Abstract
Interleukin-7 has critical and nonredundant roles in T cell development, hematopoiesis, and postdevelopmental immune functions as a prototypic homeostatic cytokine. Based on a large body of preclinical evidence, it may have multiple therapeutic applications in immunodeficiency states, either physiologic (immuno-senescence), pathologic (HIV) or iatrogenic (postchemotherapy and posthematopoietic stem cell transplant) and may have roles in immune reconstitution or enhancement of immunotherapy. Early clinical development trials in humans show that, within a short time, rhIL-7 administration results in a marked preferential expansion of both naive and memory CD4 and CD8 T cell pools with a tendency toward enhanced CD8 expansion. As a result, lymphopenic or normal older hosts develop an expanded circulating T cell pool with a profile that resembles that seen earlier in life with increased T cell repertoire diversity. These results, along with a favorable toxicity profile, open a wide perspective of potential future clinical applications.
Keywords: interleukin, cytokine, T cell
Background
IL-7 is a multifunctional, homeostatic cytokine first isolated as a 25 KDa glycoprotein produced by a murine bone marrow stromal cell line.1 IL-7 is not produced by lymphocytes but, rather, by bone marrow stroma2 as well as other cell types including thymic stroma, keratinocytes, neurons, antigen presenting cells, lymph node follicular dendritic cells, and endothelial cells. IL-7 signals through a heterodimer involving IL-7Rα and the common γ chain (γc). IL-7Rα is shared with TSLP and the γc receptor is shared with IL-2, IL-4, IL-9, IL-15, and IL-21. IL-7 plays a critical, nonredundant role in the development of T cells. This is directly demonstrated in murine models wherein IL-7R knockout mice have arrest of T cell development at a double positive stage3 and IL-7 deficient mice are profoundly lymphopenic with thymic cellularity reduced 20-fold.4 In contrast, mice transgenic for IL-7 develop T cell lymphoproliferative/autoimmune diseases and T cell lymphomas.5,6 The effects of IL-7 on T cell development are multifactorial, involving antiapoptotic and proliferative effects on developing lymphocytes,7,8 promotion of V(D)J rearrangement of T cell receptor genes,9 and provision of trophic and costimulatory signals for mature T cells.10
In human T cell development, IL-7’s critical role is confirmed by analysis of three groups of patients with severe combined immunodeficiency (SCID) who have mutations involving the IL-7 receptor or its signaling pathway. Children with X-linked SCID (T-NK cell deficient, but spared B cells) have a defect of the γ-chain of the IL-7 receptor,11 children with autosomal recessive SCID (T-NK cell deficient but spared B cells) not infrequently show mutations of the Jak-3 tyrosine kinase,12,13 and kindreds with a defective α-chain of the IL-7 receptor are severely T cell deficient, but have normal NK cells and B cells.14 Current concepts hold that the T cell deficiency in each of these clinical entities relates to absent or defective IL-7 signaling during T cell development, whereas NK cell deficiency results from absent or defective IL-15 signaling, which coexists when the genetic defect involves the γ-chain of the IL-7 receptor or the Jak-3 tyrosine kinase.
