Abstract
Astrocytes provide support for neurons, regulate metabolic processes, and influence neuronal communication in a variety of ways, including through the homeostatic regulation of glutamate. Following 2-hour cocaine or methamphetamine self-administration (SA) and extinction, rodents display decreased levels of basal glutamate in the nucleus accumbens core (NAcore), which transitions to elevated glutamate levels during drug seeking. We hypothesized that, like cocaine, this glutamate ‘overflow’ during methamphetamine seeking arises via decreased expression of the astroglial glutamate transporter GLT-1, and withdrawal of perisynaptic astroglial processes (PAPs) from synapses. As expected, methamphetamine self-administration and extinction decreased the level of contact made by PAPs in the NAcore, yet did not impact glutamate uptake, GLT-1 expression, or the general structural characteristics of astrocytes. Interestingly, systemic administration of N-acetylcysteine (NAC), a drug that both upregulates GLT-1 and promotes glialglutamate release, reduced cued methamphetamine seeking. In order to test the impact of astrocyte activation and the induction of glial glutamate release within the NAcore, we employed astrocyte-specific expression of designer receptors exclusively activated by designer drugs (DREADDs). We show here that acute activation of Gq-coupled DREADDs in this region inhibited cued methamphetamine seeking. Taken together, these data indicate that cued methamphetamine seeking following two-hour SA is not mediated by deficient glutamate clearance in the NAcore, yet can be inhibited by engaging NAcore astrocytes.
Introduction
Like many other drugs of abuse methamphetamine alters glutamatergic transmission within the corticostriatal pathway (Kalivas, 2009; Parsegian and See, 2014; Schwendt et al., 2012). Within this circuit, in the nucleus accumbens core (NAcore), drug-induced disruptions in astrocyte structure and function are directly linked to relapse vulnerability (Scofield et al., 2016a). These astrocentric alterations can be generally described as disruptions in the glutamate homeostasis, including augmented basal extrasynaptic glutamate levels (Baker et al., 2003; Lominac et al., 2012; Parsegian and See, 2014), deficient astroglial cystine-glutamate exchange (Knackstedt et al., 2009; Trantham-Davidson et al., 2012) and reduced glutamate clearance (Gipson et al., 2013; Knackstedt et al., 2010; Reissner et al., 2014; Shen et al., 2014; Trantham-Davidson et al., 2012). In addition, astrocytes display drug-induced alterations in their morphological properties, with reductions in synaptic insulation and retraction of fine perisynaptic astrocyte processes observed following drug exposure (Scofield et al., 2016b; Testen et al., 2018). We explore the drug-induced sequela of astrocytic dysfunction further below.
Basal extrasynaptic glutamate tone is maintained largely by astroglial glutamate release, carried out in part (Scofield, 2018), by the glial cystine-glutamate exchanger (XC−) (Moussawi et al., 2011a). In the NAcore, basal glutamate levels are decreased following two-hour, colloquially referred to as ‘short access’, cocaine (Baker et al., 2002), and meth self-administration (SA) (Parsegian and See, 2014). One consequence of decreased extrasynaptic glutamate tone in the NAcore is the disinhibition of glutamatergic cortical terminals, due to decreased activation of presynaptic release-regulating mGluR2/3 autoreceptors (Moran et al., 2005). As such, reduced tonic activation of mGluR2/3 in the NAcore is an important aspect of the molecular basis of methamphetamine relapse vulnerability, as this adaptation would serve to exacerate glutamate release during drug seeking, discussed in detail below. Consistent with this hypothesis, systemic activation of mGluR2/3 has been shown to inhibit cued methamphetamine (Caprioli et al., 2015; Kufahl et al., 2013), cocaine (Peters and Kalivas, 2006), nicotine (Liechti and Markou, 2007), heroin (Bossert et al., 2005), and ethanol seeking (Zhao et al., 2006). Further, xCT, the catalytic subunit of xC-, is downregulated in the NAcore following cocaine (Knackstedt et al., 2010) and nicotine (Knackstedt et al., 2009) self-administration (SA) and extinction. Thus, we hypothesized that, like cocaine (Baker et al., 2002), decreased basal glutamate levels in the NAcore following methamphetamine SA and extinction could arise from decreased efficacy of astroglial cystine-glutamate exchange.
Using rodent models of relapse, elevated NAcore glutamate levels are observed during seeking of cocaine, heroin, nicotine, and methamphetamine, which have been directly linked to the neural mechanisms underlying relapse (Kalivas, 2009; Scofield et al., 2016a). Following cocaine (Knackstedt et al., 2010), heroin (Shen et al., 2014), and nicotine (Gipson et al., 2013) SA and extinction training, glutamate release in the NAcore during drug seeking has been accompanied by concomitant reductions in astroglial glutamate clearance, due to drug-induced downregulation of the glutamate transporter GLT-1, primarily expressed in astrocytes (Scofield et al., 2016a). Interestingly, cocaine decreases GLT-1 expression in the NAcore 24 hours after short access SA, yet this downregulation is further exacerbated following 45 days of withdrawal (Fischer-Smith et al., 2012), suggesting that both drug exposure, and a period of extinction or abstinence, contribute to alterations in NAcore astrocytic glutamate clearance. Given that enhanced levels of NAcore glutamate are also observed during methamphetamine seeking (Parsegian and See, 2014), we postulated that expression of GLT-1 would be reduced in the NAcore following methamphetamine SA and extinction.
We also hypothesized that, like cocaine (Scofield et al., 2016b), methamphetamine SA and extinction would reduce the extent of synaptic contact made by perisynaptic astrocytic processes (PAPs) in the NAcore. We posit that withdrawal of these processes from NAcore synapses may contribute to deficient glutamate clearance and to overall cue-induced glutamate spillover (Hirase et al., 2014; Okubo and Iino, 2011). This hypothesis was constructed in light of the fact that enhanced access of synaptically released glutamate to non-synaptic compartments in the NAcore would significantly contribute to relapse biology, given the current model of cue-induced transient plasticity underlying the induction of drug seeking (Kalivas, 2009; Scofield and Kalivas, 2014; Smith et al., 2014; Smith et al., 2015).
In light of the connection between decreased basal glutamate levels and relapse vulnerability(Scofield et al., 2016a), we hypothesized that restoration of basal glutamate levels via activation of NAcore glial glutamate release would serve as an effective means of inhibiting cued methamphetamine relapse. The usage of glial mechanisms to restore glutamate tone and inhibit relapse was selected as astroglial glutamate release occurs at lower magnitudes than conventional synaptically released glutamate (Lee and Haydon, 2007; Sahlender et al., 2014). Accordingly, glutamate of glial origin is more likely activate the release limiting presynaptic mGluR2/3 autoreceptors discussed above, as opposed to postsynaptic mGluR5 receptors, that when activated promote relapse (Baker et al., 2003; Kupchik et al., 2012; Moran et al., 2005; Scofield et al., 2015b). One means for the induction of astroglial glutamate release is the systemic administration of the antioxidant drug N-acetylcysteine (NAC), which has been shown to inhibit cue- and drug-primed reinstatement to cocaine (Murray et al., 2012; Reichel et al., 2011), heroin (Zhou and Kalivas, 2008) and nicotine seeking (Ramirez-Nino et al., 2013). NAC has also shown some human clinical efficacy with reduced self-reported craving and cue reactivity in clinical trials across several classes of addictive substances (Roberts-Wolfe and Kalivas, 2015) including methamphetamine (Mousavi et al., 2015). Mechanistically, NAC upregulates NAcore GLT-1 and xCT following drug exposure (Knackstedt et al., 2010), with restoration of GLT-1 expression and glutamate clearance being critical for its ability to inhibit cocaine seeking ((Reissner et al., 2015), however see (Logan et al., 2018)). In addition, NAC restores basal glutamate levels (Moussawi et al., 2011b), likely due to its ability to induce glutamate release in astrocytes by providing a substrate for XC−, pushing forward astroglial glutamate release (Moussawi et al., 2011b). As an extension of our previous glial glutamate release studies with NAC, we have utilized activation of Gq-DREADD in NAcore astrocytes as a potential means for the selective induction of glial glutamate release in order to inhibit cued cocaine seeking. Indeed, acute activation of Gq-DREADD prior to reinstatement reduced cued cocaine seeking, an effect that was dependent on activation of mGluR2/3 (Scofield et al., 2015a).
In the current study, we sought first to determine to what extent drug-induced impacts on astroglial physiology observed with other drugs of abuse extend to methamphetamine, in order to refine our understanding of the involvement of NAcore astrocytes in methamphetamine relapse biology (Scofield et al., 2016a). Specifically, we investigated the impact of two-hour methamphetamine SA and extinction on GLT-1 function and expression as well as several aspects of astrocyte structure and astrocyte-synapse interaction. Next, we sought to determine if restoration of basal glutamate levels by engaging astrocytic release of glutamate in the NAcore, either chronically during extinction with NAC or acutely prior to relapse testing with astrocyte-specific Gq-DREADD activation, serves as an effective means to inhibit cued relapse.
