Abstract
Introduction
The purpose of the study was to evaluate the palliative advanced practice radiation therapy (APRT) role with respect to the impact on waiting times for patients from referral to radiation treatment delivery, the ability of the APRT to define palliative radiation therapy fields and patient satisfaction. The evaluation of the impact of the APRT role and referral pathway on patient waiting times has been previously published.
Methods
Patients were allocated to two different pathways; APRT and standard. Patients in the APRT pathway had their radiotherapy treatment managed by the APRT including defining their palliative fields blinded to the radiation oncologist (RO).
Results
Of the 150 palliative patients, 94 had their radiation therapy managed by the APRT and 56 were managed through the standard pathway. 82/92 APRT defined fields were accepted by the RO.
Conclusions
Inter‐observer variability between the APRT and the RO in defining palliative radiation therapy fields is similar to that reported in the literature between clinicians. With previously published reduced wait times from referral to treatment for palliative patients, the establishment of the APRT role is justified.
Keywords: Advanced practice, inter‐observer variability, palliative, quality improvement, radiation therapist
Introduction
Worldwide access to radiotherapy is unacceptably low with upfront investment and additional skilled professionals needed to prevent unnecessary suffering and deaths from cancer.1 Demand for palliative radiotherapy is on the increase so an increasing burden on the radiation oncology workforce is expected.1 Interest in the basic service of palliative radiotherapy is sometimes overshadowed by exciting new technological advances in radiotherapy which come with ever expanding time pressures on radiation oncologists (RO). To maintain an effective and timely palliative radiotherapy service, we need to maximise the skills of our existing health professionals. This can be done by qualifying staff to work across professional boundaries within multidisciplinary teams.2 In Australia, role expansion or advanced practice has proven to be successful in other allied health professions but is only beginning to be formally executed in radiation therapy.3, 4
For over 10 years advanced practice in radiation therapy has been an established practice in the UK and Canada. The development and integration of advanced practice in radiation therapy internationally was seen to address problems such as oncology staff shortages, expansion and improvement of cancer services, career development and staff retention and recruitment. Aligning with international models, the development of advanced practice in radiation therapy in Australia has been identified as an opportunity to extend clinical roles and to improve coordination of care, efficiency and productivity.5
In 2014 a palliative advanced practice radiation therapy (APRT) role was established at Radiation Oncology Princess Alexandra Raymond Terrace (ROPART), Australia to fill a gap in our palliative service.6
An evaluation of the palliative APRT role sought to assess the impact on waiting times for patients from referral to radiation treatment delivery, the ability of the APRT to define palliative radiation fields on digitally reconstructed radiographs (DRR) compared to a RO and the impact of the APRT involvement in patient satisfaction. Our hypotheses are that patients managed by the APRT would have reduced wait times from referral to treatment, palliative fields defined by the APRT would be similar to those defined by the RO with no noted difference in patient satisfaction with care. This would justify the APRT role in the department by demonstrating that the palliative patient's radiotherapy treatment from referral had been streamlined. The evaluation of the impact of the APRT role and referral pathway on patient waiting times has been previously published.6
We now report on the assessment of the field delineation.
Methods
Ethics approval for this project was obtained through Princess Alexandra Hospital and Health Services. (HREC/13/QPAH/713).
Potential participants were consecutive patients referred for palliative RT at ROPART between October 2014 and March 2015.
For field definition, patients were allocated into two different pathways. Those allocated to the first pathway (APRT managed) consisted of patients who were referred either directly to the APRT6or to one of four nominated ROs. This group of patients had all aspects of their radiation therapy, from referral to treatment, managed by the APRT in consultation with the treating RO. Those allocated to the second pathway (standard managed) consisted of patients who were referred to the remaining six ROs in the department. This group of patients were managed as per the department's standard referral to treatment pathway without any involvement of the APRT. Pathway allocation is shown in Figure 1.
Figure 1.