With regard to B cell development, IL-7 was first recognized as an important maturation and differentiation factor for pre-B cells in murine models15 and IL-7 is an essential factor for supporting B lymphopoiesis ex vivo.2 Moreover, IL-7 transgenic mice show expansion of immature B cells,5,6 and humans treated with rhIL-7 show expansions in immature B cells within the bone marrow.16 However, IL-7 does not appear to be essential for human B cell development, since patients with SCID due to γc,JAK3, or IL-7Rα mutations, can have normal or even elevated numbers of peripheral blood B cells.11–14 Thus, while IL-7 participates in normal B cell development ex vivo,17 it does not appear to be strictly required for B cell development in humans. IL-7 also stimulates egress of primitive hematopoietic cells from bone marrow, and it has been used successfully as a mobilization agent in mice, resulting in long lasting, full tri-lineage engraftment in mice transplanted with rhIL-7 mobilized peripheral blood, a property that may be clinically exploitable.18
In addition to its critical role in T cell lymphopoiesis and its effect on developing B cells, IL-7 also plays a central role in peripheral T cell homeostasis. As discussed above, IL-7R shares the receptor common γ-chain (CD132) with several other cytokines. Within this family, cytokines can be classified as activating versus homeostatic. IL-2 is a prototypic activating cytokine, while IL-7 is a prototypic homeostatic cytokine. IL-2 selectively signals activated T cells, is secreted by activated T cells and IL-2 signaling upregulates its own receptor (IL-2Rα; CD25) thus amplifying the IL-2 response during immune activation. In contrast, IL-7Rα (CD127) is expressed on resting T cells but is downregulated following signaling by IL-7 itself, other prosurvival cytokines (IL-2, IL-4, IL-6, IL-15)19 or following T cell receptor (TCR) ligation. This tight regulation of IL-7Rα expression is congruent with the homeostatic role of IL-7, as it presumably prevents T cells that have already received a prosurvival signal from competing with other cells for its utilization.20 Moreover, while production of activating cytokines occurs in the context of immune activation, IL-7 is continuously produced and available to resting T cells within the lymphoid niche. This continuous availability of IL-7 provides essential trophic signals for homeostatic proliferation and survival of naive T cells, since naive T cells adoptively transferred into an IL-7 deficient host rapidly disappear.21
Many studies have documented that IL-7 therapy can dramatically increase peripheral T cell numbers, primarily through augmentation of homeostatic peripheral expansion.19,22,23 Briefly, homeostatic peripheral expansion is T cell receptor driven cycling, mediated primarily by low-affinity antigens. While augmentation of thymic output has also been recently suggested,24 it remains controversial and very difficult to establish in the context of human trials. Regulatory CD4+CD25hi T cells (Tregs) express low IL-7Rα levels25,26 and unlike IL-2, IL-7 therapy expands total CD4+ T cells without expanding Tregs.16,27 IL-7 does not play a major role in Treg development, maintenance, and expansion; and in fact, recent evidence suggests that IL-7 may be capable of down modulating Treg activity.28 In summary, in addition to potent effects on developing lymphocytes, IL-7 is required for maintenance of mature T cell populations and supraphysiologic levels of IL-7 expand peripheral T cell populations through a process termed homeostatic peripheral expansion.
Potential Clinical Applications of IL-7 as an Immunorestorative
As individuals age, the adaptive immune system relies increasingly on the recruitment of memory cells to elicit immune responses. This is because the pool of naive T cells (i.e., the source of the wide diversity of specificities for antigen) decreases considerably with age. Furthermore, immune injury, whether physiologic (thymic involution with advancing age, immuno-senescence), pathologic (e.g. progressive immune depletion with HIV infection), or iatrogenic (following immune depleting therapy such as chemotherapy or irradiation), induces profound limitations in the natural pathways of T cell immune reconstitution. Immune reconstitution occurs through two primary pathways. The thymic pathway, which predominates in children, generates new T cells from pluripotent hematopoietic stem cells that home to the thymus and undergo expansion, differentiation, and selection. The resulting T cells, which bear a naive phenotype, display a diverse T cell receptor (TCR) repertoire and are poised to recognize an array of foreign antigens. In contrast, thymic-independent homeostatic peripheral expansion predominates in adults.29 Homeostatic peripheral expansion results in a skewed T cell repertoire, which is poorly diversified and limited mostly to T cells that encounter their specific antigen during the period of immune reconstitution. Furthermore, homeostatic peripheral expansion is unable to restore numbers of CD4+ T cells to pretreatment levels.30
Deficits in immune reconstitution are evident in multiple clinical settings wherein patients experience lymphocyte depletion. For instance, patients with human immunodeficiency virus infection, patients following allogeneic stem cell transplantation and older patients following high-dose cytotoxic therapy for cancer,31–37 show incomplete or prolonged periods of lymphopenia before full immune reconstitution. Individuals older than 45 to 50 years of age, who experience lymphocyte depletion, are likely to continue to have profound deficits in naїve T cells for the rest of their lives. While moderate immune competence can be accomplished through thymic-independent homeostatic peripheral expansion, new pathogens, or pathogens with high rates of mutations such as the influenza virus, may be a cause of substantial morbidity. Indeed, immune responses to immunizations such as influenza are diminished in elderly patients and subjects immunized after cancer chemotherapy appear to remain at increased risk of infection compared to controls (relative risk of developing protective titers ranges from 0.55 to 0.75, compared to a risk of 1 for normal control individuals).38 Likewise, in HIV-infected individuals, antibody responses following immunization to T-dependent antigens are significantly decreased and correlate with the CD4 count.39,40 Finally, cancer patients with limited immune reconstitution may also be at increased risk for tumor recurrence and are poor candidates for active immunotherapy strategies, which could potentially contribute to diminish disease recurrence.35
Because of the prevalence of long-term immune dysfunction in patients with age associated, iatrogenic or virus induced lymphopenia, there is great interest in administering immunorestoratives to hasten the capacity to restore normal immune function. RhIL-7 appears capable of substantially augmenting homeostatic peripheral expansion with preferential expansion of naive T cells, which bear the most diverse T cell receptor repertoires. Indeed, even athymic mice show fully restored immunocompetence with rhIL-7 therapy.41 Thus, while it is not clear that rhIL-7 can reverse age, disease, or therapy-associated thymic involution, rhIL-7’s capacity to augment naive cell proliferation may accomplish significant diversification of the T-cell receptor repertoire and restore near normal T cell diversity even in the absence of robust thymopoiesis.
