3.59 | 24.39 ± 3.64 | 24.21 ± 3.58 | 0.391 | Student's t-test |
| Weight (kg) | 1940 | 65.59 ± 12.90 | 65.54 ± 12.16 | 65.61 ± 13.06 | 0.929 | Student's t-test |
| Dyslipidemia, n (%) | 1944 | 1512 (77.8) | 316 (89.0) | 1196 (75.3) | < 0.001 | Fisher's exact test |
| ACS type | 1944 | < 0.001 | Fisher's exact test | |||
| STEMI, n (%) | 1195 (61.5) | 153 (43.1) | 1042 (65.6) | |||
| NSTEMI, n (%) | 309 (15.9) | 68 (19.2) | 241 (15.2) | |||
| UA, n (%) | 440 (22.6) | 134 (37.7) | 306 (19.3) | |||
| Creatinine (mg/dL) | 1886 | 1.025 ± 1.143 | 1.304 ± 1.845 | 0.963 ± 0.904 | < 0.001 | Welch's t-test |
| eGFR (mL/min/1.73 m2)§, n (%) | 1886 | < 0.001 | Mann–Whitney U test | |||
| < 15 | 39 (2.1) | 16 (4.7) | 23 (1.5) | |||
| 15 to < 30 | 30 (1.6) | 7 (2.0) | 23 (1.5) | |||
| 30 to < 60 | 538 (28.5) | 121 (35.2) | 417 (27.0) | |||
| 60 to < 90 | 1034 (54.8) | 166 (48.3) | 868 (56.3) | |||
| ≥ 90 | 245 (13.0) | 34 (9.9) | 211 (13.7) | |||
| Lipid profile (Visit 1) | ||||||
| LDL-cholesterol (mg/dL)# | 1827 | 121.2 ± 39.7 | 103.6 ± 38.0 | 125.1 ± 39.0 | < 0.001 | Student's t-test |
| LDL-cholesterol (mg/dL) (calculated) | 1767 | 99.4 ± 31.9 | 90.6 ± 30.5 | 101.3 ± 31.9 | < 0.001 | Student's t-test |
| Total cholesterol (mg/dL) | 1803 | 165.2 ± 38.4 | 156.6 ± 38.6 | 167.1 ± 38.2 | < 0.001 | Student's t-test |
| HDL cholesterol (mg/dL) | 1831 | 41.1 ± 11.7 | 40.9 ± 11.0 | 41.1 ± 11.8 | 0.705 | Student's t-test |
| Triglycerides (mg/dL)† | 1838 | 109.0 (22, 967) | 109.0 (36, 552) | 109.0 (22, 967) | 0.946 | Wilcoxon rank sum test |
| Lipoprotein(a) (mg/dL)† | 1378 | 21.20 (0.41, 159.0) | 21.00 (0.60, 158.0) | 21.25 (0.41, 159.0) | 0.666 | Wilcoxon rank sum test |
| Apolipoprotein A1 (mg/dL) | 1427 | 108.8 ± 20.8 | 110.7 ± 19.7 | 108.3 ± 21.0 | 0.103 | Student's t-test |
| Apolipoprotein B (mg/dL) | 1425 | 86.5 ± 22.2 | 81.0 ± 22.2 | 87.6 ± 22.1 | < 0.001 | Student's t-test |
| Atherosclerotic risk factors (baseline) | ||||||
| Medical history of hypertension (n, %) | 1944 | 1427 (73.4) | 301 (84.8) | 1126 (70.9) | < 0.001 | Fisher's exact test |
| Medical history of diabetes mellitus (n, %) | 1944 | 679 (34.9) | 152 (42.8) | 527 (33.2) | < 0.001 | Fisher's exact test |
| History of smoking (n, %) | 1944 | < 0.001 | Fisher's exact test | |||
| Current smoker | 739 (38.0) | 89 (25.1) | 650 (40.9) | |||
| Previous smoker | 541 (27.8) | 129 (36.3) | 412 (25.9) | |||
| None | 664 (34.2) | 137 (38.6) | 527 (33.2) | |||
Estimated glomerular filtration rate < 60 (CKD stage 3–5).
At first measurement after hospitalization.
Median (min, max).
Conversion factor for creatinine from mg/dL to µmol/L, 88.4.
ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NSTEMI, non-ST elevation myocardial infarction; SD, standard deviation; STEMI, ST elevation myocardial infarction; UA, unstable angina.
Table 2. Medications used by patients at the time of acute coronary syndrome in patients overall and in those with and without a history of CAD.
| Total | History of CAD | No history of CAD | p value (history of CAD vs no history of CAD) | Statistical test | |
|---|---|---|---|---|---|
| Previous medication, N (%) | 1944 | 355 | 1589 | ||
| Lipid-modifying medications | |||||
| Any lipid-lowering therapies | 626 (32.2) | 232 (65.4) | 394 (24.8) | < 0.001 | Fisher's exact test |
| Any statin therapy | 531 (27.3) | 212 (59.7) | 319 (20.1) | < 0.001 | Fisher's exact test |
| Intensive statin therapy§ | 31 (5.8) | 15 (7.1) | 16 (5.0) | 0.348 | Fisher's exact test |
| Other lipid-lowering therapy (without statin) | 96 (4.9) | 20 (5.6) | 76 (4.8) | 0.496 | Fisher's exact test |
| Ezetimibe | 40 (2.1) | 16 (4.5) | 24 (1.5) | 0.001 | Fisher's exact test |
| Other medications | |||||
| Antihypertensive | 995 (51.2) | 271 (76.3) | 724 (45.6) | < 0.001 | Fisher's exact test |
| Antiplatelet | 450 (23.1) | 267 (75.2) | 183 (11.5) | < 0.001 | Fisher's exact test |
| Antiglycemic# | 382 (19.7) | 92 (25.9) | 290 (18.3) | 0.001 | Fisher's exact test |
| Antiglycemic (insulin) | 77 (4.0) | 30 (8.5) | 47 (3.0) | < 0.001 | Fisher's exact test |
| Oral anticoagulant | 69 (3.5) | 36 (10.1) | 33 (2.1) | < 0.001 | Fisher's exact test |
| Anti-arrhythmic | 54 (2.8) | 27 (7.6) | 27 (1.7) | < 0.001 | Fisher's exact test |
Intensive statin therapy was defined as atorvastatin ≥ 20 mg, rosuvastatin ≥ 10 mg, or pitavastatin ≥ 4 mg.
Oral agent and injections except insulin.
CAD, coronary artery disease
Lipid Profile
The mean and SD LDL-C level at first measurement after hospitalization was 121.2 (39.7) mg/dL; 30.3%, 6.6%, and 7.7% of all patients had an LDL-C level < 100 mg/dL, < 70 mg/dL, and ≥ 180 mg/dL, respectively. Lipid parameters measured at Visit 1 are shown in Table 1. Among all patients with available data, 49.6% of 1831 patients had an HDL-C level ≥ 40 mg/dL; 21.9% of 1838 patients had a triglyceride level ≥ 150 mg/dL; and 13.4% of 1378 patients had a lipoprotein(a) level ≥ 50 mg/dL (Tables 1 and 3).
Table 3. Summary statistics of baseline lipid profile in patients overall and in those with and without a history of CAD.
