Abstract
Background
A reduction in renal angiomyolipoma volume observed with everolimus (EVE) treatment in patients with tuberous sclerosis complex (TSC) has been postulated to translate to clinical benefit by reducing the risk of renal hemorrhage and chronic renal failure.
Methods
The long-term effects of EVE on renal function (∼4 years of treatment) were examined in patients treated with EVE in the Phase 3 EXIST-1 and EXIST-2 studies. Patients in EXIST-1 had TSC and subependymal giant cell astrocytoma (SEGA), and patients in EXIST-2 had renal angiomyolipoma and a definite diagnosis of TSC or sporadic lymphangioleiomyomatosis. EVE was administered at 4.5 mg/m2/day, with adjustment to achieve target trough levels of 5–15 ng/mL in EXIST-1 and at 10 mg/day in EXIST-2. Estimated glomerular filtration rate (eGFR) and creatinine levels were assessed at baseline, at Weeks 2, 4, 6, 8, 12 and 18, then every 3 months thereafter. Proteinuria was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Results
A total of 111 patients from EXIST-1 and 112 patients from EXIST-2 were included in this analysis. Respective mean ages at EVE initiation were 10.5 [standard deviation (SD) 6.45] and 33.2 (SD 10.29) years, and 3.6% and 37.5% of patients had undergone prior renal intervention. Mean baseline eGFR was 115 and 88 mL/min/1.73 m2 in EXIST-1 and EXIST-2, respectively. Overall, mean eGFR remained stable over time in both studies, with an decline in renal function mostly confined to some patients with severely compromised renal function before treatment. Patients with prior renal intervention exhibited low eGFR values throughout the study. The incidence of proteinuria increased after initiating treatment with EVE and was mostly Grade 1/2 in severity, with Grade 3 proteinuria reported in only two patients. Measurements of proteinuria were limited by the use of urine dipstick tests.
Conclusions
The use of EVE does not appear to be nephrotoxic in patients with SEGA or renal angiomyolipoma associated with TSC and may preserve renal function in most patients.
ClinicalTrials.gov identifiers NCT00789828 and NCT00790400
Keywords: mTOR inhibition, renal function, tuberous sclerosis
INTRODUCTION
Tuberous sclerosis complex (TSC), a hereditary disorder, affects ∼1 million people worldwide [1]. TSC1 and TSC2 mutations result in increased activation of mammalian target of rapamycin complex 1 (mTORC1), leading to increased metabolism, proliferation and tumor growth [2]. Up to 80% of patients with TSC develop renal angiomyolipomata [1]—tumors comprising blood vessels, smooth muscle-like cells and adipose-like tissue [3–5]. Ultrastructural analysis and immunohistochemical and biochemical expression of pericyte-associated proteins by angiomyolipomata suggest that they are derived from vascular pericytes, mesenchymal perivascular cells located on the abluminal surface of capillaries involved in the regulation of microvascular stability, development and function [6]. mTORC1 signaling has been shown to play an important mechanistic role in renal hypertrophy [7, 8]. In preclinical models, deletions in TSC2 result in abnormal renal cell polarity and the development of cysts [9, 10].
Renal angiomyolipomata are the most common cause of TSC-related mortality in adults [11, 12]. Compared with sporadic renal angiomyolipomata, those associated with TSC usually occur in multiples and are larger, bilateral and more likely to grow [13]. Aneurysms develop frequently from these tumors and may lead to life-threatening spontaneous hemorrhage [14]. Renal angiomyolipoma >3 cm, aneurysm >0.5 cm and renal angiomyolipoma growth increase the risk of renal hemorrhage [14, 15]. Patients with renal angiomyolipomata are also at risk for hypertension and renal failure, which leads to increased health care utilization and associated costs [12, 13, 16]. Guidelines recommend first-line treatment with a mammalian target of rapamycin (mTOR) inhibitor for asymptomatic, growing renal angiomyolipoma >3 cm in diameter; however, these guidelines also caution that long-term benefits and safety data are needed [17]. Proteinuria is a known adverse event (AE) associated with mTOR inhibitors, and screening is warranted [18].
Everolimus (EVE), an mTOR inhibitor, has demonstrated efficacy in TSC-associated disorders, including renal angiomyolipoma [19–22]. The effect of EVE on renal function was assessed in patients with TSC in the Phase 3 EXIST-1 and EXIST-2 studies. The primary endpoints for these two studies were subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma response rate, respectively, and EVE demonstrated significant benefit over placebo (PBO) [20, 21]. In addition, in an exploratory subset analysis of patients in EXIST-1 with renal angiomyolipoma, EVE demonstrated a greater reduction in total renal angiomyolipoma volume versus PBO (angiomyolipoma response rate 53.3% versus 0%) [23]. Following positive results in the double-blind core phases, patients receiving PBO were offered open-label EVE in the extension phases [24, 25].
Some preclinical studies have suggested that mTOR activity that is too high or too low may result in kidney injury [26, 27]. In order to better assess this question in the TSC population, the long-term effects of EVE (∼4 years) on renal function in patients from EXIST-1 and EXIST-2 are reported here.
MATERIALS AND METHODS
Study design
EXIST-1 and EXIST-2 were randomized, double-blind, PBO-controlled, Phase 3 studies [20, 21, 24, 25]. EXIST-1 (NCT00789828) examined EVE for treating TSC-associated SEGA [20, 24] and EXIST-2 (NCT00790400) examined EVE for treating renal angiomyolipoma associated with TSC or sporadic lymphangioleiomyomatosis (LAM) [21, 25]. Methods from EXIST-1 and EXIST-2 have been published previously; in brief, both studies comprised double-blind core phases followed by open-label extension phases, with patients being followed for 4–5 years [24, 25]. This retrospective analysis examined long-term renal function data collected prospectively throughout both studies.