Aging, in and of itself, even in the absence of lymphodepleting chemotherapy also poses a risk for diminished immune competence and preclinical data suggests that rhIL-7 therapy could be therapeutic in this setting. Age-associated abnormalities of both T cell and B cell compartments have been well described,42,43 and humoral immune responses and immunoglobulin class switch are downregulated in aged mice and humans.44,45 There are many examples of a significant decrease in vaccine responses in the elderly population (tetanus and tick-borne encephalitis,46 pneumococcal vaccine,47 influenza).48–53 Therefore, methods for improving vaccine efficiency in aged populations are critically needed. Given IL-7’s potent capacity to augment responses to immunization and to augment repertoire diversity, it is plausible to consider IL-7 therapy as a means for augmenting vaccine responsiveness in elderly populations.
Potential Role for IL-7 in Tumor-directe Immunotherapy
By enhancing immune reconstitution or expanding the immune cell repertoire, IL-7 could also have a significant role in enhancing immunotherapy for cancer.19,22,23 IL-7 augments effector and memory responses to vaccination in mice54 with preferential enhancement of responses to weak subdominant antigens, and improves survival of the CD8+ memory cell pool. In preclinical models, IL-7 therapy augments antitumor responses leading to improved survival when combined with antitumor vaccines.54,55 When combined with tumor cell immunotherapy, IL-7 significantly prolongs the survival of tumor-bearing mice. This enhanced antitumor protection correlates with an increased number of activated dendritic cells and T cells in lymphoid tissues and increased activated effector T cells in the tumor microenvironment.55
The role of IL-7 in immunotherapy may go beyond the well-described enhancement of T cell numbers and T cell repertoire diversity, decreased apoptosis and increased sensitivity to CD3 trigger.16 In a murine tumor model,28 IL-7 enhanced cytotoxic activity, increased the number of IL-17 producing CD4+ T cells, and increased serum cytokine levels (IL-6, IL-1α, IL-1β, IL-12, tumor necrosis factor-α, C-C chemokine ligand-5 (RANTES), macrophage inflammatory protein-1α). Moreover, IL-7 appeared to inhibit Treg function, and increased refractoriness to inhibitory signals by abrogating TGF-β induced inhibition of CD8+ T cell proliferation and mediating anti-TGF-β effects through down modulation of Cbl-b expression. Finally, previous work has demonstrated that IL7-Rα+ expression on activated T cells is associated with increased central memory cell generation56 and IL7-Rα+ expression on adoptively transferred antigen-specific T cells correlates with better survival in vivo.57,58 Thus, it is possible that rhIL-7 therapy could augment the effectiveness of adoptive T cell therapy for cancer by improving survival of IL-7-Rα+ central memory populations and diminishing the competitiveness of senescent IL-7-Rα− populations. Finally, recent studies have suggested that rhIL-7 can diminish PD-1 expression on activated CD8+ populations,28 an effect which would be predicted to enhance CD8+ T cell survival in vivo.