| Lipid profile | Total | History of CAD | No history of CAD | p value | Statistical test |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | (history of CAD vs no history of CAD) | ||
| LDL-C (mg/dL) at first measurement after hospitalization | 1827 | 332 | 1495 | ||
| < 100 | 553 (30.3) | 173 (52.1) | 380 (25.4) | < 0.001 | Fisher's exact test |
| < 70 | 120 (6.6) | 50 (15.1) | 70 (4.7) | < 0.001 | Fisher's exact test |
| ≥ 180 | 141 (7.7) | 12 (3.6) | 129 (8.6) | 0.001 | Fisher's exact test |
| LDL-C (mg/dL) (calculated) at Visit 1 | 1767 | 312 | 1455 | ||
| < 100 | 998 (56.4) | 212 (67.9) | 786 (54.0) | < 0.001 | Fisher's exact test |
| < 70 | 254 (14.4) | 66 (21.2) | 188 (12.9) | < 0.001 | Fisher's exact test |
| ≥ 180 | 39 (2.2) | 3 (1.0) | 36 (2.5) | 0.134 | Fisher's exact test |
| HDL-C (mg/dL) at Visit 1 | 1831 | 327 | 1504 | 0.807 | Fisher's exact test |
| < 40 | 922 (50.4) | 167 (51.1) | 755 (50.2) | ||
| ≥ 40 | 909 (49.6) | 160 (48.9) | 749 (49.8) | ||
| Triglycerides (mg/dL) at Visit 1 | 1838 | 327 | 1511 | 0.825 | Fisher's exact test |
| < 150 | 1435 (78.1) | 257 (78.6) | 1178 (78.0) | ||
| ≥ 150 | 403 (21.9) | 70 (21.4) | 333 (22.0) | ||
| Lp(a) (mg/dL) at Visit 1 | 1378 | 240 | 1138 | 0.922 | Mann–Whitney U test |
| < 30 | 919 (66.7) | 162 (67.5) | 757 (66.5) | ||
| 30 to < 50 | 275 (20.0) | 43 (17.9) | 232 (20.4) | ||
| ≥ 50 | 184 (13.4) | 35 (14.6) | 149 (13.1) |
CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a)
Table 4 shows the relationship between history of CAD and LDL-C levels at first measurement after hospitalization. Patients with a previous history of CAD had lower mean LDL-C levels, were notably more likely to have LDL-C levels < 100 mg/dL (52.1% vs 25.4%), and were more likely to be on statin therapy (59.7% vs 20.1%) than those without a previous history of CAD (Table 2).
Table 4. Relationship between history of CAD and LDL-C level at first measurement after hospitalization among patients overall and according to lipid-lowering therapy.
| History of CAD§ |
No history of CAD§ |
Statistical tests# (patients with/without CAD), p values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD LDL-C mg/dL |
< 100 mg/dL (< 2.59 mmol/L) (%) |
< 70 mg/dL (< 1.81 mmol/L) (%) |
n | Mean ± SD LDL-C mg/dL | < 100 mg/dL (< 2.59 mmol/L) (%) |
< 70 mg/dL (< 1.81 mmol/L) (%) |
Mean difference (Student's t-test) |
Rates of LDL-C < 100 mg/dL (Fisher's exact test) |
|
| All patients at hospitalization | 332 | 103.6 ± 38.0 | 173 (52.1) | 50 (15.1) | 1495 | 125.1 ± 39.0 | 380 (25.4) | 70 (4.7) | < 0.001 | < 0.001 |
| No lipid-lowering therapy | 116 | 120.6 ± 40.5 | 37 (31.9) | 11 (9.5) | 1131 | 130.1 ± 39.6 | 234 (20.7) | 41 (3.6) | 0.014 | 0.009 |
| Any lipid-lowering therapy | 216 | 94.5 ± 33.3 | 136 (63.0) | 39 (18.1) | 364 | 109.6 ± 32.9 | 146 (40.1) | 29 (8.0) | < 0.001 | < 0.001 |
| Any statin therapy | 196 | 93.1 ± 33.2 | 128 (65.3) | 39 (19.9) | 294 | 107.5 ± 31.3 | 125 (42.5) | 25 (8.5) | < 0.001 | < 0.001 |
| Intensive statin therapy† | 12 | 96.2 ± 35.2 | 7 (58.3) | 2 (16.7) | 16 | 112.9 ± 34.1 | 5 (31.3) | 0 (0.0) | - | - |
| Fibrate therapy | 5 | 93.0 ± 16.5 | 2 (40.0) | 0 (0.0) | 27 | 118.9 ± 29.1 | 5 (18.5) | 2 (7.4) | - | - |
| Ezetimibe therapy | 14 | 105.9 ± 44.2 | 6 (42.9) | 3 (21.4) | 22 | 109.0 ± 47.2 | 9 (40.9) | 6 (27.3) | - | - |
| EPA/DHA therapy | 23 | 83.3 ± 31.9 | 16 (69.6) | 6 (26.1) | 41 | 108.0 ± 31.6 | 18 (43.9) | 4 (9.8) | - | - |
| Other lipid-lowering therapy (no PCSK9i) | 20 | 100.2 ± 32.1 | 11 (55.0) | 2 (10.0) | 29 | 110.5 ± 49.5 | 14 (48.3) | 4 (13.8) | - | - |
Patients with available LDL-C level at the time of ACS diagnosis.
Statistical comparisons were carried out only for n ≥ 100.