Patients
In EXIST-1, eligible patients were 0–65 years of age with a definite diagnosis of TSC according to consensus criteria [28, 29] and growing SEGAs, with one or more target SEGA ≥1 cm in diameter assessed by multiphasic magnetic resonance imaging (MRI) [20, 24, 30]. Patients must have been medically stable and unlikely to require surgery for SEGAs, with no critical hydrocephalus or imminent cerebral herniation [20].
Eligible patients in EXIST-2 had a definite diagnosis of TSC or sporadic LAM per consensus criteria [28, 29, 31], and were ≥18 years old with one or more renal angiomyolipoma ≥3 cm in diameter. Patients requiring angiomyolipoma-related surgery or with renal angiomyolipoma-related bleeding or embolization during the previous 6 months or those with severely impaired lung function were excluded [21, 25, 32].
Local independent ethics committees at each center approved the protocols and the studies were conducted in accordance with the principles of Good Clinical Practice, Declaration of Helsinki and all local regulations. Safety data were reviewed every 6 months by an independent data monitoring committee appointed in each study and all patients (or their legal representatives) provided written informed consent before enrolment [20, 21].
Treatment
Patients were originally randomly assigned 2:1 to receive EVE or PBO in a double-blind phase and then could receive open-label EVE in an extension phase. In EXIST-1, patients received EVE at a starting dose of 4.5 mg/m2/day, with adjustment to achieve target trough levels of 5–15 ng/mL [20, 24]. In EXIST-2, patients received EVE 10 mg/day, with dose modifications allowed based on tolerability [21, 25].
Endpoints and assessments
In EXIST-1, the primary endpoint was SEGA response rate, defined as the proportion of patients with ≥50% reduction from baseline in SEGA volume (in the absence of worsening of nontarget SEGAs, new lesions ≥1 cm in diameter and new or worsening hydrocephalus) [20, 24, 30]. Secondary endpoints included time to and duration of SEGA response, renal angiomyolipoma response rate (proportion of patients with ≥50% reduction from baseline in renal angiomyolipoma volume) and safety [20, 24].
In EXIST-2, the primary endpoint was the renal angiomyolipoma response rate, defined as the proportion of patients with ≥50% reduction from baseline in the sum of volumes of all target renal angiomyolipoma lesions (in the absence of new target lesions >20% increase from nadir in kidney volume or Grade ≥2 renal angiomyolipoma-related bleeding). Secondary endpoints included time to and duration of renal angiomyolipoma response, time to progression and safety [21, 25].
In both studies, estimated glomerular filtration rate (eGFR) and creatinine levels were assessed at baseline and Weeks 2, 4, 6, 8, 12 and 18, then every 3 months thereafter. Protein was assessed by urine dipstick by the investigator at baseline and study visits at Weeks 4, 8, 12, 18 and 24, then every 12 weeks thereafter. Results were confirmed by a central laboratory in the event of relevant abnormalities. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula [33] for patients ≥18 years of age or the ‘bedside’ Schwartz formula [34] in those <18 years. Creatinine and proteinuria were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 [35].
Statistical methods
For both studies, all EVE data from both the double-blind core phase and the open-label extension were combined and analyses were performed on all patients who received one or more dose of EVE. Baseline demographics and patient characteristics were summarized using descriptive statistics {mean [standard deviation (SD)], median (range)}. Baseline was defined as the last available assessment on or before the first dose with EVE. The mean eGFR values and percentage eGFR change from baseline over time as well as median eGFR per baseline chronic kidney disease (CKD) stage were calculated. The proportions of patients with severe renal impairment (GFR <30 mL/min/1.73 m2, CKD Stage 4/5) or with Grade 3 or 4 serum creatinine or proteinuria (per NCI CTCAE version 3.0) at any time during treatment with EVE were determined and urine dipstick protein results over time were summarized. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Patients
Table 1 shows baseline demographics and clinical characteristics of the 111 and 112 patients in EXIST-1 and EXIST-2, respectively, who received one or more dose of EVE and are included in this analysis. Because of the age at which SEGAs typically appear, all patients who received EVE in EXIST-1 were <30 years of age. Angiomyolipomata more commonly cause symptoms in adulthood, therefore entry criteria for EXIST-2 restricted patients to those ≥18 years of age. EXIST-1 enrolled more males than females, whereas two-thirds of the patients in EXIST-2 were female. Sixty-four percent of patients in EXIST-1 also had evidence of renal angiomyolipoma, but lesions were smaller than those in patients in EXIST-2. Approximately one-third of patients (38%) in EXIST-2 had undergone renal interventions before recruitment; 23 patients had undergone prior embolization, 20 had prior nephrectomy (total or partial), 4 had surgical removal of a lesion and 1 had thermoablation. In EXIST-1, four patients had undergone a prior renal intervention (nephrectomy).
Table 1.