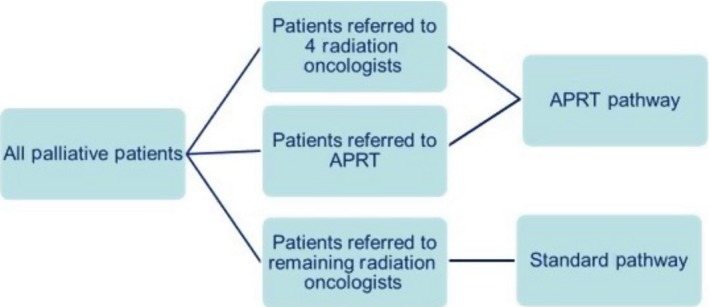
Pathway allocation. APRT, advanced practice radiation therapist.
All patients managed by the APRT were contacted prior to the initial clinic appointment to assess symptom burden, performance status, social situation and patient preference for planning and treatment on the same or an alternative day. The APRT then arranged consult, planning and treatment appointments, liaising with other allied health disciplines as needed. Where possible the patient's visits to the department were kept to a minimum by attempting to consult, plan and administer the first fraction of radiotherapy in one or two visits. The palliative APRT had access to all imaging and histology and where possible attended the patient's initial consultation with the RO. Once a treatment plan was formulated by the RO the details including patient positioning, dose and fractionation were discussed with the APRT.
The standard pathway involved any internal or external referrers phoning or faxing a referral directly to one of the departments six remaining ROs. All appointments and patient contact was made by administration staff under the guidance of the RO. Patients were given the next available appointment for consultation, planning and treatment unless directed by the RO.
Field definition
Planning CT scans were imported into the 3D volume based planning system by planning RTs and an isocenter placed at an approximate field centre on the DRR. The APRT and RO both had access to all patient imaging and any prior radiation treatment details at the time of field definition. Both were blinded to the others field definition.
Once the final RO plan was submitted for planning, a comparison was made of the APRT and RO treatment fields. The RO was then asked to deem the APRT's field definition clinically acceptable or unacceptable and document the reason if the field was unacceptable. Clinical acceptability meant that although field definitions may differ, the clinical outcome measure would not be meaningful to the patient. The distance from the isocenter to each field border for all field definitions marked by the APRT and the RO were recorded.
Statistical analysis
Statistical descriptions of continuous variables were presented as mean and standard deviation (SD) or median and inter‐quartile range (IQR) depending on the distribution of the data. Normality was assessed using Shapiro‐Wilk test. Categorical variables were described using frequencies and percentages. The mean of the distance from the isocenter to each field border (i.e. superior, inferior, right and left) was calculated using all definitions marked by the APRT and RO and compared using a t‐test. All analyses were performed using the R statistical software and p‐values were two‐tailed with p < 0.05 considered statistically significant.
Results
Between October 2014 and March 2015, 150 consecutive patients were referred for palliative radiotherapy. Of the 150 palliative patients 94 had their radiation therapy managed by the APRT and 56 were managed through the standard pathway. Table 1 outlines patient's characteristics.
Table 1.
Patient characteristics
| Characteristic | APRT managed | Standard management |
|---|---|---|
| N = 94(%) | N = 56(%) | |
| Gender | ||
| Male | 44 (47) | 36 (64) |
| Female | 50 (53) | 20 (36) |
| Age (years) | ||
| Median (range) | ||
| ≤55 | 13 (14) | 12 (21) |
| 56–74 | 60 (64) | 25 (45) |
| ≥75 | 21 (22) | 19 (34) |
| Primary diagnosis | ||
| Bladder | 2 (2) | 3 (5) |
| Prostate | 21 (23) | 12 (21) |
| Colorectal | 2 (2) | 2 (4) |
| Lung | 28 (31) | 15 (26) |
| Breast | 25 (27) | 9 (16) |
| Gynae | 7 (7) | 1 (2) |
| Renal | 3 (2) | 4 (7) |
| Other | 6 (6) | 10 (19) |
APRT, advanced practice radiation therapist.