Intensive statin therapy was defined as atorvastatin ≥ 20 mg, rosuvastatin ≥ 10 mg, or pitavastatin ≥ 4 mg.
Conversion factor for LDL-C from mg/dL to mmol/L, 0.0259.
ACS, acute coronary syndrome; CAD, coronary artery disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LDL-C, low-density lipoprotein cholesterol; PCSK9i, proprotein convertase subtilisin/kexin 9 inhibitor; SD, standard deviation
In the additional analysis using the Suita score, 355 (18.3%) of 1944 patients with a history of CAD were categorized as the secondary prevention group and 1589 patients without history of CAD were categorized as the primary prevention group. The latter were further categorized as high-risk (n = 1368 [86.1%]), medium-risk (190 [12.0%]), and low-risk (31 [2.0%]) groups. In the secondary prevention group, 173 (52.1%) patients achieved LDL-C target < 100 mg/dL. In the primary prevention high-risk group, 578 (45.8%) achieved LDL-C target < 120 mg/dL. The majority of primary prevention patients were categorized as high-risk.
Comorbidities and cardiovascular risk factors for patients overall are summarized in Supplemental Fig. 3.
Supplemental Fig. 3.
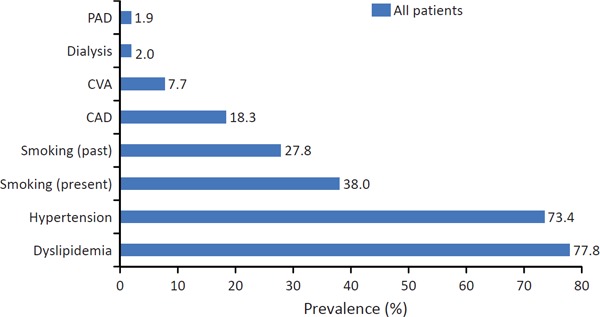
Comorbidities and cardiovascular risk factors for patients overall
CAD, coronary artery disease; CVA, cerebrovascular accident; DM, diabetes mellitus; PAD, peripheral artery disease
Laboratory Parameters in Patients with and without DM
The distribution of LDL-C levels at first measurement after hospitalization among patients with and without DM according to their previous history of CAD (positive or negative) is shown in Fig. 1. In general, the proportions of patients with an LDL-C level < 100 mg/dL and < 70 mg/dL were higher among those with DM vs those without DM, regardless of their previous history of CAD. The difference was especially notable among patients with a previous history of CAD with an LDL-C level < 100 mg/dL (with vs without DM: 57.2% vs 48.1%) and < 70 mg/dL (with vs without DM: 19.3% vs 11.8%).
Fig. 1.
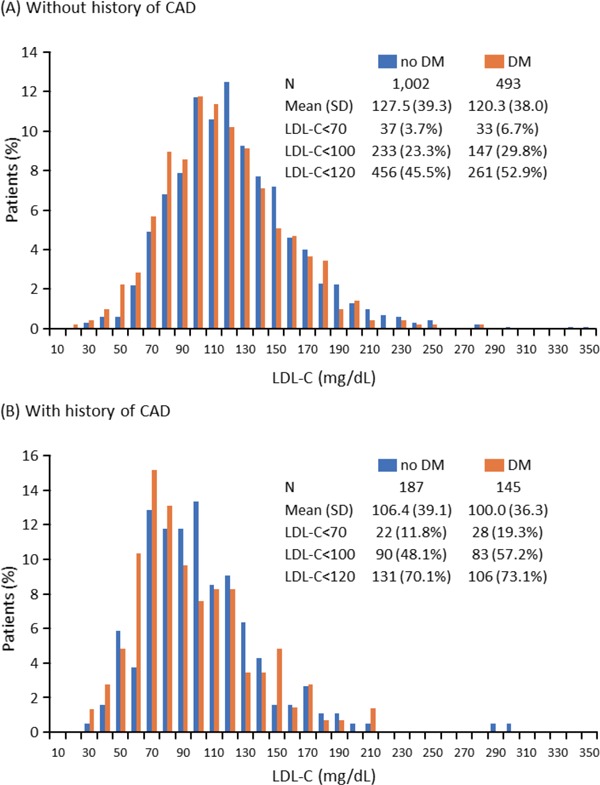
Distribution of LDL-C levels at first measurement after hospitalization among patients with and without DM without history of CAD (A) and in those with history of CAD (B)
Conversion factor for LDL-C from mg/dL to mmol/L: 0.0259
CAD, coronary artery disease; DM, diabetes mellitus; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation
A comparison of the results of lipid profile after stabilization (i.e., Visit 1) among patients with and without DM is shown in Supplemental Table 1. The levels of most lipid parameters were significantly higher among patients without DM than those with DM, except for triglyceride level, which was significantly higher in those with DM (Supplemental Fig. 4).