Baselineademographics and disease characteristics
| Characteristics | EXIST-1 | EXIST-2 |
|---|---|---|
| (n = 111) | (n = 112) | |
| Age (years) | ||
| Mean (SD) | 10.5 (6.4) | 33.2 (10.3) |
| Median (range) | 9.5 (1.1–27.4) | 32.2 (18.1–61.6) |
| Age categories (years), n (%) | ||
| <3 | 18 (16.2) | 0 |
| 3–<10 | 41 (36.9) | 0 |
| 10–<18 | 34 (30.6) | 0 |
| ≥18 | 18 (16.2) | 112 (100) |
| <30 | 111 (100) | 49 (43.8) |
| ≥30 | 0 | 63 (56.3) |
| Male, n (%) | 64 (57.7) | 39 (34.8) |
| Race, n (%) | ||
| White | 104 (93.7) | 99 (88.4) |
| Asian | 0 | 11 (9.8) |
| Other | 7 (6.3) | 2 (1.8) |
| Diagnosis of TSC, n (%) | 111 (100.0) | 107 (95.5) |
| Presence of SEGA, n (%) | 111 (100.0) | 55 (49.1) |
| Presence of renal angiomyolipoma, n (%) | 71 (64.0) | 111 (99.1) |
| Prior renal angiomyolipoma-related interventions,bn (%) | 4 (3.6) | 42 (37.5) |
| Renal angiomyolipoma lesions ≥1 cm, n (%) | ||
| 0 | 54 (48.6) | 2 (1.8) |
| 1–5 | 33 (29.7) | 43 (38.4) |
| 6–10 | 8 (7.2) | 67 (59.8) |
| Patients with one or more evaluable lesion,cn (%) | 38 (34.2) | 110 (98.2) |
| Sum of volumes of target lesionsc (cm3) | ||
| Median | 10 | 92 |
| Range | 0.5–198.1 | 2.8–1611.5 |
| GFR (mL/min/1.73 m2) | ||
| Mean (SD) | 115 (27.9)d | 88 (31.9)e |
| CKD stage at baseline, n (%) | ||
| 1 | 94 (84.7) | 47 (42.0) |
| 2 | 13 (11.7) | 40 (35.7) |
| 3 | 3 (2.7) | 20 (17.9) |
| 4 | 0 (0.0) | 3 (2.7) |
| Missing | 1 (0.9) | 2 (1.8) |
Baseline is defined as the last available assessment on or before the start date of EVE treatment.
Includes embolization and partial nephrectomy.
Evaluable target renal angiomyolipoma lesions ≥1 cm.
n = 100.
n = 98.
At study completion (2 October 2014 and 4 February 2015, for EXIST-1 and EXIST-2, respectively), the median duration of EVE exposure was ∼47 months in both studies. Nearly 75% of the patients completed the studies per the protocol (Table 2) [24, 25].
Table 2.
Patient disposition
| EXIST-1 | EXIST-2 | |
|---|---|---|
| (n = 111) | (n = 112) | |
| Duration of EVE exposure (months), (median (range) | 47 (2–58) | 46.9 (<1–64) |
| Completed per protocol, n (%) | 82 (73.9) | 83 (74.1) |
| Discontinued, n (%) | 29 (26.1) | 29 (25.9) |
| AE | 10 (9.0)a | 9 (8.0)b |
| Administrative problems | 7 (6.3) | 2 (1.8) |
| Lost to follow-up | 3 (2.7) | 1 (0.9) |
| Patient withdrawal of consent | 6 (5.4) | 7 (6.3) |
| Disease progression | 1 (0.9)c | 5 (4.5)d |
| New treatment for indication under studye | 1 (0.9) | 2 (1.8) |
| Abnormal laboratory value | 0 | 1 (0.9)f |
| Death | 1 (0.9) | 1 (0.9) |
| Protocol deviation | 0 | 1 (0.9) |
AEs leading to discontinuation in EXIST-1 were Acinetobacter bacteremia, aggression, anemia, azoospermia, blood alkaline phosphatase level increase, focal segmental glomerulosclerosis, need for neurosurgery, neutropenia, pneumonia, pneumothorax, sinusitis, stomatitis and viral infection (one patient each, 0.9%).
AEs leading to discontinuation in EXIST-2 were angioedema, bronchospasm, convulsion, diarrhea, hypersensitivity, localized edema, malaise, pancreatic carcinoma, nasal sinus cancer, proteinuria, rhabdomyolysis and skin toxicity (one patient each, 0.9%).
SEGA progression defined as one or more of the following: increase from nadir of ≥25% in SEGA volume to a value greater than baseline, unequivocal worsening of nontarget SEGA lesions, appearance of a new SEGA lesion ≥1.0 cm in longest diameter and new or worsening hydrocephalus.
Renal angiomyolipoma progression defined as one or more of the following: ≥25% increase from nadir in angiomyolipoma volume, ≥20% increase from nadir in the volume of either kidney with a value greater than baseline, appearance of new angiomyolipoma ≥1 cm and Grade ≥2 angiomyolipoma-related bleeding.
Patient sought other treatment for disease under study.
Laboratory abnormality leading to discontinuation was blood phosphorus decreased.
Renal function
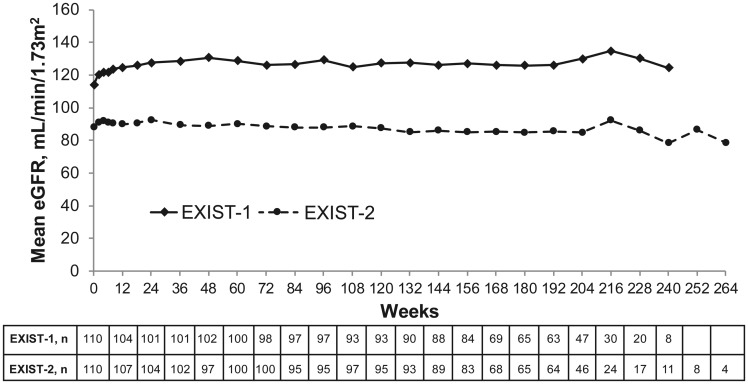
Overall, mean eGFR remained stable over time in both studies (Figure 1). Mean eGFR values were lower in EXIST-2 than in EXIST-1. One patient in EXIST-1 and eight in EXIST-2 experienced severe renal impairment (GFR <30 mL/min/1.73 m2) at least once postbaseline. All of these patients had considerably compromised renal function before starting EVE (i.e. Stage 3/4 CKD) and eight exhibited continued renal function decline (Table 3). In other patients, including 27 with eGFR <60 mL/min/1.73m2, eGFR mostly remained stable or improved throughout treatment with EVE.