Materials and Methods
Catheter surgery.
Male Sprague Dawley rats (N=71) were obtained from Charles River (300g), and were individually housed in a 12:12 reverse light:dark cycle room with ad lib access to food and water. Prior to implantation of jugular catheters, animals were anesthetized with ketamine (66 mg/kg), xylazine (1.3 mg/kg), and equithesin (all administered i.p), and given Ketorolac (analgesic; 4.0 mg/kg, s.c.). Silastic catheters were inserted into the right jugular vein. The catheter was guided subcutaneously to the back and attached to an infusion harness (Instech Solomon) for i.v. drug delivery. Cefazolin (antibiotic) (10 mg/0.1 ml) was infused post-surgically, and for 3 days during recovery with 0.1 ml 70 U/ml heparinized saline. Given the ability of β-lactam antibiotics to influence GLT-1 expression (Rasmussen et al., 2011), it is important to note that the cefazolin treatment regimen used in our study is below the necessary dose (100 mg/kg) to impact GLT-1 expression (Rao et al., 2015), and was administered prior to methamphetamine SA and extinction.
Gq-DREADD and LCK-GFP viral infusion surgery.
Following implantation of intravenous jugular catheters, a subset of animals received virus microinjections into the NAcore as described previously (Scofield et al., 2015a). Briefly, 1μl of rAAV5/GFAP-HA-hm3D-IRES-mCitrine (GFAP-Gq-DREADD), or rAAV5/GFAP-LCK-GPP (GFAPLCK-GFP) obtained from the UNC vector core at ~1×1012 viral particles/ml was stereotaxically infused bilaterally into the NAcore (AP + 1.5 mm; ML ± 1.8 mm; DV − 7.5 mm from bregma, at a 6° angle) using 33 gauge injectors (plastics one) at a rate of 0.1μl/min, followed by a 10-min diffusion time. All procedures were conducted in accordance with the ‘Guide for the Care and Use of Laboratory Rats’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina.
Methamphetamine self-administration, extinction, and reinstatement.
Animals self-administered methamphetamine as described previously (Reichel et al., 2011) using standard operant chambers (Med Associates, St Albans, VT) and was conducted as described previously (Peters et al., 2016; Scofield et al., 2015d). Briefly, following recovery from catheter surgery, rats were assigned to methamphetamine or yoked-saline control groups and began SA. Methamphetamine hydrochloride (Sigma, St Louis, MO), was dissolved in sterile 0.9% saline. Rats self-administered methamphetamine in 2-hour sessions for 10–14 days (criterion of greater than 10 infusions per day) on a fixed-ratio (FR)-1schedule of reinforcement. A response on the active lever resulted in a presentation of a 5 second-duration conditioned-cue complex consisting of a tone (78db, 4.5 kHz) and white light stimulus above the active lever followed by a 2-sec infusion (20 μg/50 μl bolus). Responses on the inactive lever resulted in no programmed consequence. Yoked saline control animals received a 50 μl saline infusion whenever the matched subject received a self-administered methamphetamine infusion. Following SA, rats underwent extinction. The number of extinction sessions is discussed in the results section for each experiment. During extinction training, neither active nor inactive lever presses had programmed consequences. Once responding decreased to an average of < 25 presses on the active lever over 2 days of extinction, rats were tested for cue-induced reinstatement. During cued reinstatement testing, active lever pressing resulted in the contingent presentation of the light and tone stimulus (same duration and frequency as during SA) complex but was not concomitant with infusion of drug.
GLT-1 protein quantification.
A subset of methamphetamine and yoked-saline animals were sacrificed for GLT-1 protein quantification via rapid decapitation 24 hours after the final extinction session. Brain tissue was rapidly removed, and the NAcore was dissected on ice. Tissue was then transferred to tubes for immediate homogenization via sonication in HEPES buffer with protease inhibitors. Following sonication, subcellular fractionation was performed to obtain a crude membrane rich fraction. Wes™ by ProteinSimple (Bio-Techne) was used to quantify NAcore protein levels as described previously (O’Neill et al., 2006; Scofield et al., 2015c). As an example, a 1 μg/μl lysate is diluted to 0.1 μg/μl by combining 2.5 μl lysate, 2.5 μl fluorescent master mix and 7.5 μl 0.1x sample buffer for a total volume 18.75 μl. The reaction mix was then heated at 70 °C for 10 minutes. Lysate mixture, blocking reagent, primary antibodies, secondary antibodies, chemiluminescent substrate, and wash buffer were then dispensed into designated wells. Stacking load time was 18 seconds and load time was 9 seconds. Data were analyzed using Compass software (ProteinSimple) with the “Gaussian fit” function. The primary antibodies were GLT-1 (Abcam, 41621, 1:2000) and Calnexin (Enzo Life Sciences, ADI-SPA-860-F, 1:1000). GLT-1 signals were normalized to that of calnexin (Reissner et al., 2015) (Garcia-Fuster et al., 2009). We chose calnexin as a loading control as it was the loading control used in our previous GLT-1 downregulation studies (Reissner et al., 2015), and because it is well-validated for use in Wes™(Barry and McGinty, 2017; Siemsen et al., 2018b). More importantly, GLT-1 is a 62 kDa protein, thus GLT-1 peaks would overlap with that of other loading controls such as beta-actin, a 42 kDa protein. Finally, studies show that actin is regulated by psychostimulant exposure (Toda et al., 2010; Toda et al., 2006), making calnexin the best choice for our study. Data are expressed as a percent of averaged yoked-saline values.
Radiolabeled glutamate uptake assay.
Glutamate uptake was measured using an in vitro slice preparation as described previously (Trantham-Davidson et al., 2012). Briefly, rats were decapitated 24 hours following the last extinction session and NAcore tissue was dissected and sliced into 250 × 250 μm sections using a Mcillwan tissue chopper. Slices were incubated at 37°C in either oxygenated Krebs–Ringer’s solution phosphate buffer (in mM: 140 NaCl, 1.3 CaCl2, 1.2 KH2PO4, 5 HEPES, 10 glucose, and 1 MgCl2, pH 7.4) to quantify Na+-dependent uptake, or an identical buffer in which NaCl was replaced with 140 mM choline chloride to measure Na+-independent uptake. Uptake was stimulated by the addition of L-[3H] glutamate (40 nM, 51 Ci/mM; PerkinElm) to slices in the presence of 3 μM L-glutamate. The reaction was conducted at 37°C for 15 min and terminated by ice-cold, Na+-free buffer. Slices were solubilized with 1% SDS and radioactivity determined using a liquid scintillation counter. Protein content was measured using a bicinchoninic acid assay protein assay (Pierce) and counts per million per milligram of protein calculated.
Immunohistochemistry.
Animals used for immunohistochemistry were anesthetized and perfused with 200 mL 0.1M PB followed by 200 mL 4% formaldehyde. Coronal slices containing the NAcore (100 μm) were blocked with 2% NGS and 2% Triton-X 100 in 0.1M PBS (PBST) for 1.5 hr. Sections were then incubated in primary antibodies diluted in PBST with shaking overnight at 4°C. For DREADD studies, mouse monoclonal HA antibody 1:1000, Covance 16B12 was used. For colocalization studies, a chicken anti-GFP (Abcam, 1:1000, Ab13970) and rabbit anti-Synapsin-1 (Abcam, 1:500, Ab8) were used. For GFAP structural analysis studies, Rabbit anti-GFAP primary antibody was used (Abcam, 1:2000, ab7260). Following primary antibody incubation, sections were washed 3X for 5 min in PBST. Secondary incubation was performed at room temperature for five hours with species-appropriate Alexa 488, 594, or 647 conjugated antibodies (1:2000, Life Technologies) in PBST. We have previously demonstrated that 5-hour secondary incubation improves antibody penetration in 100μm slices, providing homogenous signal intensity throughout the section (Scofield et al., 2016b; Siemsen et al., 2018a). Slices were then washed again 3X for 5 min in PBST prior to mounting with Pro Long Gold Antifade (Life Technologies).
Confocal microscopy and the astrocyte synaptic contact assay.