Supplemental Table 1. Lipid profile after stabilization (i.e., Visit 1) in patients with and without DM.
| DM | No DM | p value | |
|---|---|---|---|
| LDL-C (calc), mg/dL | 94.2 (29.6) | 102.3 (32.8) | < 0.001§ |
| LDL-C (direct), mg/dL | 96.6 (30.4) | 104.4 (33.5) | < 0.001§ |
| TC, mg/dL | 159.2 (36.4) | 168.4 (39.1) | < 0.001§ |
| HDL-C | 39.1 (10.5) | 42.2 (12.1) | < 0.001§ |
| TG | 113.0 (26, 665)‡ | 107.0 (22, 967)‡ | 0.001# |
| Apolipoprotein A1 | 106.5 (19.3) | 110.0 (21.4) | 0.002§ |
| Apolipoprotein B | 84.9 (22.1) | 87.4 (22.3) | 0.041† |
| Lipoprotein(a) | 20.0 (1.0, 148.0)‡ | 23.0 (0.41, 159.0)‡ | 0.003# |
Data are presented as mean (standard deviation).
Welch's t-test;
Wilcoxon rank sum test;
Student's t-test;
median (Min, Max).
Conversion factors for LDL-C, TC, and HDL-C from mg/dL to mmol/L, 0.0259; TG from mg/dL to mmol/L, 0.0113; apolipoprotein A1 and B from mg/dL to g/L, 0.01; and lipoprotein(a) from mg/dL to µmol/L, 0.0357.
DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides
Supplemental Fig. 4.
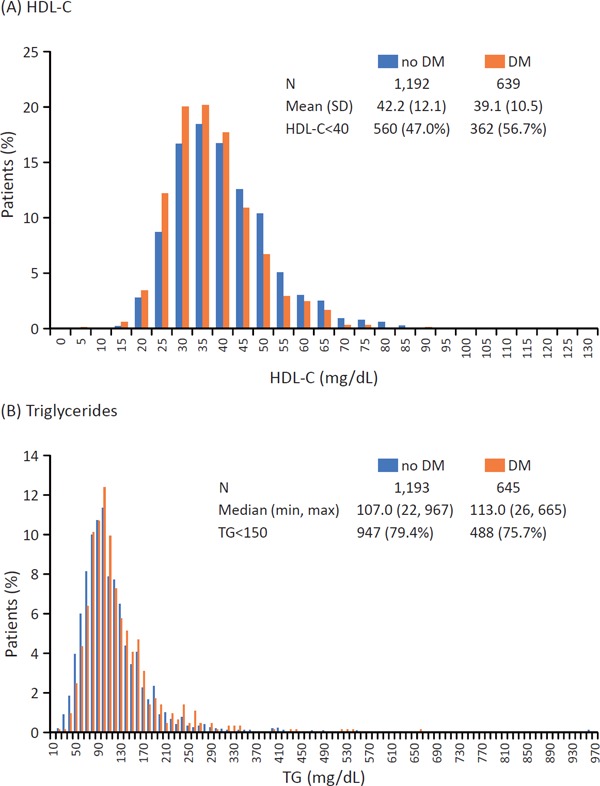
Distribution of HDL-C (A) and triglycerides (B) at Visit 1 in patients with and without DM
Conversion factors for HDL-C from mg/dL to mmol/L, 0.0259; and TG from mg/dL to mmol/L, 0.0113.
DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; SD, standard deviation; TG, triglycerides
The status of glycemic control in DM patients in terms of HbA1c and FBG is shown in Supplemental Fig. 5. The mean and SD HbA1c and FBG were 7.38% (1.43%) and 144.8 (46.2) mg/dL, respectively, among patients with DM. Furthermore, 51.3% and 61.3% of patients with DM had an HbA1c ≥ 7.0% and FBG ≥ 125 mg/dL, respectively.