FIGURE 1.
Mean eGFR over time in the EXIST-1 and EXIST-2 studies. The MDRD formula [33] was used to estimate GFR for patients ≥18 years of age and the ‘bedside’ Schwartz formula [34] was used to estimate GFR in patients <18 years of age.
Table 3.
Individual trends over time in patients with eGFR <30 mL/min/1.73 m2 at any time throughout the EXIST-1 and EXIST-2 studies
| Patient | Treatment group | eGFR, mL/min/1.73 m2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline a | Month b 1 | Month b 3 | Month b 6 | Month b 12 | Month b 18 | Month b 24 | Month b 30 | Month b 36 | Month b 42 | Month b 48 | Month b 54 | ||
| EXIST-1 | |||||||||||||
| 1 | EVE | 53.4 | 61.7 | 51.6 | 53.1 | 43.1 | 41.5 | 36.9 | 27.1 | 32.7 | 24.0 | − | − |
| EXIST-2 | |||||||||||||
| 2 | EVE | 48.8 | 42.7 | 37.6 | 35.7 | 37.5 | 33.1 | 39.7 | 40.2 | 34.3 | 28.5 | 25.2 | 26.3 |
| 3 | EVE | 51.2 | 48.8 | 31.3 | 42.2 | 41.2 | 46.7 | 37.7 | 21.6 | 30.7 | 27.9 | 28.3 | 24.5 |
| 4 | EVE | 50.2 | 50.7 | 53.5 | 48.6 | 47.0 | 45.1 | 43.8 | 40.6 | 34.5 | 29.4 | 28.9 | 30.3 |
| 5 | EVE | 35.8 | 33.5 | 33.5 | 33.4 | 33.3 | 29.4 | 27.7 | 24.9 | 26.2 | 27.5 | 27.5 | 27.4 |
| 6 | EVE | 29.2 | 29.2 | 21.3 | 26.0 | 25.9 | 22.2 | 17.1 | 17.8 | 19.3 | 19.2 | − | − |
| 7 | Placebo | 23.0 | 24.3 | 23.2 | 22.3 | 20.6 | 17.9 | 18.2 | 15.0 | 11.9 | − | − | − |
| 8 | Placebo | 24.3 | 20.2 | 30.5 | 21.9 | 24.7 | 25.7 | 22.8 | 24.8 | 21.7 | − | − | − |
| 9 | Placebo | 42.8 | 42.7 | 39.6 | 36.9 | 36.8 | 36.7 | 23.4 | 20.5 | 17.5 | − | − | − |
All eGFR measurements were done centrally and timings of measurements are approximate.
Refers to the last measurement of eGFR before starting EVE.
One month = 30.4 days. Values come from the measurement on the day closest to the end of the month for each patient.
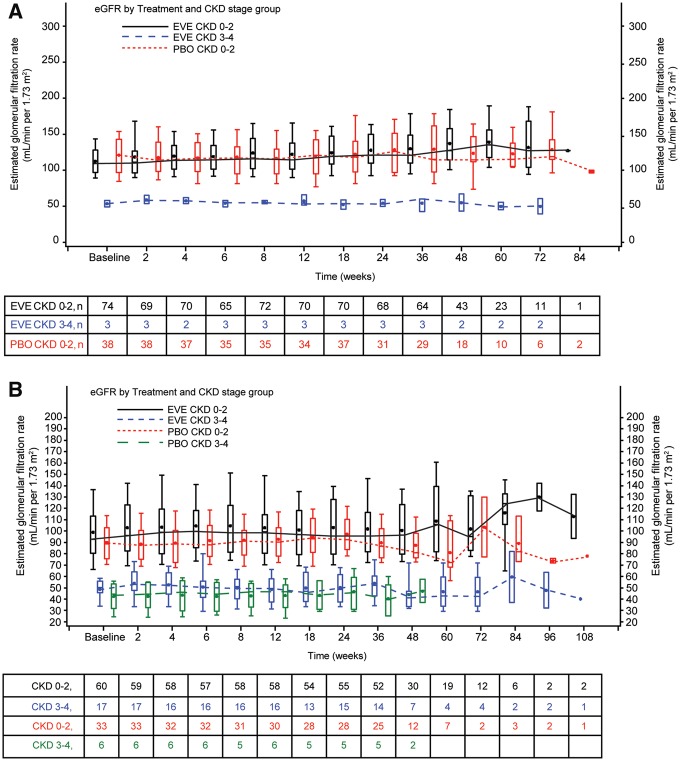
During the PBO-controlled double-blind phases, patients with CKD Stage 3/4 at baseline maintained lower eGFR throughout the double-blind phases of both studies compared with patients with baseline CKD Stages 0–2. The median eGFR among patients with CKD Stage 3/4 was ∼40–50 mL/min/1.73 m2 over the double-blind period (Figure 2a and 2b). Although some individual patients with compromised renal function experienced worsening eGFR over time, eGFR generally did not deteriorate throughout the double-blind phases in patients receiving EVE.
FIGURE 2.
Boxplots of the change in eGFR based on treatment and baseline CKD stage during the double-blind phases of (A) EXIST-1 and (B) EXIST-2. The MDRD formula [33] was used to estimate GFR for patients ≥18 years of age and the ‘bedside’ Schwartz formula [34] was used to estimate GFR in patients <18 years of age.