Astrocyte imaging and subsequent colocalization assay was performed as described previously, (Scofield et al., 2016b; Testen et al., 2018). Briefly, astrocytes with clear boundaries were selected for imaging provided they could be fully visualized throughout the z-series with no obvious interruption of LCK-GFP signal. An average of 5 full astrocytes per animal across several coronal sections were imaged along with synaptic puncta. Laser power and optical parameters were kept constant between groups for all cells imaged and astrocyte and synaptic puncta imaging was performed by an investigator blinded to treatment group. Acquisition of astrocyte images was performed on a Leica SP8 laser scanning confocal super resolution microscope with HyD detectors and a 63X objective (NA 1.4). Images were acquired at 2048 × 2048, with a pinhole of 1 Airy unit, and a z-step size of 0.3 micron generating data sets with a voxel dimension of 60nm × 60nm × 300 nm. The resulting data sets for individual astrocytes were comprised of an average of 150 individual optical sections per cell. Following acquisition, images were deconvolved using Huygens software embedded in the Leica SP8 (Scientific Volume Imaging) and imported into Bitplane, Imaris (Zurich, Switzerland). Once in Imaris, an experimenter blinded to treatment group isolated boundaries of individual cells using the surface function to generate a region of interest (ROI). Both volume and surface area of individual astrocytes were calculated from this ROI. Given that these outputs are heavily impacted by the minimum resolution of the individual optical system and the amount of sampling (Mandelbrot, 1967), morphometric data are expressed as astrocyte surface area in μm2 over astrocyte volume in μm3, as others have done (Chvatal et al., 2007).
Astrocyte synaptic contact was assessed by measuring colocalization of the virally labeled astrocyte plasma membrane signal with the synaptic marker Synapsin-1. Given our empirically determined resolution limit for the SP8 is 150nm, colocalization of the astrocyte signal with the synaptic marker can be used as an index of synaptic insulation. With our super resolution confocal system, synaptic contact or colocalization is scored when astrocyte processes were fewer than 150nm from the synaptic marker. See supplemental video 1 for an example Z-series data set video depicting red Synapsin-1, green astrocyte plasma membrane, and white colocalized voxels or contact. To avoid any potential bias, signal intensity thresholds for colocalization analyses were automatically determined using the Costes method (Costes et al., 2004). Following mathematical derivation of intensity thresholds, the resulting volume (μm3) of ‘colocalized’ voxels was calculated and normalized to the volume (μm3) of the corresponding astrocyte. This calculation yields an index of single cell astrocyte-synaptic insulation or proximity, defined as % ROI colocalization. For synapse number analysis, we isolated all puncta within and around the astrocyte ROI, with a cut off of 400nm, as this is the maximum reported distance at which a PAP can impact glutamate clearance (Pannasch et al., 2014). The number and location of synaptic puncta were automatically determined by Imaris using center point signal intensity for the Synapsin-1 signal. For synapses number analyses, values were normalized to the corresponding astrocyte volume to control for cell to cell differences overall dimensions.
High-throughput GFAP structural analyses.
To determine whether methamphetamine alters the structure of astrocytes we imaged fields of GFAP+ cells in the NAcore and used Imaris to quantify several aspects of the astrocytic GFAP arbor. Three to four images of fields of GFAP-positive astrocytes were imaged per animal (n=1016 astrocytes/24 images/7 animals for methamphetamine, and n=830 astrocytes/21 images/6 animals for saline) using the above-mentioned confocal system using a 63X oil-immersion objective (1.4 N.A.) and 1.04X digital zoom. Images were acquired using a frame size of 4096×4096, 1 Airy Unit pinhole, and a z-step size of 0.2 micron generating data sets with a voxel dimension of 43nm × 43nm × 200nm. These parameters were selected in order to achieve the more accurate digital reconstruction of GFAP signal. During structural analyses, images were digitally reconstructed in Imaris software. Z-stacks were initially cropped to 12 μm (60 optical sections) in order to isolate fields of cells with homogenous signal intensity, and to reduce file size. Fields of GFAP arbors were skeletonized using a semi-automated, approach with the filament analysis package in Bitplane Imaris. In order to isolate individual astrocyte cells, seed point diameter was set to 12 μm. In instances where Imaris failed to automatically detect an individual cell or placed two starting points on a single cell, filaments were manually corrected. We used a minimum diameter for GFAP branches of 1 μm when digitally mapping each individual astrocyte. This value was selected as it was well within the resolution limit of our confocal system, and produced the most accurate semi-automated reconstructions. Once a field of cells is skeletonized, each field was inspected for accuracy, and corrections were made manually when necessary. The following output variables were then exported from Imaris: number of astrocytes per field, total sum of GFAP filament length (in μm), number of dendrite branches, mean diameter of GFAP filament, and sum of Sholl’s (1 μm radii) intersections. As each image data set contained multiple cells, all variables are expressed as an average of each astrocyte cell contained within a particular data set, these values were then averaged for the 3–4 data sets collected for each animal.
NAC administration.
A subset of rats received NAC (100 mg/kg, i.p. in saline) or vehicle injections during extinction. The pH of NAC was adjusted to 7.4. Both groups received NAC or vehicle for the entire period of extinction training, as described previously (Reichel et al., 2011). This treatment regimen was selected in light of studies demonstrating that cue-induced nicotine seeking is inhibited by chronic (15 days), yet not subchronic, NAC injections (Powell et al., 2019). NAC was injected immediately before placement into the chamber for either extinction or reinstatement sessions.
Gq-DREADD activation of astrocytes.
A subset of animals was injected with clozapine-N-oxide (CNO; 0 or 3 mg/kg in sterile saline + 5% DMSO, i.p.) or vehicle prior to reinstatement testing as described previously (Scofield et al., 2015a). After the first reinstatement session, animals re-entered extinction training for 2–5 days and were tested for cued reinstatement a second time. Cued reinstatement testing was conducted in a randomized, intake balanced cross-over design where each animal received CNO or Vehicle prior to one of two total reinstatement sessions.
Statistical Analyses
When comparing two groups for a single dependent variable, two-tailed Student’s t-test was used. When comparing active lever presses between NAC treated and vehicle-treated animals, a two-way mixed-model ANOVA was used with reinstatement (extinction versus reinstatement) as a within-subjects variable and treatment (vehicle versus NAC) as a between-subjects variable followed by Bonferroni-corrected pairwise comparison test when a significant interaction was observed. DREADD data was analyzed with a two-way repeated measures ANOVA in a randomized crossover design with reinstatement (extinction versus reinstatement) and treatment (CNO versus vehicle) as repeated measures. Data were tested for normality using the Shapiro-Wilk test. When data from one or more groups was sampled from a non-normally distributed population, the appropriate correction was applied to the statistical analysis as discussed in the results for each experiment. We also analyzed differences in variance between groups using the F-test for equality of variances and, when necessary, corrected for differences in variance using a Welch’s-corrected t-test as discussed in the results section for each experiment. All data is shown as the mean +/− standard error of the mean (SEM).
Results
Methamphetamine exposure does not downregulate GLT-1 expression or decrease Na+ -dependent or NA+ -independent glutamate uptake in the NAcore.
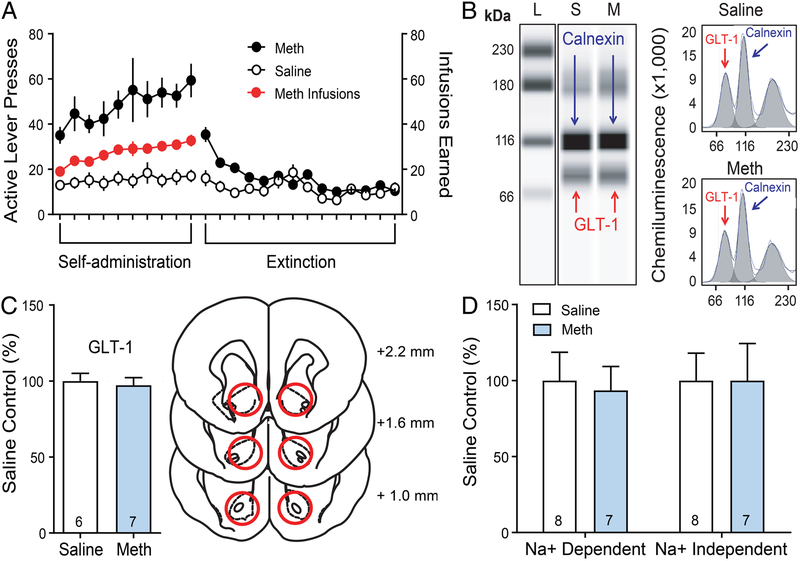
For simplicity, all of the Methamphetamine SA, extinction and yoked-saline data were combined and presented in Figure 1A. Rats (n=28) underwent methamphetamine or received yoked saline infusions and extinction (13–14 days of extinction). In a subset of rats (Meth-n=7, Saline-n=6), chemiluminescent column-based protein measurement analyses were performed to determine if Meth impacts expression of GLT-1 in the NAcore when compared to yoked-saline controls following extinction. Figure 1B depicts chemiluminescent GLT-1 and calnexin (loading control) immunoreactivity along with the electropherograms used in quantification of multiplexed protein signals. Both Meth (p=0.890) and saline (p=0.870) showed normally distributed data. Moreover, variance was not significantly different between groups [F(6,5)=1.110, p=0.928]. A two-tailed t-test revealed no significant differences in GLT-1 expression between groups [t(11)=0.390, p=0.710]. In a separate cohort of animals (Meth-n=8, Saline-n=8) we performed radiolabeled glutamate uptake assays to determine if methamphetamine SA and extinction functionally inhibits glutamate clearance in the NAcore. One Methamphetamine animal was removed following failed tissue extraction. Figure 1D depicts Na+− dependent (a measure of GLT-1 function) and Na+ -independent glutamate uptake (a measure of xC- function). For Na+−dependent uptake, both meth (p=0.440) and saline (p=0.080) showed normally distributed data according to Shapiro-Wilk test. Variance was also not different between groups [F(7,6)=1.595, p=0.585]. We show here that following 2-hour methamphetamine SA and extinction Na+− dependent [t(13)=0.260, p=0.797] glutamate uptake was unchanged. For NA+- independent glutamate uptake, there was no difference between groups in the variance [F(6,7)=1.600, p=0.549]. However, saline (p=0.810), but not Meth (p=0.008) showed normally distributed data. Thus, a Mann-Whitney U-test indicated that Na+-independent glutamate uptake was unchanged [U(67,53)=25, p=0.779).