Supplemental Fig. 5.
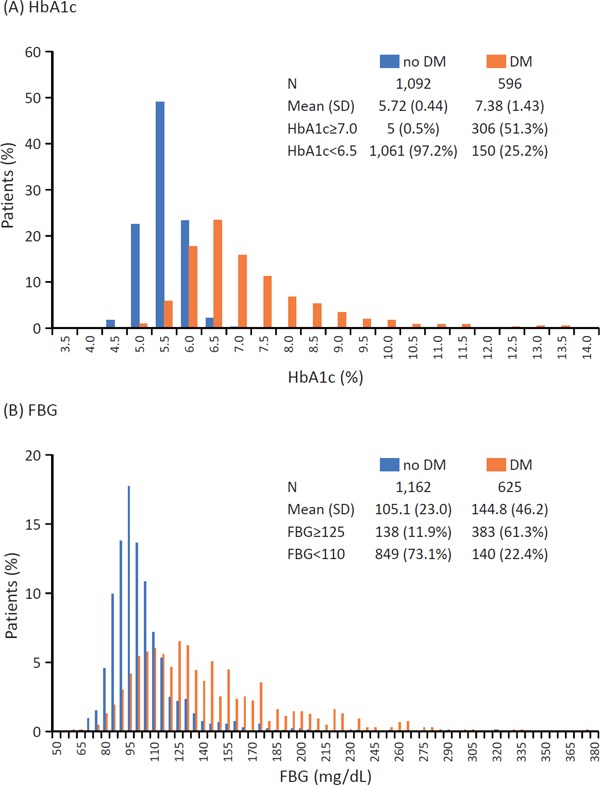
Glucose control at Visit 1 in patients with and without DM in terms of HbA1c (A) and FBG (B)
Conversion factor for FBG from mg/dL to mmol/L, 0.0555.
DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; SD, standard deviation
Discussion
The EXPLORE-J study is one of the largest ACS registries in Japan to explore the current lipid management status of ACS patients. Among the 1944 patients included in the present study, most had a history of dyslipidemia (77.8%). Among all patients in the present study and among those with a previous history of CAD, respectively, 30.3% and 52.1% had LDL-C < 100 mg/dL and 6.6% and 15.1% had LDL-C < 70 mg/dL at the first measurement after hospitalization. These findings suggest that a large proportion of patients were not meeting the recommended LDL-C target level of < 100 mg/dL for secondary prevention of CAD, and even those who had achieved target LDL-C levels still developed ACS. A reason for this may be the low rate of lipid-lowering therapy use (any statin) in the present study (27.3%). It is possible that patients are not receiving adequate doses of statins, and alternative lipid-lowering agents may be warranted in some patients; therefore, untreated patients may be more susceptible to cardiovascular events. For many patients, the current target LDL-C level for secondary prevention in Japan may be suboptimal, especially among patients with a previous history of CAD.
Regarding other lipid parameters, no notable differences were shown in the distribution of all patients according to HDL-C level (approximately 50% of the patients had HDL-C level < 40 and ≥ 40 mg/dL at Visit 1). Triglyceride levels were within the target level (< 150 mg/dL) in 78.1% of the patients.
According to the Suita score analysis, those categorized as having high risk accounted for the majority of patients (84% of the primary prevention subset) in the study population hospitalized for ACS, implying the reliability of the score as a tool to predict the likelihood of developing CAD in the future. Additionally, these findings strongly suggest that the multidisciplinary approach is important for the prevention of CAD. The low target LDL-C achievement rate in the secondary prevention/high-risk groups may suggest that more stringent LDL-C control is required. Further study to adopt the Suita score for targeting LDL-C level seems to be warranted.
In the present study, patients without a previous history of CAD had a higher mean LDL-C level than those with a previous history of CAD. These findings are consistent with those of a previous study in which the mean LDL-C levels among patients with a previous history of CAD were generally lower than those among patients without a previous history of CAD16). In both studies, the mean LDL-C levels were lower in patients with DM vs those without DM, regardless of their previous history of CAD. The reason patients with DM tend to have lower LDL-C levels in general may be owing to higher use of statins and other lipid-lowering therapies. Patients being treated for DM may also see physicians more often and be adhering to diet and exercise therapies, which would contribute to a more favorable lipid profile in general. This is evidenced by the Nationwide Lifestyle Intervention Program Targeting Metabolic Syndrome, which has shown that regular health checkups and guidance on lifestyle modifications had some success in Japan in preventing metabolic syndrome and its sequelae17, 18).