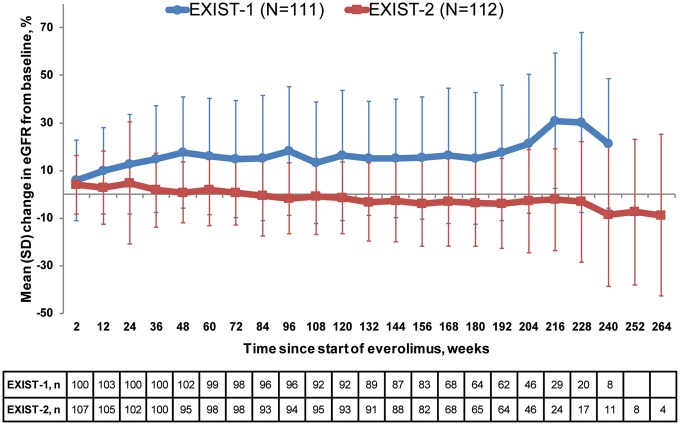
Mean eGFR remained relatively stable over time among patients in either study. Among patients in EXIST-1, eGFR generally increased over the first 48 weeks, then remained stable, whereas in EXIST-2 eGFR did not show much change over the course of the study (Figure 3).
FIGURE 3.
Mean percentage change from baseline in eGFR with SD. The MDRD formula [33] was used to estimate GFR for patients ≥18 years of age and the ‘bedside’ Schwartz formula [34] was used to estimate GFR in patients <18 years of age.
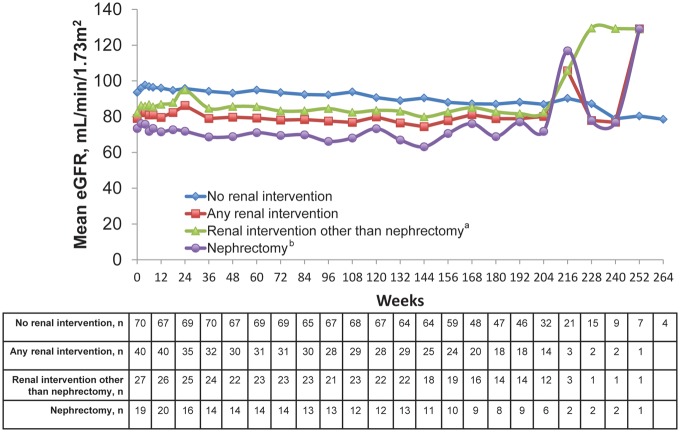
In EXIST-2, about one-third of patients had undergone renal intervention for angiomyolipoma (i.e. surgery) before entering the study. The effects of prior renal intervention on eGFR in these patients can be found in Figure 4. Overall, patients with any renal intervention had lower eGFR than those who did not have a renal intervention. Patients who had previous nephrectomy had the lowest mean eGFRs. However, eGFR appeared to remain stable over time while receiving EVE regardless of past renal interventions for treating angiomyolipoma.
FIGURE 4.
eGFR over time according to previous angiomyolipoma-related renal intervention in EXIST-2 patients. GFR was estimated using the MDRD formula [33]. aIncludes embolization, thermoablation and angiomyolipoma-related surgery other than nephrectomy. bIncludes partial and complete nephrectomy.
More than 75% of the patients in EXIST-1 had negative or trace proteinuria levels per laboratory assessment across all time points (Table 4). In EXIST-2, ≥50% of patients had negative or trace levels through Week 228. The percentage of patients in EXIST-2 with 1+ to 4+ proteinuria per laboratory assessment while on EVE was relatively stable over time, with most patients in the mild (1+) category.
Table 4.
Protein urinalysis values over time for patients from EXIST-1 and EXIST-2
| EXIST-1 | ||||||||||||
| n (%)a | Baseline (n = 111) | Week 12 | Week 24 | Week 48 | Week 72 | Week 96 | Week 120 | Week 144 | Week 168 | Week 192 | Week 216 | Week 240 |
| Negative | 81 (80.2) | 62 (61.4) | 60 (59.4) | 63 (64.3) | 57 (58.8) | 54 (59.3) | 60 (71.4) | 52 (63.4) | 48 (66.7) | 41 (71.9) | 28 (87.5) | 10 (62.5) |
| Trace | 14 (13.9) | 20 (19.8) | 26 (25.7) | 20 (20.4) | 24 (24.7) | 24 (26.4) | 16 (19.0) | 17 (20.7) | 15 (20.8) | 8 (14.0) | 2 (6.3) | 6 (37.5) |
| 1+ | 4 (4.0) | 12 (11.9) | 7 (6.9) | 7 (7.1) | 9 (9.3) | 7 (2.2) | 5 (6.0) | 5 (6.1) | 4 (5.6) | 3 (5.3) | 1 (3.1) | 0 |
| 2+ | 2 (2.0) | 2 (2.0) | 3 (3.0) | 3 (3.1) | 3 (3.1) | 2 (2.2) | 1 (1.2) | 5 (6.1) | 3 (4.2) | 4 (7.0) | 1 (3.1) | 0 |
| 3+ | 0 | 1 (1.0) | 2 (2.0) | 3 (3.1) | 3 (3.1) | 1 (1.1) | 2 (2.4) | 2 (2.4) | 2 (2.8) | 0 | 0 | 0 |
| 4+ | 0 | 3 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 101 | 101b | 101c | 98b | 97 | 91c | 84 | 82b | 72 | 57b | 32 | 16 |
| EXIST-2 | ||||||||||||
| n (%)a | Baseline (n = 112) | Week 12 | Week 24 | Week 48 | Week 72 | Week 96 | Week 120 | Week 144 | Week 168 | Week 192 | Week 216 | Week 240 |
| Negative | 70 (67.3) | 37 (35.2) | 32 (31.4) | 33 (34.4) | 29 (29.3) | 34 (35.1) | 30 (31.3) | 33 (35.9) | 22 (32.8) | 21 (32.8) | 9 (36.0) | 2 (18.2) |
| Trace | 15 (14.4) | 22 (21.0) | 25 (24.5) | 29 (30.2) | 28 (28.3) | 19 (19.6) | 24 (25.0) | 24 (26.1) | 19 (28.4) | 15 (23.4) | 5 (20.0) | 1 (9.1) |
| 1+ | 12 (11.5) | 24 (22.9) | 26 (25.5) | 18 (18.8) | 21 (21.2) | 26 (26.8) | 20 (20.8) | 23 (25.0) | 11 (16.4) | 15 (23.4) | 4 (16.0) | 2 (18.2) |
| 2+ | 6 (5.8) | 13 (12.4) | 11 (10.8) | 11 (11.5) | 18 (18.2) | 13 (13.4) | 15 (15.6) | 7 (7.6) | 11 (16.4) | 11 (17.2) | 6 (24.0) | 4 (36.4) |
| 3+ | 1 (1.0) | 9 (8.6) | 7 (6.9) | 3 (3.1) | 3 (3.0) | 5 (5.2) | 6 (6.3) | 4 (4.3) | 2 (3.0) | 2 (3.1) | 1 (4.0) | 2 (18.2) |
| 4+ | 0 | 0 | 0 | 1 (1.0) | 0 | 0 | 0 | 1 (1.1) | 1 (1.5) | 0 | 0 | 0 |
| Total | 104 | 105 | 102b | 96b | 99 | 97 | 96b | 92 | 67 b | 64 | 25 | 11 |
Since proteinuria can be transient, patients with proteinuria may not necessarily be the same individuals from one time interval to another.