RhIL-7 in Clinical Trials
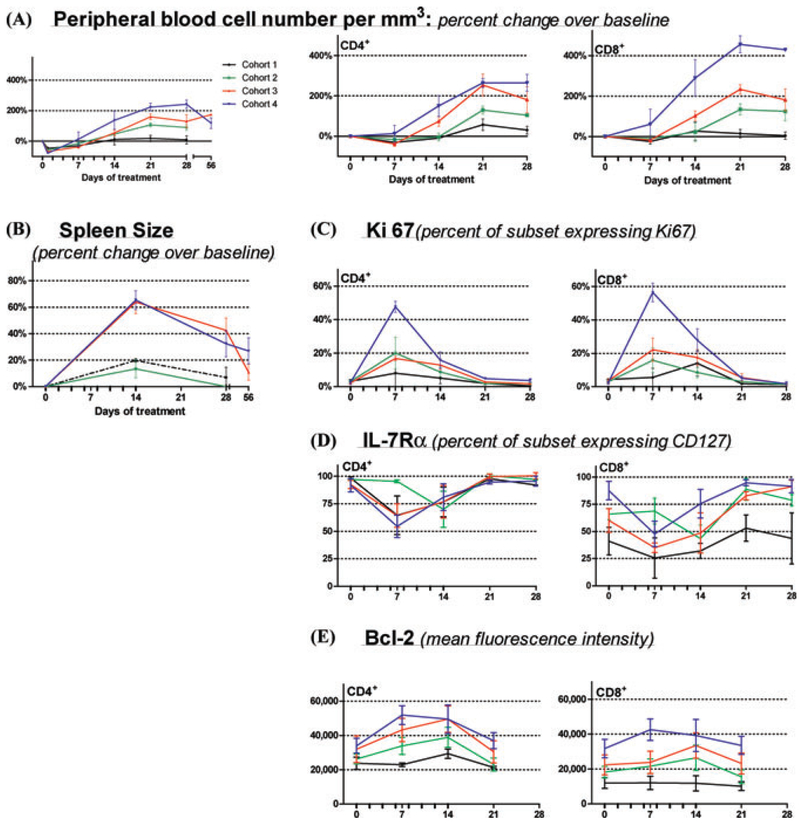
The first five studies of rhIL-7 initiated in humans evaluated an E. Coli produced, non-glycosylated rhIL-7 (“CYT99 007,” Cytheris Inc., Rockville, MD) using various phase I designs with doses ranging from 3 to 60 μg/kg in three different populations and dose schedules (see Table 1). Two trials involved oncology subjects, two trials involved HIV+ subjects, and one trial involved subjects following allogeneic transplantation for nonlymphoid malignancy. In Oncology trial 1, careful immunologic studies were performed on all IL-7 recipients allowing detailed analysis of the effects of rhIL-7 therapy in humans. All subjects treated between 10–60 mcg/kg/dose showed dose dependent increases in circulating absolute lymphocyte counts, and lymphoid organ enlargement (spleen and normal lymph nodes, but not thymus) was seen at the doses of 30 and 60 μg/kg/dose, maximum on day 14, then returning to baseline over several weeks (Fig. 1). In most subjects, CD3+ αβ and γδ cells, CD4+ and CD8+ T cells increased equally, in a clear dose-dependent fashion. The absolute cell numbers peaked one week after the end of treatment (day 21) but remained elevated for up to 2 months following the end of treatment. Similarly, increased lymphocyte counts persisted for 24 and 48 weeks after the last administered rhIL-7 dose in HIV individuals with the longest follow-up.59 There was no correlation between subject age and magnitude of the increase in CD3+, CD4+, or CD8+ T cells.
TABLE 1.
Summary of Clinical Studies with rhIL-7 “CYT 99 007”
| TRIAL | “Oncology 1” | “Oncology 2” | “HIV 1” | “HIV 2” | “Allogeneic transplantation” |
|---|---|---|---|---|---|
| Institution | NCI; ETIB, POB | NCI; SB | Case Western; Cleveland, OH, NIAID; Bethesda, MD | Hôpital Henri-Mondor, Creteil, France | MSKCC |
| Bethesda, MD | Bethesda, MD | New York, NY | |||
| Investigator | Sportès, C. | Rosenberg, S. | Lederman, M., Sereti, I. | Lévy, Y | van den Brink, M. |
| Subjects | Refractory malignancy CD3 > 300/mm3 |
Refractory metastatic melanoma | HIV: HAART > 12 mo. CD4: > 100/mm3 |
HIV : HAART > 12 mo. CD4: 100–400/mm3 |
Myeloid leukemia post allogeneic transplantation |
| Number of subjects | 16 | 12 | 17 (stratum1) 8 (stratum 2) |
14 | 1 |
| Design | Phase I: dose escalation | Phase I: dose escalation (coadministration of gp 100/MART peptide vaccines) | IL-7/placebo (random 3 to 1) 2 strata: HIV RNA: • < 50 or • 50–50,000 copies |
Phase I: dose escalation 2 strata: CD4/mm3 • 100–200 • 200–400 |
Phase I: dose escalation |
| Doses/schedule | • 3, 10, 30, 60 μg/kg/dose • every other day; • 8 doses (2 weeks) |
• 3, 10, 30, 60 μg/kg/dose • 3 times a week • 8 doses (3 weeks) |
• 3, 10, 30, 60 μg/kg/dose • single dose |
• 3, 10 μg/kg/dose • 3 times a week • 8 doses (3 weeks) |
• 3 μg/kg/dose • 3 times a week • 8 doses (3 weeks) |
| Reference | [16] | [27] | [60] | [59] |
NCI: National Cancer Institute; ETIB: Experimental Transplantation and Immunology Branch; POB: Pediatric Oncology Branch; SB: Surgery Branch; NIAID: National Institute of Allergy and Infectious Diseases; MSKCC: Memorial Sloan-Kettering Cancer Center.