While DM patients may be more aggressively treated by clinicians, these patients may still experience ACS events at lower LDL-C levels. This suggests that the target LDL-C level may require re-evaluation in Japan. Moreover, the idea of implementing a lower target LDL-C level in DM patients to reduce cardiovascular risk is supported by a previous study by Howard et al., which compared the effect of implementing aggressive vs standard LDL-C target levels (< 70 vs < 100 mg/dL) for DM patients with atherosclerosis. Howard et al. found that more aggressive LDL-C target levels provided greater benefits in terms of reducing the progression of atherosclerosis compared with standard LDL-C target levels19).
The present study has some limitations, including those inherent to the observational study design and small sample size. Serum LDL-C levels can decrease early after the development of ACS20); however, we did not assess the specific timing of LDL-C measurement. This timing could have varied among the cohorts. Not all of the study patients had a complete lipid profile at hospitalization.
Conclusions
High-risk patients presenting with ACS may require more aggressive treatment with intensive statin therapy, ezetimibe, or proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors. Further analyses are warranted as to whether more aggressive LDL-C target levels are needed in high-risk patients, along with further investigation of risk factors associated with the onset of ACS.
Upcoming data on MACE from the 2-year observation period will be evaluated to further identify high-risk patients, and will provide insight into current post-ACS cardiovascular risk factor management, familial hypercholesterolemia diagnosis, Achilles tendon thickness, and PCSK9 levels in ACS patients.
Acknowledgments
The authors thank Tamio Teramoto of Teikyo University; Shun Ishibashi of Jichi Medical University; Kotaro Yokote of Chiba University; Tomonori Okamura of Keio University; and Hiroyuki Daida of Juntendo University for collaboration and advice. The authors would also like to thank Azusa Tsukida and Yuki Tajima of Sanofi for providing support with statistical analysis, Yuki Tajima of Sanofi for providing medical writing support, Yasuyoshi Nakahigashi and Hideo Hayashi of Sanofi and Mebix, Inc. for assistance with study implementation/operation, Densuke Systems Co. Ltd. for statistical analysis, and Regeneron Pharmaceuticals, Inc. for critical review of the manuscript. Writing and editorial assistance was provided by J Ludovic Croxford, PhD, and Michelle Belanger, MD, of Edanz Medical Writing and was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Abbreviations
- ACS
acute coronary syndrome
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CAD
coronary artery disease
- CKD
chronic kidney disease
- DM
diabetes mellitus
- FBG
fasting blood glucose
- HbA1c
glycosylated hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- MACE
major adverse cardiovascular events
- NSTEMI
non-ST elevation myocardial infarction
- PAD
peripheral artery disease
- PCSK9
proprotein convertase subtilisin/kexin 9
- SD
standard deviation
- STEMI
ST elevation myocardial infarction
- UA
unstable angina
Notice of Grant Support
This study was sponsored by Sanofi and Regeneron Pharmaceuticals, both of which participated in the study design, the collection, analysis, and interpretation of data, the writing of the report, and in the decision to submit the article for publication.
Conflict of Interest
Masato Nakamura and Junya Ako have received honoraria from Astellas Amgen Biopharma and Sanofi. Hidenori Arai has received honoraria from Astellas Amgen Biopharma, Daiichi Sankyo, MSD, Kowa, Astellas, and Chugai. Atsushi Hirayama has received honoraria from Bayer, Daiichi Sankyo, Astellas Amgen Biopharma, Astellas, Sanofi, MSD, and Eisai; clinical research funding from Bayer and Daiichi Sankyo; and scholarship grants from MSD, Sanofi, Astellas Amgen Biopharma, and Daiichi Sankyo. Yoshitaka Murakami has no conflicts of interest to declare. Atsushi Nohara has received honoraria from Sanofi. Mariko Harada-Shiba has received honoraria from Astellas Amgen Biopharma, Astellas, Sanofi, and Aegerion; and scholarship grants from Aegerion and Astellas. Kiyoko Uno and Asuka Ozaki are employees of Sanofi.