Figure 1: Methamphetamine SA and extinction did not decrease GLT-1 expression or glutamate uptake in the NAcore.
A) Active lever responding and infusions earned for methamphetamine and yoked-saline animals during self-administration and extinction B) Representative bands for GLT-1 and calnexin. L denotes a molecular weight ladder while S and M represent saline and methamphetamine treated animals. Right, representative electropherograms with area under the curve shown in grey. GLT-1 protein levels were normalized to calnexin and quantified as a percent of yoked-saline control. C) Left, quantification of WES data demonstrates that GLT-1 expression was unchanged when comparing methamphetamine and saline groups. Right, schematic illustrates location of tissue punch taken for NAcore. D) Glutamate uptake was quantified as pmol/mg of protein and expressed as a % of yoked-saline control. Both sodium dependent and independent glutamate uptake were unchanged by methamphetamine SA and extinction.
Methamphetamine exposure had no impact on astrocyte GFAP cytoskeleton ultrastructure in the NAcore.
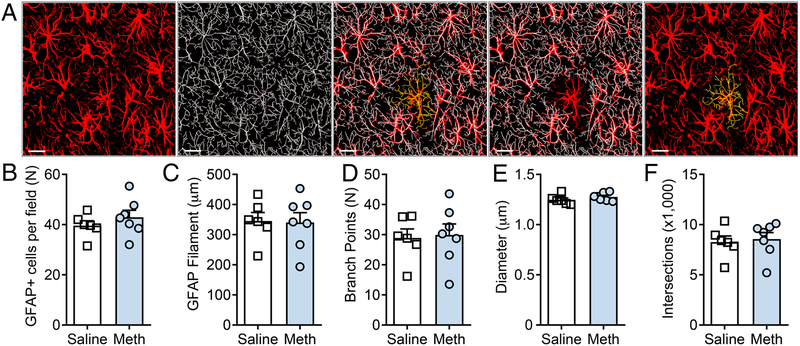
Following 14 days of extinction, rats (Meth-n=8, Saline-n=8) were sacrificed 24 hours after the last extinction session without reinstatement testing for GFAP structural analyses and assessment of synaptic contact (see below). For GFAP structural analyses, 1 meth animal and 2 saline animals were removed due to the fidelity of IHC detection of GFAP being below levels required for high magnification structural analyses. Figure 2A shows a representative field of GFAP+ astrocytes in the NAcore, and corresponding filament analysis in Imaris software. For all of the following variables analyzed, data were sampled from normal distributions for saline (p>0.05) and meth (p>0.05) rats. First, we observed no effect of methamphetamine on the number of GFAP+ cells in the NAcore after extinction [t(11)=0.953, p=0.361] (Figure 2B). Moreover, methamphetamine had no significant impact on the average GFAP total filament length [t(11)=0.114, p=0.912] (Figure 2C), the number of GFAP branch points [t(11)=0.204, p=0.842] (Figure 2D), the average GFAP filament diameter [t(11)=1.234, p=0.243] (Figure 2E), or the total number of three dimension 1 μm Sholl sphere intersections [t(11)=0.332, p=0.746] (Figure 2F). There was no difference between groups in terms of variance for all of the above variables analyzed (p=0.345, p=0.622, p=0.558, p=0.666, p=0.807, respectively). Despite the lack of an effect of methamphetamine on GFAP arbors, note the heterogeneous pattern of thickness of GFAP filament diameter detected, indicating sufficient recognition of range of cytoskeletal filament thicknesses using this assay.
Figure 2: Methamphetamine SA and extinction have no impact on GFAP structural complexity in the NAcore.
A) (left to right): 3D reconstruction of GFAP+ cells in the NAcore, detection of individual GFAP+ cells using filament extension in Imaris, and a single cell isolated from a field of GFAP+ cells in the NAcore (yellow). B-F) Methamphetamine SA and extinction failed to impact the number of GFAP+ cells per field, the average total filament length, the number of branch points, the average diameter of GFAP filament, nor the total number of Sholl’s intersections. Scale bar represents 20 microns
Methamphetamine exposure reduced astrocyte synaptic contact in the NAcore.
Although NAcore GFAP structure was unaltered in rats which underwent methamphetamine SA and extinction, it remains possible that methamphetamine engages reorganization of fine membranous astrocyte processes known to extend past the GFAP filament (Haseleu et al., 2013). In order to investigate astrocytes at the level of the plasma membrane and their interaction with synapses, we imaged NAcore astrocytes from the same animals used in the GFAP experiments described above. Prior to catheterization, NAcore astrocytes were transduced with a vector that provides astrocyte-specific membrane-restricted expression of GFP. Following methamphetamine SA and extinction, sections were immunohistochemically stained with a synaptic marker, Synapsin-1, to analyze astrocyte-synapse interaction. We then imaged astrocytes and adjacent Synapsin-1 puncta in methamphetamine (n=8) and saline controls (n=8). One meth animal was excluded from these analyses due to lack of viral expression in the NAcore.
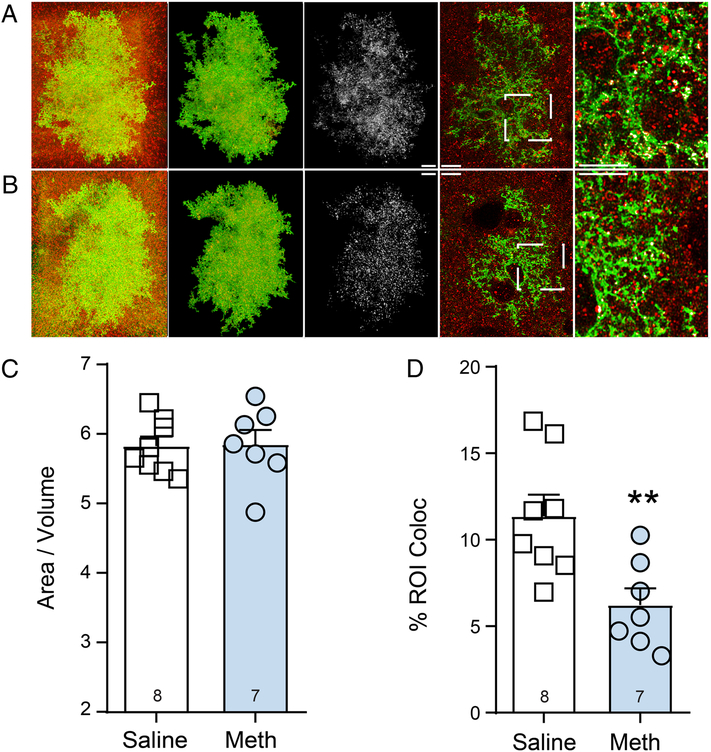
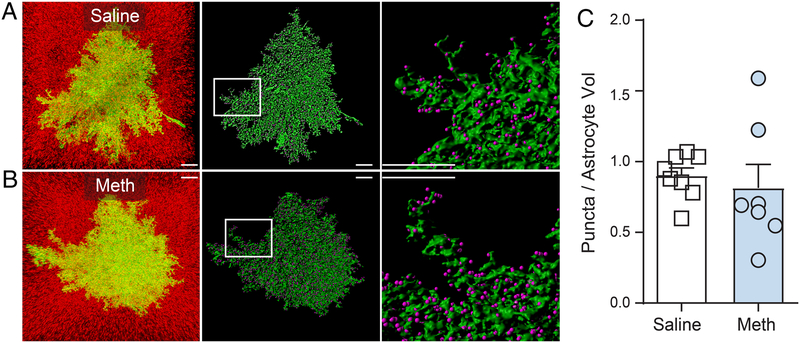
In keeping with what we have reported above for GFAP, we observed no significant alterations in the overall whole cell morphometric properties of astrocytes, expressed here as a ratio of astrocyte surface area (μm2) over volume (μm3) for each cell, [t(13)=0.112, p=0.912] (Figure 3A, B and C). For this analysis, variance was not different between groups [F(6,7)=1.917, p=0.414]. When analyzing astrocyte-synaptic contact following methamphetamine SA and extinction, it was revealed that both Meth (p=0.677) and Saline (p=0.369)-treated animals were sampled from normally distributed populations. Moreover, there was no difference in the variance between groups [F(7,6)=1.959, p=0.431]. Thus, a two-tailed t-test revealed a significant difference between groups in astrocyte-synaptic contact following methamphetamine SA and extinction [t(13)=3.165, p=0.007] (Figure 3D). We also analyzed the signal intensity of LCK-GFP and Synapsin-1. Although there was no difference between groups in the variance when analyzing the mean signal intensity within the ROI for the LCKGFP signal [F(7,6)=1.356, p=0.726], Saline (p=0.053), but not Meth (p=0.021) were sampled from normally distributed data. Therefore, a Mann-Whitney U-test correcting for non-normally distributed data indicated no difference between groups in the mean signal intensity within the ROI for the LCKGFP signal [U(67,53)=17, p=0.232]. When analyzing the mean signal intensity within the ROI for the Synapin-1 signal, we found that both Meth (p=0.999) and Saline (p=0.414) were sampled from normally distributed data. Moreover, there was no significant difference between groups in variance [F(7,6)=1.130, p=0.895]. Therefore, a two-tailed t-test indicated no significant difference between Synapin-1 signal [t(13)=0.960, p=0.354], indicating that signal intensity did not contribute to the differences in astrocytic insulation of synapses described above. Further, following methamphetamine SA and extinction, we analyzed the number of synapses within the territory of individual astrocytes. When doing so, it was revealed that both Meth (p=0.270) and Saline (p=0.417) were sampled from normally distributed data. However, there was a significant difference between groups in the variance [F(6,7)=7.755, p=0.016]. Thus, a Welch’s-corrected t-test revealed no significant alterations in the number of synapses within the territory of individual astrocytes were observed, (expressed as # of Synapsin-1 puncta / astrocyte volume μm3 [t(7.35)=0.489, p=0.638] (Figures 4A–C). Taken together these data indicate that the reduced levels astrocyte synaptic contact described above did not result from overall reductions in the number of NAcore synapses.