A total of 92 RT fields were defined by the APRT on DRRs blinded to the RO. Two patients in the APRT pathway had their fields defined with a manual mark up by the RO, so were not included in the definition study. The definition distribution is shown in Table 2.
Table 2.
Definition distribution
| Site of XRT | Delineations |
|---|---|
| No. (%) | |
| Spine | 23 (25) |
| Whole brain | 14 (15) |
| Chest (bone) | 2 (2) |
| Chest (soft tissue) | 17 (19) |
| Pelvis (bone) | 14 (15) |
| Pelvis (soft tissue) | 1 (1) |
| Abdomen (soft tissue) | 2 (2) |
| Limb | 18 (20) |
| Face | 1 (1) |
XRT, radiotherapy.
82/92 APRT defined fields were accepted by the RO. Of the acceptable defined fields 9% had at least one border that was more than 2 cm different. The largest difference in a border of a field defined by the APRT that was accepted by a RO was 4.5 cm. Overall the comparison of the mean distance from the isocenter to the individual borders of the fields marked by the APRT and the RO were not statistically significant. Distribution of distances from isocenter to individual borders is shown in Figure 2.
Figure 2.
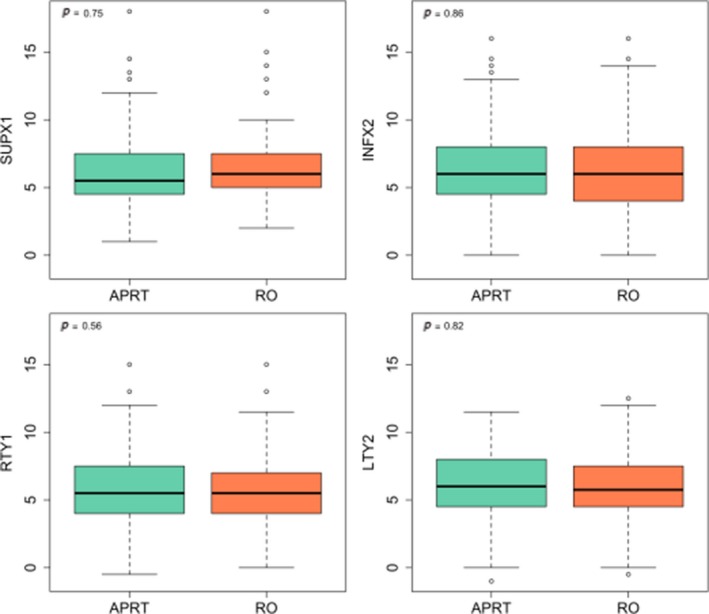
Distribution of distances from Isocenter to Individual Borders. SUPX1, superior border; INFX2, inferior border; RTY1, right border; LTY2, left border.
The reason given by the ROs for the 10 clinically unacceptable definitions are shown in Table 3.
Table 3.
Explanation of unacceptable field definitions
| Case No. | Treatment site | Soft tissue/bone | Reason for unacceptable delineation |
|---|---|---|---|
| 1 | Mediastinum | Soft Tissue | RO increased superior border 1 cm due to possible enlarged node. |
| 2 | Pelvis | Bone | GTV acceptable, RO increased PTV to cover potential microscopic disease |
| 3 | C Spine | Bone | Field size acceptable. RO decreased shielding. |
| 4 | T Spine | Bone | RO decreased superior border 2 cm due to previous field overlap. |
| 5 | Rt Hip | Bone | RO decreased medial border 2 cm considering possible future treatment to prostate. |
| 6 | Pelvis | Bone | GTV acceptable, RO increased PTV due to possible disease extension correlating with patient symptom |
| 7 | L Spine + SIJs | Bone | RO increased lateral border 1 cm to cover adjacent disease |
| 8 | Abdomen | Soft Tissue | RO decreased lateral border 4 cm to cover symptomatic disease only and minimise acute toxicity |
| 9 | Pelvis | Bone | RO decreased both lateral borders 1.5 cm to cover symptomatic disease only. |
| 10 | Pelvis | Bone | Field size acceptable. RO removed shielding considering future field matching |
GTV, gross tumour volume; PTV, planning target volume; RO, radiation Oncologist; C, cervical; T, thoracic; L, lumber; SIJ, sacroiliac joints.