References
- 1). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators : Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 2). Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS: Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA, 2016; 316: 1289-1297 [DOI] [PubMed] [Google Scholar]
- 3). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis : Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Fujiyoshi N, Arima H, Satoh A, Ojima T, Nishi N, Okuda N, Kadota A, Ohkubo T, Hozawa A, Nakaya N, Fujiyoshi A, Okamura T, Ueshima H, Okayama A, Miura K, NIPPON DATA2010 Research Group : Associations between socioeconomic status and the prevalence and treatment of hypercholesterolemia in a general Japanese population: NIPPON DATA2010. J Atheroscler Thromb, 2018; 25: 606-620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees : ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J, 2011; 32: 1769-1818 [DOI] [PubMed] [Google Scholar]
- 6). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129: S1-S45 [DOI] [PubMed] [Google Scholar]
- 7). Iso H: Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb, 2011; 18: 83-88 [DOI] [PubMed] [Google Scholar]
- 8). Iso H: A Japanese health success story: trends in cardiovascular diseases, their risk factors, and the contribution of public health and personalized approaches. EPMA J, 2011; 2: 49-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Kitamura A, Sato S, Kiyama M, Imano H, Iso H, Okada T, Ohira T, Tanigawa T, Yamagishi K, Nakamura M, Konishi M, Shimamoto T, Iida M, Komachi Y: Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: The Akita-Osaka Study. J Am Coll Cardiol, 2008; 52: 71-79 [DOI] [PubMed] [Google Scholar]
- 10). Davis KL, Meyers J, Zhao Z, McCollam PL, Murakami M: High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population: prevalence, cardiovascular event rates, and costs. J Atheroscler Thromb, 2015; 22: 1287-1304 [DOI] [PubMed] [Google Scholar]
- 11). Goto A, Goto M, Noda M, Tsugane S. Incidence of type 2 diabetes in Japan: a systematic review and meta-analysis. PLoS One, 2013; 8: e74699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Nakamura M, Uno K, Hirayama A, Ako J, Nohara A, Arai H, Harada-Shiba M: Exploration into lipid management and persistent risk in patients hospitalised for acute coronary syndrome in Japan (EXPLORE-J): protocol for a prospective observational study. BMJ Open, 2017; 7: e014427. 10.1136/bmjopen-2016-014427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ, National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association : Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation, 2004; 110: 227-239 [DOI] [PubMed] [Google Scholar]
- 14). Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y: Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the Framingham risk score: the Suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 15). Miyauchi K, Morino Y, Tsukahara K, Origasa H, Daida H, PACIFIC steering committee members : The PACIFIC (Prevention of AtherothrombotiC Incidents Following Ischemic Coronary attack) Registry: Rationale and design of a 2-year study in patients initially hospitalised with acute coronary syndrome in Japan. Cardiovasc Drugs Ther, 2010; 24: 77-83 [DOI] [PubMed] [Google Scholar]
- 16). Tani S, Yagi T, Atsumi W, Kawauchi K, Matsuo R, Hirayama A: Relation between low-density lipoprotein cholesterol/apolipoprotein B ratio and triglyceride-rich lipoproteins in patients with coronary artery disease and type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol, 2017; 16: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Matsuzawa Y: Specific health guidance, the nationwide lifestyle intervention program targeting metabolic syndrome, seems to be successful in Japan. J Atheroscler Thromb, 2018; 25: 304-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Tsushita K, S Hosler A, Miura K, Ito Y, Fukuda T, Kitamura A, Tatara K: Rationale and descriptive analysis of specific health guidance: the nationwide lifestyle intervention program targeting metabolic syndrome in Japan. J Atheroscler Thromb, 2018; 25: 308-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, Howard WJ, Lee ET, Mete M, Poolaw B, Ratner RE, Russell M, Silverman A, Stylianou M, Umans JG, Wang W, Weir MR, Weissman NJ, Wilson C, Yeh F, Zhu J: Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA, 2008; 299: 1678-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Arnold SV, Kosiborod M, Tang F, Zhao Z, McCollam PL, Birt J, Spertus JA: Changes in low-density lipoprotein cholesterol levels after discharge for acute myocardial infarction in a real-world patient population. Am J Epidemiol, 2014; 179: 1293-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]