Figure 3: Methamphetamine SA and extinction reduces PAP insulation of NAcore synapses.
A) and B) Astrocytes from saline A) and Methamphetamine B) were imaged along with adjacent Synapsin-1 puncta. From left to right the images shown depict a raw Z-series (red and green) in the first column, the same z-series where the astrocyte has been isolated with adjacent Synapsin-1 puncta in the second column, colocalization or contact of the astrocyte and synaptic marker (white) in the third column, an individual optical section from the Z-series on the left showing astrocyte in green Synapsin-1 in red and white coloc or contact in the fourth column, and finally an inset panel enlarged to show in detail of synaptic contact made by NAcore astrocytes in the fifth column. Scale bar represents 10 microns C) Overall morphometric properties of astrocytes are unchanged by methamphetamine SA and extinction. D) Synaptic insulation is reduced by following methamphetamine SA and extinction **p<0.01 compared to saline.
Figure 4: Methamphetamine SA and extinction does not alter the number of synapses within the territory of individual astrocytes.
A) and B) From left to right, Isolated NAcore astrocytes for saline A) and methamphetamine B) are shown with the full field of synaptic puncta, next Space filling models (green) are shown with punctate Synapsin-1 signal detection performed using the Imaris spots function (purple spots), the boxed region depicts the area shown in the inset panel to the right. Scale bar represents 10 microns C) There were no significant differences in the number of puncta within individual astocyte territories across treatment groups. Number of puncta were normalized to the volume of each individual cell in μm3.
Chronic NAC treatment during extinction inhibited cue-induced methamphetamine seeking.
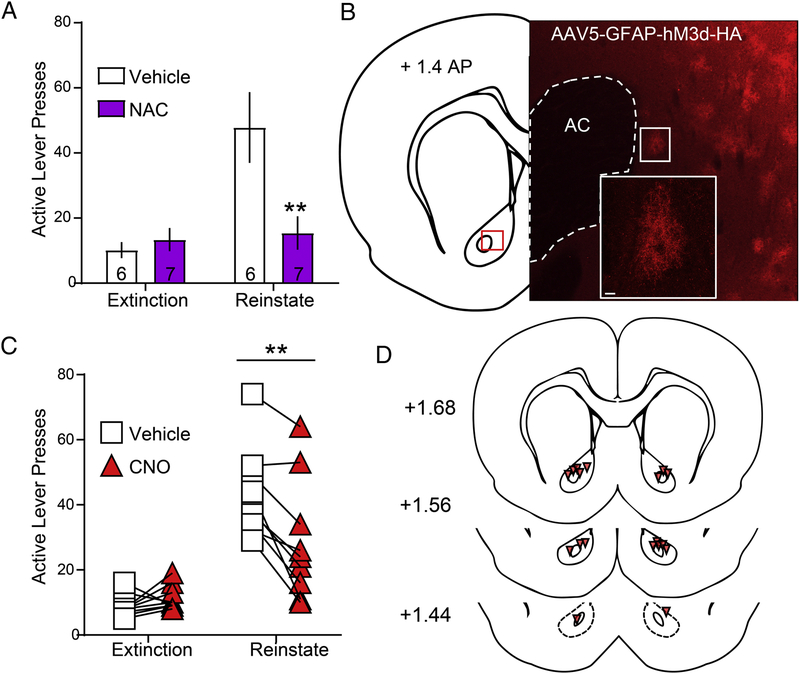
We next tested the impact of chronic systemic NAC treatment, a drug shown to promote normalization of glutamate homeostasis through the induction of astrocyte glutamate release via cystine-glutamate exchange (Kupchik et al., 2012; Moussawi et al., 2009). During extinction of methamphetamine SA, rats (n=13) received daily injections of either saline (0.3 ml, n=6) or N-acetylcysteine (NAC) (100 mg/kg, n=7). Rats underwent 15–22 extinction sessions. The number of extinction sessions required to extinguish lever pressing behavior was sampled from non-normally distributed data for both Saline (p=0.005) and NAC (p=0.002)-treated animals. However, a Mann-Whitney U-test indicated no significant difference between groups regarding the number of sessions required to extinguish lever pressing behavior [U(40,51)=19, p=0.877] (data not shown). We also analyzed the number of infusions earned, and NAC (p=0.615), but not Saline (p=0.011)-treated animals were sampled from normally distributed data, although there was no difference between groups in the variance [F(5,6)=2.268, p=0.348]. Thus, a Mann-Whitney U-test indicated no significant differences between groups in the number of infusions earned over the last three days of Meth SA [U(36,55)=15, p=0.422] (data not shown). Further, we confirmed that vehicle (p=0.170, p=0.830) and NAC (p=0.124, p=0.252) extinction and reinstatement data, respectively, were sampled from normally distributed populations. Moreover, there was no difference in the variance between these two treatment groups when comparing extinction [F(6,5)=2.450, p=0.344] or reinstatement [F(5,6)=3.820, p=0.134] lever pressing. We show here that chronic NAC treatment inhibited cued methamphetamine seeking. A two-way (treatment × session type) repeated-measures ANOVA revealed a main effect of NAC treatment [F(1,11)=6.100, p=0.031], a main effect of reinstatement [F(1,11)=9.644, p=0.010] and a treatment × session interaction [F(1,11)=7.761, p=0.018]. Bonferroni-corrected pairwise comparison test revealed a significant difference between vehicle and NAC during reinstatement (p=0.002), but not extinction (p>0.999, Figure 5A).
Figure 5: Chronic NAC during extinction or acute activation of GFAP-hM3D in NAcore suppresses cue-induced methamphetamine seeking.
A) Akin to what has been observed with other drugs of abuse, NAC treatment significantly reduced cued responding during methamphetamine reinstatement, p<0.01 B) Shown on the left is an atlas plate with a red square depicting the localization of the inset panel on the right. The micrograph shows a field of Gq-DREADD transduced astrocytes at low magnification (scale bar represents 100 microns). The inset panel depicts a higher magnification image of an individual cell within the low magnification plane to confirm the non-neuronal cellular identity of the transduced cell consistent with our previous reports (Scofield et al., 2015b). C) CNO significantly reduced active lever pressing during cued methamphetamine reinstatement when compared to vehicle controls **p<0.01 compared to vehicle.
Acute activation of Gq-DREADD in NAcore astrocytes inhibited cue-induced methamphetamine seeking.