Figure 1.
Effects of rhIL-7 Therapy on Circulating lymphocytes and Spleen Size. RhIL-7 injections indicated by tick marks on X-axis along with baseline, days 7, 14, 21, and 28 data points (additional data points are shown for total lymphocytes count only: day 1 for all and day 55–90 for subjects treated with 30 or 60 μg/kg/d). Mean value for each cohort (± SEM) are plotted: 3 μg/kg/d (black); 10 μg/kg/d (green); 30 μg/kg/d (red); 60 μg/kg/d (blue). (A) Absolute lymphocyte count from complete blood counts (left panels) and percent change in absolute numbers over baseline (right panels) for CD3+/CD4+ and CD3+/CD8+ cells the respective subsets are shown. (B) Spleen size: percent changes from the pretherapy bidimensional product by CT scan. (C, D, and E) CD3+/CD4+ (left panels) and CD3+/CD8+ subsets (right panels). (C) “Ki-67”: percentage of cells expressing Ki-67 (flow cytometry). (D) “IL-7Rα” expression as mean fluorescence intensity via flow cytometry. (E) “bcl-2” expression as mean fluorescence intensity (after subtracting background staining for each subset) via flow cytometry.
The effects of rhIL-7 on T cell expansion could be attributed to a combination of increased cell cycling and diminished programmed cell death but were self-limited by downregulation of IL-7Rα. At baseline, 1%–3% of circulating CD4+ and CD8+ T cells were in cycle (expressing Ki-67). Therapy resulted in a dramatic, dose-dependent increase in cycling frequency: in subjects treated with 60 μg/kg/dose, >40% of CD4+ peripheral T cells and >55% of CD8+ peripheral T cells expressed Ki-67 at day 7. T cell cycling substantially declined by day 14, coincident with maximum IL-7Rα downregulation despite sustained pharmacologic serum IL-7 levels (continued rhIL-7 administration through day 14). As a surrogate for decreased apoptosis, bcl-2 expression was increased and remained elevated throughout the rhIL-7 administration, likely contributing to the increase in T cell numbers. Indeed, at the time of peak lymphocyte expansion, one week after the end of the treatment (day 21), cell cycling had returned to baseline values.16
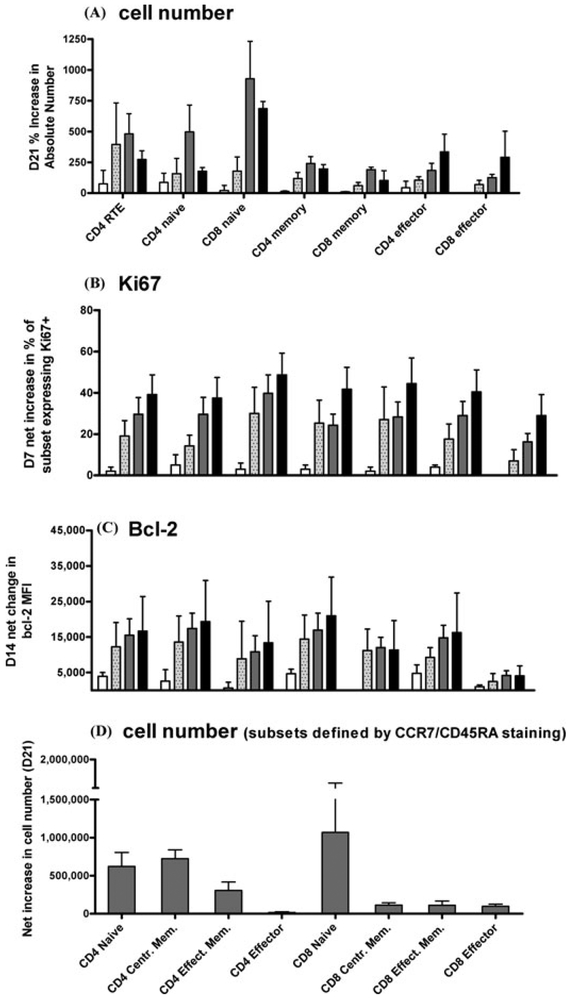
Detailed studies of T cell subsets16 showed that rhIL-7 preferentially expanded the CD4+ recent thymic emigrants (CD4+/CD31+/CD45RA+), naive (CD4+/CD27+/CD45RA+), and central memory (CD4+/CD45RA−/CCR7+) T cells as well as CD8+ naive (CD8+/CD27+/CD45RA+) T cells (Fig. 2). This T cell expansion resulted in a statistically significant increase in T cell repertoire diversity for both CD4+ and CD8+ T cells along with a decrease in the proportion of terminally differentiated effector T cells. It is also noteworthy that rhIL-7 preferentially expanded naive and central memory subsets, both of which are important in initiating lymph node germinal center formation. Tregs showed markedly less proliferation and expansion compared to other CD4+ T cells, resulting in a decrease in the proportion of circulating regulatory T cells. Therefore, within the short time frame of the study, rhIL-7 therapy induced marked expansion of circulating T cells exhibiting a rejuvenated profile resembling that seen early in life with minimal Treg expansion. These lymphocytes remained functional with conserved or increased in vitro responsiveness to anti-CD3 stimulation. Ongoing studies are underway to determine whether combining antiviral therapy with rhIL-7 may augment viral clearance in hepatitis C infection and whether rhIL-7 therapy can safely augment immune reconstitution following T cell depleted allogeneic SCT.
Figure 2.