Discussion
Despite palliative RT being an effective and efficient treatment for symptomatic metastatic cancer, access to timely treatment is not always available to patients. In some cases delays in receiving treatment can impact survival7 and increase unnecessary anxiety in patients.8 In response to this problem dedicated multidisciplinary rapid response clinics have been developed around the world to provide patients with a streamline service which involves faster referral to treatment times and fewer hospital visits.9, 10, 11, 12, 13, 14 Often these clinics are restricted to patients with metastatic disease in one organ, that is, palliative bone, lung or brain.13, 15, 16 or can only be accessed by a proportion of patients due to limited clinic time and other commitments of the members of the multidisciplinary team.10
Development of APRT roles in Australia is relatively new and has taken guidance from the UK and Canadian models. In UK the catalyst for the development of these roles was a shortage of radiation oncologists, the need for career extension and improved patient pathways.17 In Canada these roles have been implemented to ease the ever‐growing workload of Radiation Oncologists due to increasingly complicated treatments and technology. Establishment of these roles improved wait times and access to radiotherapy for palliative patients.18
The APRT palliative role evaluated in this study was established to expand the scope of the existing Rapid Response Palliative Radiotherapy clinic. Development of this role has shown to be beneficial to patients by improving wait times from referral to treatment, decreasing hospital visits and improving continuity of care.6 The role of the APRT was expanded to include field definition on DRR with the premise that this would reduce planning times and allow ROs additional time to address their expanding workload. This has been shown in Canada, in an advanced practice SBRT role, where the clinical specialist RT reviewed the day 1 CBCT and treatment reviewed SBRT patients, in place of the RO. This resulted in time saving for the RO.19
There are many studies recommending dose and fractionation for optimum symptom control in patients with metastatic cancer.20, 21 Apart from guidelines from International Bone Metastases Consensus Working Party for defining field borders on spinal and long bone metastases22 there is a lack of evidence and consensus when it comes to defining an appropriate CTV for short course palliative radiotherapy.23 Unlike radical radiotherapy a holistic approach is needed when prescribing palliative treatments considering factors such as toxicity versus symptom control, previous and future treatment, prognosis and performance status of the patient.
There is significant inter‐observer variability between clinicians when defining palliative fields.23, 24, 25 This is the result of many factors. Interpreting symptom origin is not always clear in patients with multiple sites of disease so clinical judgement, matching physical and radiological findings and thorough consultation is essential. Different clinical conclusions can result in variation of treatment volumes. In patients with extensive disease the question of ‘where to start and where to stop’ and balancing treatment toxicity with symptom control can also result in a discrepancy between clinicians.
Grabarz et al. demonstrated the presence of variation in simple palliative field definition between three radiation oncologists and two radiation oncology fellows over nine treatment cases. The nine cases included patients with soft tissue disease, bone disease or a combination of both. Treatment fields were defined by a GTV with uniform expansions and demonstrated a percentage overlap ranging from 55% to 88%.23
In a study by Rose et al, a dedicated palliative RT defined treatment fields blinded to the RO on simple bone metastases. A comparison was done for 11 cases with respect to the differences in field size and border placement. Overall there was no statistically significant difference between the mean RO irradiated area and the mean RT irradiated area. However, they did report three significant discrepancies in field definitions. They report that because of the lack of randomised evidence supporting optimal treatment planning for bone metastases the differences between two of the violated delineations could come under acceptable inter‐observer variation.25
In this study, the field definitions included patients with soft tissue disease, bone disease or a combination of both. Of the ten unacceptable fields defined by the APRT no commonalities in the differences from the RO definitions were found. In cases 1 and 2, a single border was extended by the RO to encompass small asymptomatic or perceived microscopic disease. In cases 4 and 5, the RO decreased a single border by 2 cm as the APRT had not considered previous or possible future radiotherapy. Both these cases occurred early in the study and in neither case, did the wider margin cover a critical structure.