In order to directly assess the impact of glial glutamate release on cued methamphetamine relapse, we transduced NAcore astrocytes with a GFAP promoter driven Gq-DREADD AAV5 vector and performed methamphetamine SA (n=13), extinction (14 days) and relapse testing as described above. 4 animals were removed from analyses; 2 for expression outside of the NAcore and 2 for lack of viral expression. A representative micrograph of astrocytic Gq-DREADD expression in the NAcore (red) is shown in Figure 5B. We observed that CNO significantly reduced active lever pressing during cued methamphetamine reinstatement when compared to vehicle controls. While extinction lever pressing was non-normally distributed for both vehicle (p=0.034) and CNO (p=0.001), reinstatement lever pressing was normally distributed for vehicle (p=0.128) and CNO (p=0.158). There was also no difference between groups in variance during extinction [F(8,5)=1.626, p=0.614] or reinstatement [F(8,8)=1.806, p=0.421]. As expected, a two-way repeated measures ANOVA revealed a main effect of session [F(1,8)=25.080, p=0.001], an effect of CNO treatment [F(1,8)=17.120, p=0.003], and a treatment × session interaction [F(1,8)=16.110, p=0.004]. Bonferroni-corrected multiple comparison tests revealed that there was no difference between treatments in active lever pressing during extinction (p>0.999), but that CNO treatment significantly reduced active lever pressing during cued reinstatement (p=0.008, Figure 5C). NAcore DREADD expression was verified in all animals (see histology in Figure 5D).
Discussion
We show here that the functional properties of GLT-1 and xC- are unchanged in the NAcore following two-hour methamphetamine SA and extinction (Figure 1). Further, methamphetamine exposure had no impact on NAcore astrocyte GFAP cytoskeleton ultrastructure (Figure 2) or the overall properties of the astrocyte plasma membrane at the level of individual cells (Figure 3A–C). However, we did observe a significant reduction in the extent of synaptic contacts made by NAcore astrocytes (Figure 3D), consistent with what has been reported following cocaine SA and extinction (Scofield et al., 2016b; Testen et al., 2018). In the second phase of the study, we investigated activation of glial glutamate release and activation of Gq-DREADD in astrocytes, as a means to inhibit cued methamphetamine seeking. We show here that systemic NAC treatment during extinction reduced cue-induced methamphetamine seeking (Figure 5A), consistent with what has been reported for cocaine (Murray et al., 2012; Reichel et al., 2011), heroin (Zhou and Kalivas, 2008) and nicotine (Ramirez-Nino et al., 2013). Moving specifically to the NAcore, we demonstrate that acute activation of Gq-DREADD in NAcore astrocytes prior to cued reinstatement also inhibits methamphetamine seeking, consistent with what has been reported for cocaine (Scofield et al., 2015b).
We were initially surprised at the lack of an effect of methamphetamine on Na+− dependent (a measure of GLT-1 function) and Na+ -independent (a measure of glial cystine-glutamate exchange (Trantham-Davidson et al., 2012)) glutamate uptake in the NAcore, especially given the previously reported microdialysis data detailing both a decrease in basal glutamate levels as well as meth seeking-induced elevations in NAcore glutamate (Parsegian and See, 2014). However, our data demonstrating a lack of impact on GLT-1 expression or NAcore glutamate clearance after methamphetamine exposure are in agreement with what has been found by others using non-contingent amphetamine administration (Sidiropoulou et al., 2001) or abstinence-based methamphetamine withdrawal as opposed to extinction (Szumlinski et al., 2017).
We observed no effect of methamphetamine SA and extinction on the structure or complexity of the arbors of the cytoskeletal protein GFAP, indicating no gross differences in general aspects of astrocyte morphology and no upregulation of canonical reactive astroglia (Verkhratsky and Nedergaard, 2018). Following cocaine SA and extinction, both GFAP and GLT-1 are downregulated in the NAcore, which is accompanied by a functional disruption of glutamate clearance (Reissner et al., 2015; Scofield et al., 2016b). Given that GFAP−/− mice show an inability to traffic GLT-1 to the surface of astrocytes following stimulation of PKA by dibutyryl cAMP, (Hughes et al., 2004), GFAP expression likely regulates GLT-1 distribution in vivo. Although we did not perform GFAP protein quantification, we did not observe methamphetamine-induced alterations of GFAP structure or GFAP process diameter, consistent with a lack of an impact of short-access methamphetamine SA and extinction on both GFAP and GLT-1 in the NAcore.
Others have shown that non-contingent administration of, several large doses of methamphetamine (10 mg/kg, once every two hours) (Broening et al., 1997), or a sensitization regimen of methamphetamine (Narita et al., 2005), elevates GFAP immunoreactivity in the NAcore. Yet, paradoxically repeated increasing doses of chronic methamphetamine did not (Simoes et al., 2008). Moreover, chronic methamphetamine abusers display the same levels of GFAP-positive reactive astrocytes in the striatum as healthy (Kitamura et al., 2010). Given that we found no change in GFAP process diameter or structure after methamphetamine SA and extinction, we suspect that alterations in GFAP immunoreactivity are more likely to occur following non-contingent administration of high dose methamphetamine. In our model of contingent methamphetamine SA and extinction, animals are allowed to titrate drug intake. We hypothesize that this model better represents human patterns of methamphetamine abuse and addiction. As such, the lack of striatal astrogliosis observed in the human postmortem studies discussed above is consistent with our hypothesis.
We demonstrate here that methamphetamine SA and extinction caused a significant reduction in the extent of synaptic contact made by astrocytes in the NAcore. At first glance, the lack of an effect of methamphetamine on GFAP filament structure, combined with methamphetamine-induced retraction of astrocyte processes from synapses may seem counterintuitive. However, it is well documented that GFAP filaments do not extend into the distal portion of astrocytic processes where most synaptic contacts are made (Scofield, 2018). In the membranous non-GFAP containing zone of the perisynaptic astrocyte process, actin cycling dynamics involving proteins like cofilin and ezrin, regulate the aspects of PAP extension and retraction crucial for activity-dependent remodeling of synaptic insulation (Lavialle et al., 2011). Thus, the overall GFAP structural profile and the dynamic association of PAPs with synapses are readily dissociable, with the latter having a more profound effect on glutamate transmission (Scofield, 2018). In a potentially parallel mechanism, hippocampal astrocytes retract from dendritic spines following high frequency stimulation (Perez-Alvarez et al., 2014). Perhaps astroglial processes react similarly in the NAcore following altered patterns of neurotransmitter release and signaling following extended bouts of psychostimulant exposure. Recently observed dopamine-mediated alterations in astrocyte structure shown in culture systems support the hypothesis that drug-induced release of neuromodulators can act to shape astrocyte interaction with synapses (Galloway et al., 2018). In light of data from our lab and others, we posit that methamphetamine- and cocaine-induced alterations of astrocytic insulation of NAcore synapses serve as a potent maladaptive response to drug exposure linked to relapse vulnerability (Bernardinelli et al., 2014; Haber and Murai, 2006; Testen et al., 2018).
Electron microscopy studies demonstrate that astrocytes commonly partially cover synapses, with less than half of the hippocampal synaptic interface contacted astrocytic processes (Ventura and Harris, 1999; Witcher et al., 2007) Given that astrocytes serve as a barrier for diffusion of transmitters to the extrasynaptic space (Sykova, 2001), the extent of contact with synapses coordinates the physical boundaries for glutamatergic signaling at the level of individual synapses (Sykova, 2004). This in turn impacts the extent and type of glutamate receptor activation when transmitter is released during exposure to methamphetamine conditioned cues. Indeed, mathematical models illustrate that alterations in synaptic coverage have a profound effect on the activation profile of perisynaptic receptors (Rusakov, 2001). Thus, methamphetamine withdrawal-induced PAP retraction could contribute to the glutamatergic signaling underlying cue-induced methamphetamine seeking. One likely target for glutamate escaping from the synaptic cleft as a result of reduced synaptic cradling in the NAcore are postsynaptic mGluR5 receptors. Consistent with this hypothesis, selective activation of these receptors has been directly linked to relapse (Kalivas, 2009; Kumaresan et al., 2009).
We show here that chronic, systemically-administered NAC during extinction of methamphetamine SA decreased cued reinstatement of drug seeking in male rats. In this study, we chose a chronic NAC treatment paradigm for the following reasons: 1) Daily injections of NAC (100 mg/kg, i.p.) during extinction provides an enduring attenuation of cue- or cue plus cocaine prime-induced reinstatement, even after discontinuing treatment for two weeks (Reichel et al., 2011), 2) Although an acute injection of NAC reduces ethanol self-administration, this effect is only seen at 100 mg/kg (Lebourgeois et al., 2018), and 3) Daily NAC injections (100 mg/kg, i.p.) during extinction inhibits cue-induced reinstatement with either NAC on board or 2 weeks after discontinuing treatment, an effect that occurs via by increasing inhibitory tone on mGluR2/3 presynaptic auto receptors in the NAcore (Moussawi et al., 2011b). Mechanistically, previous studies have linked NAC-induced inhibition of cocaine seeking to restoration of NAcore GLT-1 expression (Reissner et al., 2015). However, viral overexpression of GLT-1 at levels that significantly enhance glutamate clearance was not sufficient for the inhibition of cocaine seeking (Logan et al., 2018). Together, these data suggest that NAC-mediated inhibition of relapse may involve additional molecular mechanisms beyond the restoration of GLT-1 expression in the NAcore. One potential mechanism for NAC-mediated inhibition of methamphetamine seeking is the activation of glutamate release from astrocytes. As described above, NAC serves as a substrate for astroglial cystine-glutamate exchange and promotes glial glutamate release from xC-. We expect that NAC decreased cued methamphetamine seeking via this mechanism, by restoring basal glutamate levels in the NAcore. Indeed, NAC-induced glial glutamate release has been previously shown to activate presynaptic mGluR2/3 in the NAcore and decreases synaptic release probability during cocaine seeking (Kupchik et al., 2012; Moran et al., 2005). In support of this hypothesis, our current study shows that selective activation of Gq-DREADD in NAcore astrocytes, a manipulation also shown to induce glutamate release from astrocytes (Scofield et al., 2015b), inhibits cued methamphetamine seeking.