RhIL-7 induces preferential expansion of Naive T cells and CD4+ Memory T Cells (with cycling and bcl-2 expression increase across subsets).16 Subjects treated at 3 μg/kg/d (white), 10 μg/kg/d (speckled), 30 μg/kg/d (grey) and 60 μg/kg/d (black). The time points shown represent the point of maximal increase for each parameter: (A) Percent increase in absolute circulating cell number (/mm3) over pretherapy value for each subset at Day 21 following rhIL-7. (B) Net increase from baseline in the proportion of Ki67+ cells in each subset at day 7 of rhIL-7 therapy. (C) Net increase in bcl-2 mean fluorescence intensity in each subset at day 14. (D) Net increase in cell number at day 21 in subsets defined by CCR7 and CD45RA surface expression as follows: Naive (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector memory (CCR7−CD45RA−), and effector RA (CCR7−CD45RA+).
Summary
RhIL-7 has now entered clinical trials and shows clear evidence for biologic activity that largely mirrors the results founds in mice and primates. IL-7 potently expands T cell populations, predominantly through enhanced peripheral T cell cycling. As a result, lymphopenic hosts show normalization of T cell numbers with rhIL-7 therapy, and normal hosts show supranormal T cell numbers with rhIL-7. In general, both CD4 and CD8 populations are expanded in vivo, with a tendency toward enhanced CD8 expansion. Both naive and memory subsets undergo expansion, but there appears to be a preferential expansion of naive subsets and little if any expansion of senescent memory populations. T cell receptor repertoire diversity can be enhanced by rhIL-7 therapy, even in the absence of direct thymopoietic effects. Tregs are not targeted by rhIL-7, leading to a relative reduction in Treg frequency when rhIL-7 therapy is administered. RhIL-7 shows promise as an immunorestorative for aged individuals or individuals who have experienced iatrogenic or disease induced lymphocyte depletion. It also is active as a vaccine adjuvant in preclinical models, and clinical trials are underway to determine whether it may augment viral clearance in the context of chronic viral infection when used in conjunction with antiviral therapy. Future studies will seek to determine whether rhIL-7 may enhance the effectiveness of tumor vaccines and/or adoptive immunotherapy for cancer.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Goodwin RG, Lupton S, Schmierer A, et al. 1989. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc. Natl. Acad. Sci. USA 86: 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudo T, Ito M, Ogawa Y, et al. 1989. Interleukin 7 production and function in stromal cell-dependent B cell development. J. Exp. Med 170: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peschon JJ, Morrissey PJ, Grabstein KH, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med 180: 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeden-Jeffry U, Vieira P, Lucian LA, et al. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med 181: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher AG, Burdet C, Bunce C, et al. 1995. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int. Immunol 7: 415–423. [DOI] [PubMed] [Google Scholar]
- 6.Samaridis J, Casorati G, Traunecker A, et al. 1991. Development of lymphocytes in interleukin 7-transgenic mice. Eur. J. Immunol 21: 453–460. [DOI] [PubMed] [Google Scholar]
- 7.Maraskovsky E, O’Reilly LA, Teepe M, et al. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor- deficient mice but not in mutant rag-1−/− mice. Cell 89: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 8.Akashi K, Kondo M, Freeden-Jeffry U, et al. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 9.Muegge K, Vila MP & Durum SK. 1993. Interleukin-7: A cofactor for V(DJ) rrearrangement of the T-cell receptor á gene. Science 261: 93–95. [DOI] [PubMed] [Google Scholar]
- 10.Yeoman H, Clark DR & DeLuca D. 1996. Development of CD4 and CD8 single positive T cells in human thymus organ culture: IL-7 promotes human T cell production by supporting immature T cells. Dev. Comp. Immunol 20: 241–263. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Yi H, Rosenblatt HM, et al. 1993. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73: 147–157. [DOI] [PubMed] [Google Scholar]