n = 1 with missing values.
n = 2 with missing values.
Safety/tolerability
One patient in EXIST-1 and one patient in EXIST-2 experienced Grade 3 elevations in serum creatinine [>3.0 × upper limit of normal (ULN)]. Grade 1 or 2 serum creatinine elevations (>ULN to 3.0 × ULN) occurred in 3.6 and 15.2% of patients in EXIST-1 and EXIST-2, respectively. Only two of the serum creatinine elevation events in EXIST-1 and seven in EXIST-2 were reported as AEs. Proteinuria was classified as an AE by the investigator in 3.6% of EXIST-1 and 17.9% of EXIST-2 patients during the course of the studies; this was mostly Grade 1 or 2 (1+ or 2+ to 3+ on a urine dipstick test) in severity. Grade 3 proteinuria (4+ on a urine dipstick test) was reported as an AE in only two patients in EXIST-2; both were suspected to be related to treatment and one led to study discontinuation but resolved after dose reduction/interruption. This patient was the only patient between the two studies that permanently discontinued treatment because of proteinuria.
DISCUSSION
Previously published reports from the EXIST-1 and EXIST-2 studies [20, 21, 23–25, 32] have demonstrated that the use of EVE was associated with >40–50% reduction in renal angiomyolipoma volume with continued volume reduction over time. The current data add to our knowledge, demonstrating that renal function was generally stable over ∼4 years of EVE treatment. A decline in renal function, with progression to Stage 4 CKD, was confined to only those patients with compromised renal function at baseline (Table 3). As a result, there is the need for ongoing close monitoring of renal function in these patients as with any patient with advanced CKD. On average, the lower eGFR values reported in EXIST-2 as compared with EXIST-1 likely reflect differences in disease severities and trajectories of renal angiomyolipoma and patient age in these two patient populations.
The natural course of kidney function decline in TSC is not well known, although some existing data in larger populations of TSC patients suggest that kidney function declines more rapidly than in the general population. A retrospective database analysis of renal involvement in patients with TSC in the UK found that the prevalence of Stage 3 or higher CKD in the TSC population was more than five times greater than in the UK general population [relative risk 5.4 (95% confidence interval 3.7–8.0); P < 0.001]. The estimated prevalence of CKD increased from 3.1% in 18- to 24-year-olds to 41.2% in 45- to 54-year-olds in the TSC population, compared with 0.1–2.9%, respectively, in the UK general population [36].
As would be expected based on the differing enrolment criteria of the studies, renal disease was more severe in patients in EXIST-2 than in EXIST-1. Renal angiomyolipoma burden at baseline was greater in EXIST-2; 7% of those in EXIST-1 and 60% of those in EXIST-2 had six or more renal angiomyolipoma lesions measuring ≥1 cm and 37.5% of the patients in EXIST-2 had undergone prior renal angiomyolipoma-related interventions (Table 1), with nephrectomy (full or partial) in 20 patients (18%). By contrast, in EXIST-1, 4 of 111 patients (3.6%) had undergone a prior renal intervention. In patients who undergo total nephrectomy, renal function is typically halved [37], which could have contributed to the lower eGFR in the patients in EXIST-2 compared with EXIST-1 (Figure 1), and is one of the reasons why clinical guidelines recommend avoidance of surgical and embolic or ablative therapies where possible in patients with renal angiomyolipoma [17]. Kidneys with a large renal angiomyolipoma burden can still contribute significantly to renal function [38], therefore the decision to undergo nephrectomy should be balanced against loss of function. Our data indeed show the difference that previous nephrectomy had on eGFR in patients in EXIST-2, but no significant decline in eGFR over time was observed during treatment in these patients. The effects of age likely contributed to the differences between the observed eGFRs in each study (Figure 3). With regards to the increase in percentage change of eGFR seen in EXIST-1, it is possible we may be observing that predominantly younger patients with TSC renal disease and early impairment of GFR improve on EVE. However, later time points have fewer patients due to discontinuations or not reaching that length of treatment by the cutoff date, so caution should be exercised in interpreting the results of the latest time points of the figures showing eGFR. It should also be noted that although both studies estimated GFR in adults using the MDRD equation, because the MDRD formula is believed to overestimate GFR in children, the Schwartz formula was used to estimate GFR in patients <18 years of age (the majority of the population in EXIST-1). Among other differences with the MDRD equation, the Schwartz formula includes patient height as a variable instead of age [33, 34].