During the study it became evident that PTV margins and expansions to field borders were often inconsistent between ROs. This is demonstrated in cases 6 and 7 where wider margins were used by the RO to cover additional asymptomatic bony disease whereas cases 8 and 9 the APRT covered asymptomatic bony disease with the RO tightening the margins to cover symptomatic disease only. In most of the unacceptable cases the question lies ‘would they have been acceptable by another RO’ and would the unaccepted field definitions come under the auspices of clinician inter‐observer variability. In none of the APRT unacceptable defined fields was symptomatic disease missed or normal tissue tolerance exceeded due to previous treatment in the same area.
Unacceptable field definitions were more prevalent in the first 3 months of the study (60% vs. 40%) and it is expected that over time and with more experience the variability between RO and APRT should reduce to that of variability between ROs, if there is good communication between RO and APRT at time of clinic regarding symptoms, imaging and treatment plan. Continued mentoring and ongoing education of the APRT is important to further improve clinical acumen. It is expected that with time an additional benefit of an experienced palliative APRT will be to complement the training of radiation oncology registrars in palliative radiation therapy.
An internal assessment of satisfaction with care was conducted during the study to ensure the new pathway was not impacting on patient experience. No difference in patient satisfaction with care was noted which could be due to the overall standard of care for palliative patients in the department being multidisciplinary and it is a priority that this cohort of patients is treated holistically. Patient satisfaction has been shown to be related to multidisciplinary care26 so in this situation the patient received care and education from nursing staff (including a dedicated palliative nurse who saw all patients), RT planning and treatment staff, administration and medical staff.
A limitation of the study is that it was carried out in a single institution. As this study is evaluating the only palliative APRT role nationally a collaborative study would have to involve international palliative advanced practitioners.
Conclusion
The inter‐observer variability between the APRT and the RO in defining palliative radiation therapy fields is similar to that documented in the literature between clinicians. These results, combined with the reduction in wait times for patients to receive palliative radiotherapy from referral and reduced hospital visits from consult to first radiotherapy treatment,6 justify the establishment of the APRT role.
The implementation of the role in this department will form the basis of a further publication with the intention of helping to direct other departments to initiate similar roles and to co‐ordinate a national curriculum for palliative APRT training. Evaluation of the role with respect to health economics will form the basis of further research.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the Allied Health Workforce Advice and Coordination Unit Models of Care Development funding which allowed for the development of the Advanced Practice Radiation Therapist role in palliative radiation therapy.
J Med Radiat Sci 66 (2019) 96–102
References
- 1. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015; 16: 1153–86. [DOI] [PubMed] [Google Scholar]
- 2. Eddy A. Advanced practice for therapy radiographers; discussion paper. Radiography 2008; 14: 24–31. [Google Scholar]
- 3. Carter MA, Owen‐Williams E, Della P. Meeting Australia's emerging primary care needs by nurse practitioners. J Nurse Pract 2015; 11: 647–52. [Google Scholar]
- 4. Broome K. Is it time for an Australian advanced practice framework for occupational therapists? Aust Occup Ther J 2015; 62: 210–3. [DOI] [PubMed] [Google Scholar]