- 12.Macchi P, Villa A, Giliani S, et al. 1995. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377: 65–68. [DOI] [PubMed] [Google Scholar]
- 13.Russell SM, Tayebi N, Nakajima H, et al. 1995. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270: 797–800. [DOI] [PubMed] [Google Scholar]
- 14.Puel A, Ziegler SF, Buckley RH, et al. 1998. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat. Genet 20: 394–397. [DOI] [PubMed] [Google Scholar]
- 15.Sudo T, Nishikawa S, Ohno N, et al. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA 90: 9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sportes C, Hakim FT, Memon SA, et al. 2008. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med 205: 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittel BN & LeBien TW. 1995. The growth response to IL-7 during normal human B cell ontogeny is restricted to B-lineage cells expressing CD34. J. Immunol 154: 58–67. [PubMed] [Google Scholar]
- 18.Grzegorzewski KJ, Komschlies KL, Jacobsen SE, et al. 1995. Mobilization of long-term reconstituting hematopoietic stem cells in mice by recombinant human interleukin 7. J. Exp. Med 181: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fry TJ, Moniuszko M, Creekmore S, et al. 2003. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101: 2294–2299. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Yu Q, Erman B, et al. 2004. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21: 289–302. [DOI] [PubMed] [Google Scholar]
- 21.Tan JT, Dudl E, LeRoy E, et al. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98: 8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu YW, Memon SA, Sharrow SO, et al. 2004. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 104: 1110–1119. [DOI] [PubMed] [Google Scholar]
- 23.Storek J, Gillespy T III, Lu H, et al. 2003. Interleukin-7 improves CD4 T-cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood 101: 4209–4218. [DOI] [PubMed] [Google Scholar]
- 24.Beq S, Nugeyre MT, Ho Tsong FR, et al. 2006. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J. Immunol 176: 914–922. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Putnam AL, Xu-Yu Z, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddiki N, Santner-Nanan B, Martinson J, et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med 203: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. 2006. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother 29: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini M, Calzascia T, Elford AR, et al. 2009. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat. Med 15: 528–536. [DOI] [PubMed] [Google Scholar]
- 29.Mackall CL, Granger L, Sheard MA, et al. 1993. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood 82: 2585–2594. [PubMed] [Google Scholar]
- 30.Mackall CL, Bare CV, Granger LA, et al. 1996. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J. Immunol 156: 4609–4616. [PubMed] [Google Scholar]
- 31.Mackall CL, Fleisher TA, Brown MR, et al. 1994. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 84: 2221–2228. [PubMed] [Google Scholar]
- 32.Hakim FT, Cepeda R, Kaimei S, et al. 1997. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood 90: 3789–3798. [PubMed] [Google Scholar]
- 33.Mackall CL, Fleisher TA, Brown MR, et al. 1997. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 89: 3700–3707. [PubMed] [Google Scholar]
- 34.Mackall CL 2000. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells 18: 10–18. [DOI] [PubMed] [Google Scholar]
- 35.Hakim FT. & Gress RE. 2005. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology. Eur. J. Immunol 35: 3099–3102. [DOI] [PubMed] [Google Scholar]
- 36.Hakim FT, Memon SA, Cepeda R, et al. 2005. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest 115: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sportes C, McCarthy NJ, Hakim F, et al. 2005. Establishing a platform for immunotherapy: clinical outcome and study of immune reconstitution after high-dose chemotherapy with progenitor cell support in breast cancer patients. Biol. Blood Marrow Transplant 11: 472–483. [DOI] [PubMed] [Google Scholar]