Reduction of the renal angiomyolipoma burden without evidence of renal function impairment may slow or halt the progression of CKD and reduce the risk of hemorrhage. The mechanism of EVE-associated amelioration of premature GFR decline in TSC is unknown. In particular, it is unknown whether it is due to a direct effect on renal angiomyolipomas. mTOR has been implicated in podocyte maintenance and glomerular disease associated with diabetic nephropathy, with activation of mTORC1 resulting in effaced foot processes and proteinuria [26, 27, 39]. mTORC1 haplotype insufficiency (i.e. independent of renal angiomyolipomas) may provide a possible alternate mechanism for progression of CKD in TSC. Preclinical studies have demonstrated that mTORC1 appears to contribute to the development of normal-size glomeruli and podocytes, whereas decreased mTORC1 activity results in glomerular injury [26, 27]. Thus too much or too little mTOR activation may cause renal injury, but our results show that careful dose titration preserved eGFR in most patients. Only nine patients developed Stage 4 CKD (eGFR <30 mL/min/1.73 m2), and it is unclear whether this was despite EVE treatment or if EVE exacerbated their underlying renal impairment. Furthermore, the timing and effects of mTOR inhibition appear to be important, with early inhibition with rapamycin preventing renal injury caused by mTORC1 activation in podocytes [26, 39]. This suggests that continued surveillance and earlier treatment may lead to improved longer-term renal function.
Proteinuria is a known mTORC1 inhibitor–associated AE [18]. Proteinuria has previously been reported in small single-arm studies with the mTOR inhibitor sirolimus for the treatment of TSC-associated renal angiomyolipomas [40, 41], with an incidence of 27.8% reported in one study [40]. However, the majority of cases in that study were mild (trace or 1+ on a urine dipstick) in severity, with few patients (7.1%) experiencing more severe proteinuria (3+ on a urine dipstick) [40]. The presence of proteinuria in baseline laboratory analyses in EXIST-1 and EXIST-2 suggest that proteinuria may be a feature of TSC renal disease. This may be further supported by findings in the double-blind phase of EXIST-2, in that proteinuria was more common in patients receiving PBO than in those receiving EVE (8% versus 4%) [21]. In both studies, most patients had negative or trace protein values over time; however, the proportion of patients with negative results did decrease shortly after EVE initiation and the proportion with 1+ and 2+ values was higher after initiation of EVE. The proportion of patients with higher values remained at ∼6% or less after 1 year and through the end of the study.
Proteinuria is often mild, transient or variable in patients taking EVE for TSC. There is no evidence from this study or the TSC literature that it is a marker for hyperfiltration nephropathy in this situation, and the etiology may be distinct from that which occurs in renal transplantation. Not uncommonly, there is an element of tubular proteinuria in patients with TSC on mTORC1 inhibitors, and the mTORC1 pathway clearly regulates proximal tubule endocytosis [42]. However, it would seem prudent that if proteinuria is >1 g/day and rising progressively, the dose of EVE should be adjusted (reduced or temporarily suspended until proteinuria is <1 g/day). It is probable that proteinuria in this situation is partly or mainly a functional effect (perhaps mediated by inhibiting the mTOR pathway in podocytes or renal tubular cells) because, in practice, it almost always decreases or resolves on dose reduction. In patients who are likely to have hyperfiltration nephropathy for other reasons (e.g. GFR <60 mL/min/1.73 m2 and/or hypertension) it seems reasonable to use an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
Limitations to our analyses include limited numbers in this subanalysis, use of open-label data (no PBO comparison), the use of two different EVE dosing methods between the two studies, assessment of renal function only by serum creatinine (which is influenced by factors other than renal function, such as muscle mass) and calculation of eGFR by two different equations. Measurement of other biomarkers of kidney function such as cystatin C or creatinine clearance would provide useful additional information regarding kidney function in these patients. Also, the proteinuria analyses were limited in that urine creatinine was characterized by dipstick with local laboratory testing and central measurements of proteinuria were not performed. Finally, patients with severe kidney function impairment (defined as creatinine >1.5 times ULN) were excluded from study enrolment in both EXIST-1 and EXIST-2.
In conclusion, data from EXIST-1 and EXIST-2 suggest preservation of renal function as assessed by eGFR and serum creatinine elevations, except in some patients whose renal function is already severely compromised. Overall, EVE does not appear to be nephrotoxic in patients with SEGA or renal angiomyolipoma associated with TSC. However, long-term monitoring of such patients and further studies are warranted.
FUNDING
This study was funded by Novartis Pharmaceuticals. Medical writing assistance for the preparation of this manuscript was provided by Traci Stuve, Andrea Bothwell and Robert Schoen of ApotheCom, with funding from Novartis.