While low dose, non-contingent, escalating methamphetamine regimens decrease expression of GluR1, GluR2 and GluN1 in the striatum (Jayanthi et al., 2014), escalating high dose methamphetamine increases expression of GluR2 and NR2A (Simoes et al., 2008). Further, following extended access methamphetamine self-administration and forced abstinence, there is an accumulation of calcium-permeable AMPA receptors in the NAcore (Scheyer et al., 2016), which has been linked both to alterations in glutamatergic synaptic plasticity (Scheyer et al., 2018) and to the incubation of methamphetamine craving. Taken together, these data illustrate that methamphetamine can potently impact striatal glutamatergic synaptic plasticity. As discussed above, we hypothesize that NAC is enacting its inhibitory effects on methamphetamine seeking by engaging presynaptic autoreceptors to stop glutamate overflow, potentially counteracting the meth-induced alterations in glutamatergic plasticity discussed above. The cocaine literature supports a more direct role for NAC in the modulation of AMPA and NMDA currents, as the cocaine-mediated increases in the AMPA/NMDA ratio in NAcore MSNs linked to drug seeking can be reversed by chronic NAC treatment during extinction (Moussawi et al., 2011b). However, this has yet to be directly tested following methamphetamine SA.
Apart from its action in the NAcore, systemic NAC administration likely produces effects in other relevant brain regions, such as the prelimbic cortex; indirectly altering plasticity in the NAcore. In keeping with this hypothesis, meth conditioned place preference (CPP) followed by extinction decreases GLT-1 protein expression in the NAshell, but not the NAcore (Althobaiti et al., 2019) and overexpressing GLT-1 in the NAshell attenuates the induction of meth CPP (Fujio et al., 2005). Given that NAC produces its anti-relapse effects, at least in part, by elevating GLT-1 expression (Reissner et al., 2015), it remains possible that NAC also engages a more canonical GLT-1 based restoration of homeostatic glutamate systems in the NAshell, which parallels what has been reported with ceftriaxone (Althobaiti et al., 2019). It would be of interest to examine the ability of NAC to alter meth-induced adaptations in other brain regions, particularly the NAshell, in future experiments. Speaking to the further exploration of NAC’s mechanism of action, one limitation of our study is that we did not investigate the impact of NAC treatment on astrocyte synaptic contact in the NAcore. It would be interesting to determine if NAC functions by acting to repair both drug-induced deficits in glutamate homeostasis and physical astrocyte-synapse interaction, it seems likely that these phenomena are functionally linked. Indeed, we have shown in the past that ceftriaxone, a drug similar to NAC in that it both normalizes glutamate homeostasis and inhibits cocaine seeking, reverses cocaine-induced decreases in NAcore astrocyte synaptic contact (Scofield et al., 2016b).
Activation of a glial-specific Gq-DREADD enhances intracellular Ca2+ levels in astrocytes (Bull et al., 2014; Xie et al., 2015) via activation of IP3 receptors on the endoplasmic reticulum (ER) and the release of ER calcium stores. Though actively debated (Fiacco and McCarthy, 2018; Khakh and McCarthy, 2015; Savtchouk and Volterra, 2018; Scofield, 2017), IP3-mediated ER-calcium release in astrocytes is thought to promote the release of gliotransmitters, including glutamate (Navarrete et al., 2012; Scofield, 2018; Takata et al., 2011). Consistent with this hypothesis, recent reports demonstrate that calcium elevation in astrocytes evoked by stimulation of type 1 cannabinoid receptors also evokes astrocytic glutamate release in the hippocampus (Covelo and Araque, 2018). Moreover, Bull and colleagues have shown that activation of glial-expressed Gq-DREADD in the NAcore reduced motivation for ethanol following abstinence (Bull et al., 2014), and we have previously shown that activating Gq-DREADD in NAcore astrocytes inhibits cued cocaine seeking; an effect that was also sensitive to blockade of mGluR2/3 receptors (Scofield et al., 2015a). Here we show that activation of Gq-signaling in NAcore astrocytes inhibited cued methamphetamine seeking. While we suspect that the inhibition of cued methamphetamine seeking by glial DREADD activation occurs via the induction of glutamate release from astrocytes, we cannot completely rule out the possibility that additional gliotransmitters, or impacts of Gq-activation, may have contributed to the anti-reinstatement effects observed (Devaraju et al., 2013; Scofield, 2018; Shi et al., 2017). It has been recently established that CNO can back metabolize to clozapine, which can cause some sedative or off target effects (Padovan-Hernandez and Knackstedt, 2018). However, it is unlikely that these effects have contributed to our results, as we have previously shown CNO administered in the same dosing regimen does not inhibit cued sucrose seeking (Scofield et al., 2015a).
In summary, our data indicate that akin to many other drugs, methamphetamine alters aspects of astrocyte biology related to glutamatergic neuronal communication, specifically the program of astrocytic synaptic insulation. We hypothesize that the morphological plasticity of neuron-astrocyte interaction in the NAcore is a fundamental aspect of relapse vulnerability. Here, future experiments could be designed to investigate precisely which synapses astrocytes are withdrawing from in the NAcore following methamphetamine exposure, one interesting direction would be classifying changes in astrocyte interaction with synapses on neurons based off of their dopamine receptor expression profile or projection targets. Finally, we show that engaging glial glutamate release in the NAcore with NAC or activation of Gq-DREADD inhibits cued methamphetamine seeking. Additional future experiments could be conducted here to determine if treatment with NAC or activation of Gq-DREADD impacts astrocyte-synapse interaction.