- 38.Gross PA, Gould AL & Brown AE. 1985. Effect of cancer chemotherapy on the immune response to influenza virus vaccine: review of published studies. Rev. Infect. Dis 7: 613–618. [DOI] [PubMed] [Google Scholar]
- 39.Kroon FP, van Dissel JT, de Jong JC, et al. 1994. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS (London, England) 8: 469–476. [DOI] [PubMed] [Google Scholar]
- 40.Malaspina A, Moir S, Orsega SM, et al. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis 191: 1442–1450. [DOI] [PubMed] [Google Scholar]
- 41.Fry TJ, Christensen BL, Komschlies KL, et al. 2001. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 97: 1525–1533. [DOI] [PubMed] [Google Scholar]
- 42.Johnson SA & Cambier JC. 2004. Ageing, autoimmunity and arthritis: senescence of the B cell compartment – implications for humoral immunity. Arthritis Res. Ther 6: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulop T, Larbi A, Wikby A, et al. 2005. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs & Aging. 22: 589–603. [DOI] [PubMed] [Google Scholar]
- 44.Frasca D, Nguyen D, Riley RL, et al. 2003. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J. Immunol 170: 719–726. [DOI] [PubMed] [Google Scholar]
- 45.Frasca D, Riley RL & Blomberg BB. 2005. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin. Immunol 17: 378–384. [DOI] [PubMed] [Google Scholar]
- 46.Hainz U, Jenewein B, Asch E, et al. 2005. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 23: 3232–3235. [DOI] [PubMed] [Google Scholar]
- 47.Artz AS, Ershler WB & Longo DL. 2003. Pneumococcal vaccination and revaccination of older adults. Clin. Microbiol. Rev 16: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirota Y, Kaji M, Ide S, et al. 1996. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 14: 1597–1602. [DOI] [PubMed] [Google Scholar]
- 49.Gross PA, Russo C, Teplitzky M, et al. 1996. Time to peak serum antibody response to influenza vaccine in the elderly. Clin. Diagnostic Lab. Immunol 3: 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murasko DM, Bernstein ED, Gardner EM, et al. 2002. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol 37: 427–439. [DOI] [PubMed] [Google Scholar]
- 51.Fulop T Jr., Wagner JR, Khalil A, et al. 1999. Relationship between the response to influenza vaccination and the nutritional status in institutionalized elderly subjects. J. Gerontol. A Biol. Sci. Med. Sci 54: M59–M64. [DOI] [PubMed] [Google Scholar]
- 52.Nichol KL, Nordin JD, Nelson DB, et al. 2007. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med 357: 1373–1381. [DOI] [PubMed] [Google Scholar]
- 53.Simonsen L, Taylor RJ, Viboud C, et al. 2007. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 7: 658–666. [DOI] [PubMed] [Google Scholar]
- 54.Melchionda F, Fry TJ, Milliron MJ, et al. 2005. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest 115: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li B, Vanroey MJ & Jooss K. 2007. Recombinant IL-7 enhances the potency of GM-CSF-secreting tumor cell immunotherapy. Clin. Immunol 123: 155–165. [DOI] [PubMed] [Google Scholar]
- 56.Kaech SM, Tan JT, Wherry EJ, et al. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 57.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. 2005. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med 202: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powell DJ Jr., Dudley ME, Robbins PF, et al. 2005. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood 105: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy Y, Lacabaratz C, Weiss L, et al. 2009. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J. Clin. Invest 119: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sereti I, Dunham RM, Spritzler J, et al. 2009. IL-7 administration drives T cell cycle entry and expansion in HIV-1 infection. Blood 113:6304–14 [DOI] [PMC free article] [PubMed] [Google Scholar]