AUTHORS’ CONTRIBUTIONS
J.J.B., K.B., M.S., D.N.F., B.A.Z., M.D.F., E.B. and J.C.K. served as investigators on this study and received research grants (to their institutions) from Novartis. J.J.B., K.B., M.S., D.N.F., M.D.F., E.B. and J.C.K. served as consultants and/or participated in advisory boards for Novartis. J.J.B., K.B., M.S., D.N.F., M.D.F. and J.C.K. received travel honoraria from Novartis. B.A.Z. received research grants, was part of advisory boards and received travel grants from Novartis. N.B. and A.R. are employees of Novartis.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Budde K, Gaedeke J.. Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis 2012; 59: 276–283 [DOI] [PubMed] [Google Scholar]
- 2. Laplante M, Sabatini DM.. mTOR signaling at a glance. J Cell Sci 2009; 122: 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crino PB, Nathanson KL, Henske EP.. The tuberous sclerosis complex. N Engl J Med 2006; 355: 1345–1356 [DOI] [PubMed] [Google Scholar]
- 4. Curatolo P, Bombardieri R, Jozwiak S.. Tuberous sclerosis. Lancet 2008; 372: 657–668 [DOI] [PubMed] [Google Scholar]
- 5. Siroky BJ, Yin H, Bissler JJ.. Clinical and molecular insights into tuberous sclerosis complex renal disease. Pediatr Nephrol 2011; 26: 839–852 [DOI] [PubMed] [Google Scholar]
- 6. Siroky BJ, Yin H, Dixon BP. et al. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol 2014; 307: F560–F570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lieberthal W, Levine JS.. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol 2009; 20: 2493–2502 [DOI] [PubMed] [Google Scholar]
- 8. Siroky BJ, Bitzer M.. The growing importance of mTORC1-S6K1 signaling in kidney. Am J Physiol Renal Physiol 2009; 297: F583–F584 [DOI] [PubMed] [Google Scholar]
- 9. Bonnet CS, Aldred M, von RC. et al. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum Mol Genet 2009; 18: 2166–2176 [DOI] [PubMed] [Google Scholar]
- 10. Brasier JL, Henske EP.. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest 1997; 99: 194–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shepherd CW, Gomez MR, Lie JT. et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991; 66: 792–796 [DOI] [PubMed] [Google Scholar]
- 12. Eijkemans MJ, van der Wal W, Reijnders LJ. et al. Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity, and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am J Kidney Dis 2015; 66: 638–645 [DOI] [PubMed] [Google Scholar]
- 13. Umeoka S, Koyama T, Miki Y. et al. Pictorial review of tuberous sclerosis in various organs. Radiographics 2008; 28: e32. [DOI] [PubMed] [Google Scholar]
- 14. Yamakado K, Tanaka N, Nakagawa T. et al. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology 2002; 225: 78–82 [DOI] [PubMed] [Google Scholar]
- 15. O’Callaghan FJ, Noakes MJ, Martyn CN. et al. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int 2004; 94: 853–857 [DOI] [PubMed] [Google Scholar]
- 16. Vekeman F, Magestro M, Karner P. et al. Kidney involvement in tuberous sclerosis complex: the impact on healthcare resource use and costs. J Med Econ 2015; 18: 1–11 [DOI] [PubMed] [Google Scholar]
- 17. Krueger DA, Northrup H, Northrup H. et al. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49: 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pirson Y. Tuberous sclerosis complex-associated kidney angiomyolipoma: from contemplation to action. Nephrol Dial Transplant 2013; 28: 1680–1685 [DOI] [PubMed] [Google Scholar]
- 19. Krueger DA, Care MM, Holland K. et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 2010; 363: 1801–1811 [DOI] [PubMed] [Google Scholar]
- 20. Franz DN, Belousova E, Sparagana S. et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013; 381: 125–132 [DOI] [PubMed] [Google Scholar]
- 21. Bissler JJ, Kingswood JC, Radzikowska E. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 817–824 [DOI] [PubMed] [Google Scholar]
- 22. Krueger DA, Care MM, Agricola K. et al. Everolimus long-term safety and efficacy in subependymal giant-cell astrocytoma. Neurology 2013; 80: 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kingswood JC, Jozwiak S, Belousova ED. et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transplant 2014; 29: 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franz DN, Belousova E, Sparagana S. et al. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLoS One 2016; 11: e0158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bissler JJ, Kingswood JC, Radzikowska E. et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLoS One 2017; 12: e0180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fogo AB. The targeted podocyte. J Clin Invest 2011; 121: 2142–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godel M, Hartleben B, Herbach N. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 2011; 121: 2197–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roach ES, Gomez MR, Northrup H.. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 1998; 13: 624–628 [DOI] [PubMed] [Google Scholar]
- 29. Hyman MH, Whittemore VH.. National Institutes of Health consensus conference: tuberous sclerosis complex. Arch Neurol 2000; 57: 662–665 [DOI] [PubMed] [Google Scholar]
- 30. Franz DN, Belousova E, Sparagana S. et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol 2014; 15: 1513–1520 [DOI] [PubMed] [Google Scholar]
- 31. Johnson SR. The ERS guidelines for LAM: trying a rationale approach to a rare disease. Respir Med 2010; 104: S33–S41 [DOI] [PubMed] [Google Scholar]
- 32. Bissler JJ, Kingswood JC, Radzikowska E. et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant 2016; 31: 111–119 [DOI] [PubMed] [Google Scholar]
- 33. Levey AS, Coresh J, Greene T. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz GJ, Munoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute. SEER Cancer Statistics Review, 1975–2003 https://seer.cancer.gov/archive/csr/1975_2003/ (3 November 2017, date last accessed)
- 36. Kingswood C, Demuth D, Nasuti P. et al. Real-world assessment of renal involvement in tuberous sclerosis complex (TSC) patients in the United Kingdom (UK). Eur Urol Suppl 2014; 13: e318–e318a [Google Scholar]
- 37. Chapman D, Moore R, Klarenbach S. et al. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J 2010; 4: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bissler JJ, Kingswood JC.. Renal angiomyolipomata. Kidney Int 2004; 66: 924–934 [DOI] [PubMed] [Google Scholar]
- 39. Inoki K, Mori H, Wang J. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 2011; 121: 2181–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dabora SL, Franz DN, Ashwal S. et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS One 2011; 6: e23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cabrera-López C, Marti T, Catala V. et al. Assessing the effectiveness of rapamycin on angiomyolipoma in tuberous sclerosis: a two years trial. Orphanet J Rare Dis 2012; 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grahammer F, Ramakrishnan SK, Rinschen MM. et al. mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J Am Soc Nephrol 2017; 28: 230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]