- 5. Hilder B, VanDam P, Doherty K. Advanced practice radiation therapists: An Australian context. J Med Radiat Sci 2018; 65: 137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Job M, Holt T, Bernard A. Reducing radiotherapy waiting times for palliative patients: The role of the Advanced Practice Radiation Therapist. J Med Radiat Sci 2017; 64: 274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nieder C, Spanne O, Haukland E, Dalhaug A. Does time between imaging diagnosis and initiation of radiotherapy impact survival after whole‐brain radiotherapy for brain metastases? ISRN Oncol 2013; 2013: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson D, Massey T, Davies E, Jack RH, Sehgal A, Møller H. Waiting times for radiotherapy: Variation over time and between cancer networks in southeast England. Br J Cancer 2005; 92: 1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bansal M, Patel FD, Mohanti BK, Sharma SC. Setting up a palliative care clinic within a radiotherapy department: A model for developing countries. Support Care Cancer 2003; 11: 343–102. [DOI] [PubMed] [Google Scholar]
- 10. Holt TR, Yau VKY. Innovative program for palliative radiotherapy in Australia. J Med Imaging Radiat Oncol 2009; 54: 76–81. [DOI] [PubMed] [Google Scholar]
- 11. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer 2006; 14: 38–43. [DOI] [PubMed] [Google Scholar]
- 12. Casson C, Johnson J. Implementation and evaluation of a rapid access palliative clinic in a New Zealand cancer centre. J Med Radiat Sci 2014; 61: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lefresne S, Berthelet E, Cashman R, et al. The Vancouver rapid access clinic for palliative lung radiation, providing more than just rapid access. Support Care Cancer 2015; 23: 125–32. [DOI] [PubMed] [Google Scholar]
- 14. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: Impact of a dedicated service on palliative cancer care. Pract Radiat Oncol 2014; 4: 247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li KK, Sinclair E, Pope J, et al. A multidisciplinary bone metastases clinic at Toronto Sunnybrook Regional Cancer Centre – A review of the experience from 1999 to 2005. J Pain Res 2008; 1: 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danielson B, Fairchild A. Beyond palliative radiotherapy: A pilot multidisciplinary brain metastases clinic. Support Care Cancer 2012; 20: 773–81. [DOI] [PubMed] [Google Scholar]
- 17. Field LJ, Snaith BA. Developing radiographer roles in the context of advanced and consultant practice. J Med Radiat Sci 2013; 60: 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinclair E, Lau M. Establishing a place for advanced practice in palliative radiation therapy: Experiences of two urban cancer centres. J Med Imaging Radiat Sci 2013; 44: 58. [Google Scholar]
- 19. D'Alimonte L, Holden L, Turner A, et al. Advancing practice, improving care the integration of advanced practice radiation therapy roles into a radiotherapy department: A single institution experience. J Med Imaging Radiat Sci 2017; 48: 118–21. [DOI] [PubMed] [Google Scholar]
- 20. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol 2007; 25: 1423–36. [DOI] [PubMed] [Google Scholar]
- 21. Rades D, Lohynska R, Veninga T, Stalpers LJA, Schild SE. Evaluation of 2 whole‐brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer 2007; 110: 2587–92. [DOI] [PubMed] [Google Scholar]
- 22. Chow E, Hoskin P, Mitera G, et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 2012; 82: 1730–102. [DOI] [PubMed] [Google Scholar]
- 23. Grabarz D, Panzarella T, Bezjak A, McLean M, Elder C, Wong RKS. Quantifying interobserver variation in target definition in palliative radiotherapy. Int J Radiat Oncol Biol Phys 2011; 80: 1498–504. [DOI] [PubMed] [Google Scholar]
- 24. Pope K, Fitzpatrick D, Potter A, et al. Dosimetric and clinical impact of 3D vs. 2D planning in palliative radiotherapy for bone metastases. Support Care Cancer 2013; 21: 2229–35. [DOI] [PubMed] [Google Scholar]
- 25. Rose B, Fairchild A, Ghosh S, Eberle S, Campbell T, Robinson D. Virtual simulation of bone metastases by a dedicated palliative radiation therapist: A retrospective comparison pilot study. J Med Imaging Radiat Sci 2010; 41: 25–9. [DOI] [PubMed] [Google Scholar]
- 26. Wen J, Schulman KA. Can team‐based care improve patient satisfaction? A systematic review of randomized controlled trials. PLoS ONE 2014; 9: e100603. [DOI] [PMC free article] [PubMed] [Google Scholar]