Supplementary Material
Two-hour methamphetamine self-administration and extinction does not alter glutamate uptake in the NAcore
Two-hour methamphetamine self-administration and extinction decreases astrocytic insulation of NAcore synapses
Chronic NAC treatment inhibits cued-methamphetamine seeking
Acute activation activation of Gq-DREADD, specifically in NAcore astrocytes, inhibits cued-methamphetamine seeking
Acknowledgements and Disclosures
This work was supported by T32 DA007288 (MDS, BMS, KC), R01 DA033049 (CMR), 5K99 DA041462 (SS), R00 DA036569, R21 DA044479, R03 DA045881 (CDG) R01 DA012513 and R01 DA003906 (PWK) R00 DA040004 (MDS). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althobaiti YS, Alshehri FS, Hakami AY, Hammad AM, Sari Y (2019) Effects of Clavulanic Acid Treatment on Reinstatement to Methamphetamine, Glial Glutamate Transporters, and mGluR 2/3 Expression in P Rats Exposed to Ethanol. J Mol Neurosci 67:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW (2002) The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22:9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SM, McGinty JF (2017) Role of Src Family Kinases in BDNF-Mediated Suppression of Cocaine-Seeking and Prevention of Cocaine-Induced ERK, GluN2A, and GluN2B Dephosphorylation in the Prelimbic Cortex. Neuropsychopharmacology 42:1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardinelli Y, Muller D, Nikonenko I (2014) Astrocyte-synapse structural plasticity. Neural Plast 2014:232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM (2005) The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport 16:1013–1016. [DOI] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV (1997) Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse 27:153–160. [DOI] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS (2014) Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, Shaham Y (2015) Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal A, Anderova M, Kirchhoff F (2007) Three-dimensional confocal morphometry - a new approach for studying dynamic changes in cell morphology in brain slices. J Anat 210:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo A, Araque A (2018) Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraju P, Sun MY, Myers TL, Lauderdale K, Fiacco TA (2013) Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. J Neurophysiol 109:2404–2414. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD (2018) Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neurosci 38:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV (2012) Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience 210:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S (2005) Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci 22:2744–2754. [DOI] [PubMed] [Google Scholar]
- Galloway A, Adeluyi A, O’Donovan B, Fisher ML, Rao CN, Critchfield P, Sajish M, Turner JR, Ortinski PI (2018) Dopamine Triggers CTCF-Dependent Morphological and Genomic Remodeling of Astrocytes. J Neurosci 38:4846–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Clinton SM, Watson SJ, Akil H (2009) Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology 34:1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A 110:9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Murai KK (2006) Reshaping neuron-glial communication at hippocampal synapses. Neuron Glia Biol 2:59–66. [DOI] [PubMed] [Google Scholar]
- Haseleu J, Anlauf E, Blaess S, Endl E, Derouiche A (2013) Studying subcellular detail in fixed astrocytes: dissociation of morphologically intact glial cells (DIMIGs). Front Cell Neurosci 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Iwai Y, Takata N, Shinohara Y, Mishima T (2014) Volume transmission signalling via astrocytes. Philos Trans R Soc Lond B Biol Sci 369:20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML (2004) Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Brain Res Mol Brain Res 124:114–123. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, Cadet JL (2014) Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- Khakh BS, McCarthy KD (2015) Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7:a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S (2010) Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 12:57–62. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Olive MF (2013) Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology 66:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC (2009) Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW (2012) The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A (2011) Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A 108:12915–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebourgeois S, Gonzalez-Marin MC, Jeanblanc J, Naassila M, Vilpoux C (2018) Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol 23:643–652. [DOI] [PubMed] [Google Scholar]
- Lee SY, Haydon PG (2007) Astrocytic glutamate targets NMDA receptors. J Physiol 581:887–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A (2007) Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol 74:1299–1307. [DOI] [PubMed] [Google Scholar]
- Logan CN, LaCrosse AL, Knackstedt LA (2018) Nucleus accumbens GLT-1a overexpression reduces glutamate efflux during reinstatement of cocaine-seeking but is not sufficient to attenuate reinstatement. Neuropharmacology 135:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE (2012) Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 37:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot B (1967) How long is the coast of britain? Statistical self-similarity and fractional dimension. Science 156:636–638. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005) Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SG, Sharbafchi MR, Salehi M, Peykanpour M, Karimian Sichani N, Maracy M (2015) The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med 18:28–33. [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW (2011a) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW (2011b) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A 108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D (2012) N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol 17:437–440. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Tsuda M, Koizumi S, Narita M, Yajima Y, Inoue K, Suzuki T (2005) Long-lasting change in brain dynamics induced by methamphetamine: enhancement of protein kinase C-dependent astrocytic response and behavioral sensitization. J Neurochem 93:1383–1392. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Fernandez de Sevilla D, Gomez-Gonzalo M, Nunez A, Martin ED, Araque A (2012) Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol 10:e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill RA, Bhamidipati A, Bi X, Deb-Basu D, Cahill L, Ferrante J, Gentalen E, Glazer M, Gossett J, Hacker K, Kirby C, Knittle J, Loder R, Mastroieni C, Maclaren M, Mills T, Nguyen U, Parker N, Rice A, Roach D, Suich D, Voehringer D, Voss K, Yang J, Yang T, Vander Horn PB (2006) Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc Natl Acad Sci U S A 103:16153–16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Iino M (2011) Visualization of glutamate as a volume transmitter. J Physiol 589:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan-Hernandez Y, Knackstedt LA (2018) Dose-dependent reduction in cocaine-induced locomotion by Clozapine-N-Oxide in rats with a history of cocaine self-administration. Neurosci Lett 674:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K, Abudara V, Dufour A, Lubke JH, Deglon N, Knott G, Holcman D, Rouach N (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17:549–558. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE (2014) Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology 39:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A (2014) Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34:12738–12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW (2006) The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 186:143–149. [DOI] [PubMed] [Google Scholar]
- Peters J, Scofield MD, Ghee SM, Heinsbroek JA, Reichel CM (2016) Perirhinal Cortex mGlu5 Receptor Activation Reduces Relapse to Methamphetamine Seeking by Restoring Novelty Salience. Neuropsychopharmacology 41:1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GL, Leyrer-Jackson JM, Goenaga J, Namba MD, Pina J, Spencer S, Stankeviciute N, Schwartz D, Allen NP, Del Franco AP, McClure EA, Olive MF, Gipson CD (2019) Chronic treatment with N-acetylcysteine decreases extinction responding and reduces cue-induced nicotine-seeking. Physiol Rep 7:e13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Nino AM, D’Souza MS, Markou A (2013) N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 225:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Goodwani S, Bell RL, Wei Y, Boddu SH, Sari Y (2015) Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 295:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Baron DA, Kim JK, Unterwald EM, Rawls SM (2011) beta-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids 40:761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther 337:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW (2014) Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology 39:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol 20:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW (2015) Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol Disord Drug Targets 14:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA (2001) The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion. Biophys J 81:1947–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Savtchouk I, Volterra A (2014) What do we know about gliotransmitter release from astrocytes? Philos Trans R Soc Lond B Biol Sci 369:20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchouk I, Volterra A (2018) Gliotransmission: Beyond Black-and-White. J Neurosci 38:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Christian DT, Wolf ME, Tseng KY (2018) Emergence of Endocytosis-Dependent mGlu1 LTD at Nucleus Accumbens Synapses After Withdrawal From Cocaine Self-Administration. Front Synaptic Neurosci 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Dolubizno H, Stefanik MT, Murray CH, Sakas C, Wolf ME (2016) AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol Psychiatry 80:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE (2012) Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One 7:e34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD (2017) Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD (2018) Exploring the Role of Astroglial Glutamate Release and Association With Synapses in Neuronal Function and Behavior. Biol Psychiatry 84:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW (2015a) Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li, Haydon PG, Kalivas PW (2015b) Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry 78:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016a) The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68:816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW (2014) Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist 20:610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Li H, Siemsen BM, Healey KL, Tran PK, Woronoff N, Boger HA, Kalivas PW, Reissner KJ (2016b) Cocaine Self-Administration and Extinction Leads to Reduced Glial Fibrillary Acidic Protein Expression and Morphometric Features of Astrocytes in the Nucleus Accumbens Core. Biol Psychiatry 80:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM (2015c) Failure to Recognize Novelty after Extended Methamphetamine Self-Administration Results from Loss of Long-Term Depression in the Perirhinal Cortex. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM (2015d) Failure to Recognize Novelty after Extended Methamphetamine Self-Administration Results from Loss of Long-Term Depression in the Perirhinal Cortex. Neuropsychopharmacology 40:2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci 34:5649–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zhang W, Lu Y, Lu Y, Xu L, Fang Q, Wu M, Jia M, Wang Y, Dong L, Yan X, Yang S, Yuan F (2017) Aquaporin 4-Mediated Glutamate-Induced Astrocyte Swelling Is Partially Mediated through Metabotropic Glutamate Receptor 5 Activation. Front Cell Neurosci 11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou K, Chao S, Lu W, Wolf ME (2001) Amphetamine administration does not alter protein levels of the GLT-1 and EAAC1 glutamate transporter subtypes in rat midbrain, nucleus accumbens, striatum, or prefrontal cortex. Brain Res Mol Brain Res 90:187–192. [DOI] [PubMed] [Google Scholar]
- Siemsen BM, Giannotti G, McFaddin JA, Scofield MD, McGinty JF (2018a) Biphasic effect of abstinence duration following cocaine self-administration on spine morphology and plasticity-related proteins in prelimbic cortical neurons projecting to the nucleus accumbens core. Brain Struct Funct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemsen BM, Lombroso PJ, McGinty JF (2018b) Intra-prelimbic cortical inhibition of striatal-enriched tyrosine phosphatase suppresses cocaine seeking in rats. Addict Biol 23:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes PF, Silva AP, Pereira FC, Marques E, Milhazes N, Borges F, Ribeiro CF, Macedo TR (2008) Methamphetamine changes NMDA and AMPA glutamate receptor subunit levels in the rat striatum and frontal cortex. Ann N Y Acad Sci 1139:232–241. [DOI] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW (2014) Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci 17:1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Kalivas PW (2015) The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res 1628:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E (2001) Glial diffusion barriers during aging and pathological states. Prog Brain Res 132:339–363. [DOI] [PubMed] [Google Scholar]
- Sykova E (2004) Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129:861–876. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugmann M, Phillips TJ, Kippin TE (2017) Methamphetamine Addiction Vulnerability: The Glutamate, the Bad, and the Ugly. Biol Psychiatry 81:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H (2011) Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31:18155–18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testen A, Sepulveda-Orengo MT, Gaines CH, Reissner KJ (2018) Region-Specific Reductions in Morphometric Properties and Synaptic Colocalization of Astrocytes Following Cocaine Self-Administration and Extinction. Front Cell Neurosci 12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen H, Kalivas PW (2010) Inhibition of actin polymerization prevents cocaine-induced changes in spine morphology in the nucleus accumbens. Neurotox Res 18:410–415. [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW (2006) Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci 26:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA (2012) Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci 32:12406–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19:6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55:13–23. [DOI] [PubMed] [Google Scholar]
- Xie AX, Petravicz J, McCarthy KD (2015) Molecular approaches for manipulating astrocytic signaling in vivo. Frontiers in cellular neuroscience 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006) Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26:9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.